Abstract
Listeria monocytogenes is both a life-threatening pathogen of humans and a model organism that is widely used to dissect the mechanisms of innate and adaptive immune resistance to infection. Specific aspects of the immune response to systemic Lm infection can be protective, neutral, or in some cases deleterious. In this review, we seek to provide an overview of the early events during Lm infection that dictate or regulate host innate and adaptive immune responses. We highlight several recent developments that add to our understanding of the complex interplay between inflammatory responses, host susceptibility to infection, and the development of protective immunity.
Listeria and Listeriosis
Listeria monocytogenes (Lm) is a Gram-positive bacterium that replicates in the cytosol of eukaryotic cells to cause severe gastrointestinal, systemic, and central nervous system infections collectively referred to as Listeriosis. Listeriosis is often associated with food-borne epidemics and is amongst the deadliest of food-borne diseases. A recent U.S. epidemic during summer-fall 2011 resulted in 146 confirmed cases with 30 (20.5%) deaths [1]. Listeriosis is most frequently diagnosed in elderly, pregnant, or immune compromised individuals. It is thus thought that altered or deficient immune responses predispose to Listeriosis. However, it remains unclear precisely which aspects of the immune response are responsible for the increased susceptibility to severe Lm infections in these human populations.
Much of what we do know about immunity to Lm comes from studies using the murine model of Listeriosis (see Text box 1). Recent studies using this model have provided new insights into our understanding of the complex interplay between Lm and the murine host, many of which likely also have relevance to human Listeriosis. In addition, these findings likely have implications for our understanding of how immunity develops and is regulated in the context of other diseases. Below, we review several of these recent findings. We focus first on studies revealing features of innate host defenses that Lm evades, subverts, or possibly even commandeers during establishment of systemic infection. These studies suggest ways in which Lm infection creates an immunological environment favorable for early bacterial expansion and dissemination within the host. We then discuss how inflammatory and innate immune factors impact development of adaptive immunity. At least in some cases, immune responses that benefit the pathogen at early stages of infection also help to ensure development of potent adaptive immune responses.
During systemic infection, Lm are rapidly engulfed by myeloid cells
Lm that enter the bloodstream are taken up within 1-2 min of inoculation by various myeloid cells in the spleen and other tissues [2]. In the spleen, the bacteria are primarily filtered out of the blood by cells present in the marginal zone [3]. Resident myeloid cells in the marginal zone include dendritic cells (DCs) and professional phagocytes such as marginal zone and metallophilic macrophages, F4/80+ macrophages, and neutrophils [4,5]. Inflammatory stimuli also induce the recruitment of Ly6Chi inflammatory monocytes to infected tissues [6]. It seems that all of these cell types participate in the engulfment of circulating bacteria (Fig. 1). However, the fate of Lm within the different cell types is quite distinct. Some phagocytes protect the host by killing engulfed bacteria, while uptake by other myeloid cells instead correlates with increase host susceptibility. Presumably, infection of these cells creates an environment hospitable for rapid Lm replication and dissemination.
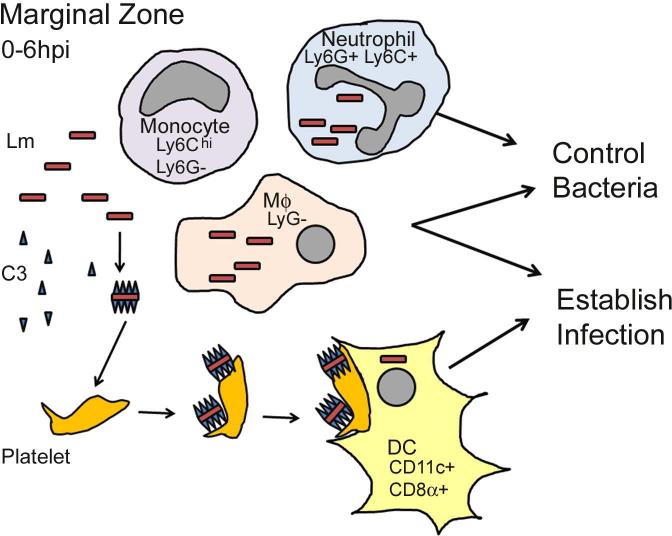
Figure 1. Immediate-early uptake of Lm from the blood.
Marginal zone phagocytes, including neutrophils, monocytes, macrophages (MΦ), and dendritic cells (DC) engulf blood-borne Lm. Ly6G+ neutrophils and Ly6G- macrophages kill phagocytosed Lm to control bacterial growth. Deposition of complement component C3b on Lm promotes bacterial binding to and aggregation of CD41+ platelets. These aggregates are phagocytosed by CD8α+CD11c+ DCs, shielding Lm from other more microbicidal phagocytes. Migration of DCs to the PALS permits increased bacterial replication during systemic infection.
Relatively few Lm within the circulation are initially phagocytosed by patrolling neutrophils [2,4]. However, these neutrophils are extremely effective at bacterial killing. Early work using antibody-mediated depletion of neutrophils with the Gr-1 monoclonal antibody (mAb) suggested that neutrophils reduce Lm burdens in the liver by 10-100 fold within 12-24 hrs post infection (hpi), whereas significant protective effects were not seen in spleens or the peritoneal cavity [7]. An important caveat to these conclusions is that the Gr-1 mAb recognizes both Ly6G and Ly6C. Ly6G expression is largely restricted to neutrophils, whereas Ly6C is expressed also by inflammatory monocytes and some lymphocytes. Three more recent studies thus investigated the effects of neutrophil depletion using the anti-Ly6G mAb 1A8 [8-10]. In two of these studies, the 1A8 mAb was administered 1 d prior to infection and increased bacterial burdens in the livers by 10-1000 fold between 1-3 d after infection. With a higher infectious dose, the 1A8 treatment also increased bacterial burdens in the spleens [8]. In the third study, the authors failed to see significant effects of 1A8 treatment on bacterial burdens [10]. However, this study used a lower amount of mAb and administered it at the time of infection, which may have enabled the neutrophils to engulf and kill Lm prior to their depletion. This study also showed that depletion of Ly6Chi cells using either CCR2-DTR mice or anti-Gr-1 caused increased susceptibility to Lm [10]. Moreover, a low dose of anti-Gr-1 mAb that selectively depleted neutrophils caused a mild increase in Lm burdens at 48 hpi, while higher doses of anti-Gr-1 also depleted Ly6Chi inflammatory cells and more dramatically increased Lm burden [2]. The early uptake of Lm by neutrophils and inflammatory monocytes thus both appear capable of increasing innate resistance. However, these cells “compete” with other myeloid cells for uptake of blood-borne Lm. Such competition appears to dramatically impact the host-pathogen interaction.
Infection of DCs helps to establish more severe systemic Listeriosis
Although Lm initially enters the spleen via the marginal zone, by 24 h post-infection (hpi) most bacteria are found in the T cell zones within periarteriolar lymphoid sheath (PALS) [4,5,11]. There has been considerable interest in defining the mechanisms for this rapid re-localization of Lm, as it precedes a dramatic burst of bacterial growth and severe systemic infection. It is now clear that most Lm present within the white pulp at 24 hpi are associated with CD8α+CD11c+ DCs (Fig. 2). To address the question of how DCs impact Lm uptake and traffic within the spleen, one group evaluated the effects of diptheria-toxin (DT) treatment in CD11c-DTR mice, which have expression of a DT receptor (DTR) transgenic in all CD11c+ populations [4]. DT treatment of these mice eliminates all splenic DC subsets, but also MOMA-1+ metallophilic and ERTR-9+ marginal zone macrophages [12]. When DT was administered prior to (but not after) Lm infection, bacterial numbers recovered from spleens (but not livers) were reduced by ~500 fold by 72 hpi [4]. The effects of DT treatment seemed due to depletion of the DC population, as inoculation of depleted mice with Lm-infected bone marrow-derived DCs (BMDCs) led to efficient spleen infection. The authors further confirmed that CD8α+ DCs are present within the splenic marginal zone and red pulp prior to infection. By quantifying bacterial cfu from sorted splenic phagocytes, it was also clear that a substantial proportion of CD8α+ DCs (but not CD8α- DCs) were infected and contained the majority of live bacteria present in the spleen by 1-3 hpi.
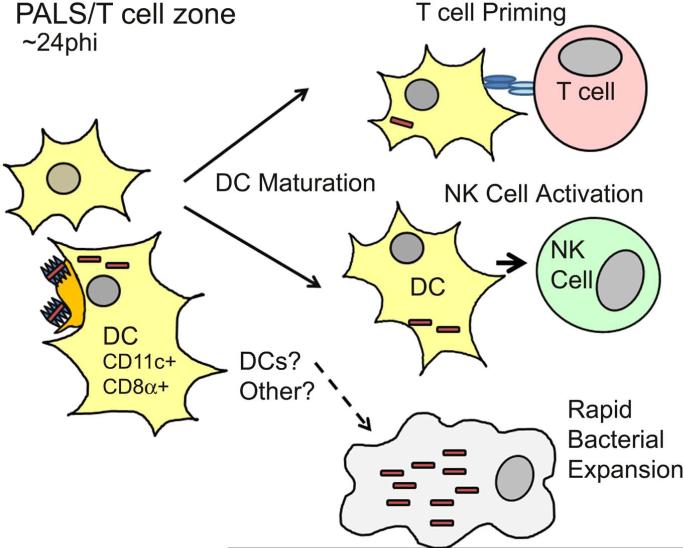
Figure 2. Consequences of Lm infection in the splenic PALS.
By 24 h post-infection (hpi), both uninfected and Lm-associated CD8α+CD11c+ dendritic cells migrate from the marginal zone into the PALS. Rapid bacterial expansion ensues in one or more cell types. Dendritic cells mature in response to inflammatory cytokines, allowing them to activate natural killer cells and prime naïve CD4+ and CD8+ T cells.
More recent work further supports the notion that CD8α+ DCs play an important role in establishment of systemic Lm infection. Mice deficient for expression the basic leucine zipper transcription factor ATF-like 3 (Batf3) lack functional CD8α+ DCs [13]. Batf3-/- mice were more susceptible to death during infection by the parasite Toxoplasma gondii [14], but survived an otherwise lethal dose of Lm [9]. These differing phenotypes suggest that, unlike T. gondii, Lm has evolved the ability to benefit from the presence of CD8α+ DCs and/or other effects of Batf3 expression. Consistent with this notion and data from the CD11c-DTR mice, spleens of batf3-/- mice harbored ~10-fold fewer Lm at 24 hpi and failed to support the rapid ~1000-fold increase in bacterial burdens seen in wt spleens by 72 hpi. However, unlike CD11c-DTR mice, Lm growth was also reduced in livers of the batf3-/- mice [9]. Since depletion of liver CD11c+ cells was also observed in CD11c-DTR mice [4], the more pronounced phenotype of batf3-/- mice may reflect earlier or more complete loss of hepatic CD103+ DCs [9]. Alternatively, the differences in liver colonization in batf3-/- and CD11c-DTR mice may reflect use of a different Lm strain, use of mice on different genetic backgrounds (129 vs. B6), or confounding secondary effects of the Batf3 deficiency. Regardless, findings with both batf3-/- and CD11c-DTR models are suggestive that splenic CD8α+DC harbor Lm early after infection of the spleen and this contributes to enhanced bacterial growth. However, data indicating that spleens of wt mice and batf3-/- mice had similar Lm burdens at 3 hpi [9], and data showing that infected bone marrow-derived macrophages (BMM) seeded splenic infection in DT-treated CD11c-DTR mice [4], argue that other myeloid cell types may also be able to support replication of splenic Lm under specific conditions.
Histology and flow cytometry revealed a substantial impact of Batf3 deficiency on migration of Lm and (Langerin+) CD8α+ DC from the splenic marginal zone to the PALS [9]. Early after infection, Lm were contained within the red pulp in both wt and batf3-/- mice. However, in wt mice only, uninfected and infected Langerin+ DCs had migrated to follicles of the PALS by 18 hpi. There was also abundant neutrophil infiltration at these sites. Antigen from Lm was detected by intracellular staining in neutrophils and inflammatory monocytes as well as CD8α+ DC. The number of infected neutrophils and monocytes staining positive for Lm antigen increased with infection dose, and was much greater in wt versus batf3-/- spleens. Surprisingly, however, the staining failed to detect Lm antigen in the CD8α+ cells from wt mice. The authors concluded that rapid intracellular bacterial growth caused death of any infected CD8α+ DCs, consistent with an observed reduction in CD8α+ DC numbers. A separate study also observed reduced DC numbers in the spleen by 48 hpi [15]. However both this and other studies reported isolation of viable Lm from CD8α+DCs at 24 hpi using sensitive cfu-based assays [4,16] [15]. Whether the reduction in splenic DC numbers is a direct consequence of bacterial replication and killing of infected DCs thus remains to be determined.
Lm uptake by CD8α+DCs was recently shown to be enhanced by deposition of the complement component C3 on the bacterial surface [16]. C3-/- mice had ~10-fold reduced splenic Lm burdens at 1 dpi and failed to increase during the first 3 dpi. These effects were independent of the C3a receptor, and opsonization of Lm with C3b prior to infection resulted in increased bacterial burdens in spleens of the C3-/- mice ~10-fold at 1 hpi. Pre-opsonization of Lm with C3b also increased recovery of viable Lm from sorted CD8α+DCs at 1 hpi. Prior to binding DCs, opsonized Lm rapidly aggregated with CD41+ platelets via the platelet protein GPIb. Depletion of platelets impaired Lm targeting to DCs and bacterial replication in the spleens. Thus, the rapid (within minutes) association of C3 and platelets with circulating Lm caused a more rapid clearance of bacteria from the blood and appeared to direct them to CD8α+ DCs. Such direction appears to “shield” the bacteria from uptake and killing by other phagocytes, such as neutrophils and macrophages. Yet, depletion of DCs did not impact the rate of Lm clearance from the blood. This suggests that in the absence of CD8α+DCs, the C3 opsonization process may direct Lm to other cells types that are presumably less-susceptible to Lm growth and establishment of systemic infection. Overall, the findings suggest that Lm initially benefits from C3 opsonization and DC uptake. However, infection of CD8α+DCs also leads to a more robust stimulation of innate and adaptive immune responses.
DC infection is important for natural killer (NK) cell activation
Migration of Lm-infected DCs into the PALS early after infection is accompanied by co-migration of natural killer (NK) cells [17]. The kinetics of NK cell activation and production of type II interferon (IFNγ) also peak at 24 hpi [18], when both cell types are clustered in the PALS [17]. Hence, it seems likely that contact with Lm-infected DCs can directly stimulate activation of NK cells within the PALS. Consistent with this model the depletion of CD11c+ cells using CD11c-DTR mice [19], or CD11c-cre x flox-stop-flox-DTR mice [17], impaired NK cell activation during Lm infection. In addition, contact with Lm-infected BMDCs stimulates naive NK cells to produce IFNγ in vitro [20]. However, while over >70% of splenic NK cells produce IFNγ by 24 hpi [17,18], only a small fraction of the total CD8α+DC population in the PALS contain Lm at 24 hpi [4,9,16]. It thus seems that NK cells need not actually contact Lm-infected DCs to become activated. Rather, an abundantly secreted bacterial protein (p60) has been found to drive the robust NK cell activation during Lm infection [18]. Purified p60 binds DCs and stimulates them to activate NK cells in the absence of other Lm factors [21]. The host cytokine Interleukin (IL)-18 was also crucial for this NK cell activation.
It is unclear how Lm benefits from activation of NK cells. NK cells contribute to defense against some viruses and tumors and, as mentioned above, are potent producers of IFNγ early after infection. Early IFNγ production by NK cells could in theory increase inflammation and macrophage activation. However, an older study found that antibody-mediated depletion of NK1.1+ cells (both NK and NKT) reduced Lm burdens following systemic infection [22]. The mechanisms for this effect have also remained undefined, but NK cells mediate immune suppressive roles in the context of neuroinflammation [23]. Perhaps NK cell activation is similarly immune suppressive during systemic Lm infection. With regards to the production of IFNγ by NK cells, production of type I IFNs during this same time period has been shown to suppress macrophage activation by IFNγ [24]. This effect of type I IFNs is one of several mechanisms that have been proposed to explain why mice deficient in IFNAR expression are ~1000 fold more resistant to Lm infection than are type I IFN-responsive mice [24-28]. Perhaps suppression of IFNγ responsiveness and/or other proposed mechanisms function in parallel with NK cell activation to increase host susceptibility to Lm.
Inflammasome activation and its impact on the innate immune response to Lm
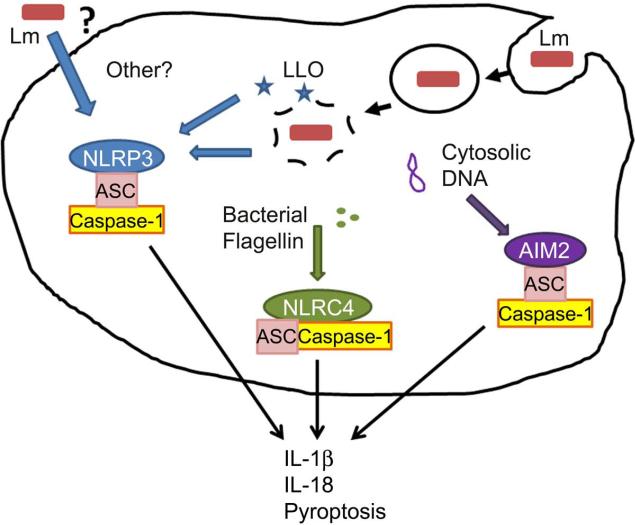
The cytokines IL-1 and IL-18 play important roles in regulating the inflammatory response and the production of other cytokines and chemokines. IL-1β and IL-18 are synthesized in the cytosol of macrophages, DCs, and other cells as biologically inactive pro-proteins. They become active when cleaved by cysteine proteases, including caspase-1 and 11, and are secreted from the cell. The activation of these proteases occurs in multi-protein complexes termed “inflammasomes,” which contain scaffold components such as ASC along with adaptor/sensor proteins that respond to various pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Recently, production of IL-1β and IL-18 by Lm infected BMM was shown to be regulated by each of the NLRP3, NLRC4, and AIM2 inflammasomes (Fig. 3).
Figure 3. Inflammasome activation by Lm.
In cultured macrophages the activity of the secreted Lm hemolysin (Hly/LLO) is essential for the ability of Lm to escape from phagosomes and growth in the cytosol of infected cells. Mutant ΔHly Lm strains elicit weak IL-1β/IL18 secretion from these cells [103-106], suggesting poor stimulation of cytosolic adaptors and/or poor growth. AIM2 was recently identified as a DNA-sensing inflammasome sensor and mediates secretion of IL-1β and IL-18 by Lm infected macrophages [105,106]. DNA presumably leaks from Lm at a low frequency during infection of the host cell cytosol. Lm flagellin is detected by the NLRC4/Ipaf inflammasome [104]. However, NLRC4 plays very little role in responding to infection by the EGD Lm strain, which suppresses flagellin expression at 37C [106]. Lm strains engineered to over-express Lm or Legionella flagellin hyperactivate the NLRC4 inflammasome [30,107]. Activation of AIM2 and NLRC4 inflammasomes in Lm-infected macrophages also induces cell death via a process termed “pyroptosis” [30,105,107,108]. In these studies, increased pyroptosis in cultured macrophages correlated with reduced bacterial burdens in vivo, suggesting that lysis of infected cells impairs Lm growth or dissemination during systemic infection. The NLRP3 inflammasome is sensitive to a variety of signals, including membrane damage and signaling from dectins or other cell surface receptors [109]. Hly may itself modulate activation of the NLRP3 inflammasome [110], though the mechanism is unclear. The Lm p60 protein also affects secretion of IL-18 [21], suggesting it may activate one or more inflammasomes.
The in vivo roles of inflammasomes and of the inflammasome-regulated cytokines IL-1β and IL-18 remain incompletely understood. In the context of Lm infection, one group reported that caspase-1 deficient (casp1-/-) mice were more susceptible, which correlated with impaired IL-18 production [29], while another reported that burdens of Lm were similar following systemic infections of control, casp1-/- and ASC-deficient mice [30]. Two commercially-available casp1-/- mouse strains were made using 129 embryonic stem cells [31,32], and a recent report revealed that the casp1 linked gene encoding caspase 11 (casp11) is non-functional in the 129 strain background [33]. Whether and how casp11 mutations impact the results of these and other studies using casp1-/- mice is unclear. With regards to IL-1β and IL-18 prior studies have also given variable results. In some settings, IL-1β reportedly promotes immunity to Lm by increasing production of IL-6 and TNFα and recruitment of neutrophils and macrophages [34-36]. Yet, responses to IL-1β were dispensable for resistance to Lm in other settings [37,38]. IL-18 can stimulate innate production of IFNγ by memory T cells and NK cells [29,39,40], and was reported to enhance Lm clearance in synergy with TNFα in the absence of IFNγ [39] Yet, in one study neutralization of IL-18 or IL-18 deficiency increased host resistance to systemic Lm infection [41]. It will be important to reconcile the roles of IL-1β and IL-18 during systemic Lm infection. Clearly these cytokines can regulate innate and adaptive immune responses, but it remains uncertain precisely how they impact pathogen burdens.
Pattern recognition and initiation of adaptive immunity to Lm
Innate immune responses play a key role in mediating the transition to effective cell-mediated immunity. The most common view is that potent innate responses are favorable for the development of potent adaptive immunity, but in recent years it has become clear that inflammatory responses also have regulatory effects on the development of adaptive immunity. As discussed below, the needs of the innate and T cell-mediated responses appear in some cases to be opposed.
Lm mutants that lack Hly or are otherwise unable to exit endosomal compartments into the cytosol are highly attenuated and fail to establish long-term protective immunity to secondary infections [42]. Similarly, mice lacking the endosomally-restricted enzyme GILT, an important component of MHC Class II antigen processing that reduces disulfide bonds in the LLO protein and thus mediates its activation, showed defects in antigen processing and poor generation of anti-bacterial immunity but more rapid control of infection [43,44]. Failure of Lm to enter the cytosol impairs T cell responses not simply due to a lack of appropriate antigen presentation. Entry of Lm into the cytosol stimulates distinct pattern recognition receptors (PRRs) and diverse transcriptional programs [45,46]. Thus, retention of Lm in the phagosome is itself immunomodulatory. Co-infection with phagosome-retained Lm prevented the generation of optimal adaptive immunity to an Lm strain with normal ability to escape phagosomes and enter the cytosol [47]. This effect was IL-10-dependent, suggesting that restricting Lm to the phagosome was actively immunomodulatory and not necessarily related to antigen sequestration [47].
The idea that triggering of PRRs in the cytosol can enhance adaptive cell-mediated responses to Lm is supported by the observation that mice lacking the cytoplasmic PRR, Nod2, are defective in their ability to generate adaptive responses in the gut to bacterial infection [48]. As mentioned above, cytosolic entry of Lm can also cause activation of one or more inflammasomes. Inflammasome activation provides enhanced adaptive immunity to a variety of pathogens [49-52]. Yet, the activation of cytosolic PRRs is not always beneficial to the adaptive response. For example, Lm engineered to stimulate increased NLRC4 inflammasome activation was more susceptible to innate response-mediated protection, as judged by faster clearance of primary Lm challenge. However, such engineering dampened adaptive cell-mediated responses, leading to impaired control of secondary Lm challenge [30,47].
The induction of adaptive immunity is similarly impacted by mutations to Lm that alter its cytosolic lifestyle, including changes in genes that control its secretion of virulence factors. For example, immunization of mice with heat-killed Lm induces the formation of CD8+ “memory” T cells that are unable to provide efficient protection from Lm rechallenge [53], whereas immunization with irradiated Lm that still retained its stimulatory properties and at least some of its cytosolic lifestyle readily induced protective CTL memory [54]. One well-studied example is the SecA2 secretion system. SecA2-mediated secretion in the cytosol promotes Lm pathogenesis, and Lm deletion mutants are attenuated [55,56]. Yet, while more readily cleared by the immune system, SecA2 deletion mutants fail to induce protective CD8+ T cell immunity [57,58]. In particular, SecA2 is required for the generation of CCL3-secreting CD8+ memory T cells in response to Lm infection [11], an important component of protective anti-Listeria immunity provided by this population [59]. These findings illustrate that the link between innate and adaptive immunity to Lm is not a simple correlation. Rather, the specific flavor of innate immune stimulation is likely to have profound effects on the outcome of the adaptive immune response.
DCs are crucial for initiating adaptive immunity to Lm
Dendritic cells (DCs) are required to initiate primary and secondary anti-Lm CTL responses and form the central lynchpin for initiation of adaptive immunity in other infectious settings [60,61]. The CD8α+ subset of DCs most efficiently presents Lm-derived antigens [62]. Additionally, the cross-priming capabilities of this DC subset are required for optimal immunity to Lm [63]. DC maturation is likely a key event in the development of protective immunity. However, the precise mechanisms of DC maturation and the specific DC functional phenotypes for the optimal induction of T cell immunity remain unclear. MyD88 activity is a critical element in the development of both innate and adaptive responses to Lm [64,65], suggesting a critical role of TLR activation in coordinating cells of the innate response as well as mediating the transition to effective T cell-dependent immunity. However, both MyD88 and type I IFN signaling may be somewhat dispensable in APCs [66]. Conversely, Lm-infected monocytes, while able to produce pro-inflammatory cytokines such as TNFα and IL-12, attenuate the ability of CD8α+ DCs to prime T cell responses [67].
The precise inflammatory environment that promotes optimal APC activation, antigen presentation and subsequent T cell immunity during Lm, as well as the mechanisms by which this is accomplished, remain incompletely defined. While much of the attention is rightly focused on Th1-type cytokines and classically restricted T cell responses, a variety of other factors have been recently identified that promote effective DC maturation and T cell priming during Lm infection. For example, IL-23, a cytokine associated with the development of Th17 responses, is required for optimal clearance of Lm [68]. IL-23 may regulate the generation of IL-17-producing γδ T cells. IL-17 produced by these cells in turn enhances DC cross-presentation [69]. Lm elicits non-classically restricted T cells responses involving the non-classical MHC Class I molecule H2-M3 [70,71]. T cells restricted to H2-M3 are activated quickly after infection and promote DC maturation and priming of conventional T cell responses [72]. Additionally, GM-CSF enhances cross-presentation by CD8α+ DCs during Lm infection [73]. Overall, emerging information suggests a complex and not necessarily intuitive interplay between innate immune responses, DC maturation and T cell priming. In particular, the requirement for cross-presentation in the generation of anti-Lm T cell responses suggests that DCs that are directly infected may not be particularly effective in priming T cell responses. Perhaps innate recognition mechanisms in the endosomal and cytosolic compartments of infected DCs counteract the signals required for their maturation and T cell priming capabilities.
The complement component C3 is required for the efficient generation of anti-Lm CTL responses, with a potentially direct role in influencing and potentiating CTL activation [74]. However, as discussed above, C3 serves as a mediator of platelet-dependent shuttling of live Lm from the blood to CD8α+ DCs in the subcapsular region of the spleen [16]. Thus, the role of C3 in promoting CTL responses may not necessarily reflect its inflammatory impact. Indeed, Lm trafficking to the PALS correlated with enhanced adaptive immunity [16], and rapid Lm associations with subcapsular DCs are followed by interactions of T cells with both infected and uninfected DCs [2]. The enhanced innate resistance of batf3-/-deficient mice also correlated with impaired adaptive immunity to Lm [9]. CD8α+ DCs therefore serve the beneficial function of inducing adaptive immunity while also serving as a cellular port of entry for bacterial spread within the spleen during bacteremia.
Other factors regulating development of anti-Lm adaptive immunity
Anti-Lm T cell responses are dominated by the presence of CTLs and Th1 cells [75]. However, the presence of Th17 cells [76], follicular helper T cells [77] and regulatory T cells (Tregs) [78] has also been documented, although their specific roles in supplementing or counteracting Th1 and CTL-mediated immunity needs to be further resolved. While Tregs do not expand during Lm infection [79], they do play an early role in impeding the priming of protective CTLs [80]. Both γδTCR+ [68] and conventional Th17 responses [81] can be detected during Lm infection, but they appear to be short-lived in comparison to Th1 responses and their generation is dependent on the route of infection [81]. The quality and duration of the initial priming event can play a role not only in the differentiation and magnitude of primary and secondary of effector Th1 responses, but also in the generation of long-lived Th1 memory cells [82,83]. However, environmental factors also play an important role, as cytokines present at the time of T cell priming can have a profound impact on the generation of both T cell effector and memory responses. Additionally, the presence of CD4+ T cell “help” at the time of CTL priming and during memory CTL maintenance influence both the size and functional capacity of long-lived immune protection provided by memory CTLs [84,85,86,87]. The amount of IL-12 and other inflammatory cytokines regulates the differentiation of short-lived effector CTL [88-91], while IL-2 plays a key role in the generation of Th1 and CTL-mediated protective immunity [77,92-94]. While Lm has served as an immensely helpful model system for understanding fundamental aspects of effector and memory T cell differentiation, further work is needed to understand the specific mechanisms that control the effective presentation of antigen to promote T cell priming while promoting robust innate immune responses. In particular, a better understanding of the mechanisms controlling the maturation, antigen presentation and T cell priming capabilities of DCs during Lm infection will provide better insight into the nature and protective functions of the complex adaptive T cell response that they induce.
Concluding remarks
A common theme of several recent studies highlighted in this review is that of a trade-off between the requirements for initiating innate and adaptive responses to Lm, and potentially other intracellular bacterial infections as well. Certain innate immune responses appear to be crucial for creating an environment in which Lm can rapidly replicate within the host and establish a high titer systemic infection. Presumably these responses are beneficial to the bacterium and there is evidence that bacterial factors may drive certain of these responses. However, other “pro-bacterial” innate immune responses, such as complement fixation and bacterial uptake by CD8α+ DCs, play also important roles in priming of protective adaptive immune responses. These findings provide a platform for understanding how the pathogenesis of Lm infection ultimately contributes to the establishment of CTL and Th1 responses. They also suggest several steps where defects in innate or adaptive immunity may render human hosts more susceptible to infection by Lm and other pathogens.
Text box 1 - The mouse model of systemic Listeriosis.
The systemic murine infection model first developed in the early 1960s by George Mackaness remains the most prevalent for studying immune responses to Lm [95]. In this model, Lm are inoculated intravenously (i.v.) directly into the bloodstream. Low dose Lm infections are cleared within 7-10 d after infection of immune competent naïve mice. This clearance correlates well with development of potent CTL responses as well as the formation of Th1 effector cells [96,97]. Deficiency in either MHC class I or MHC class II delays clearance of Lm infection [98], and mice lacking all T cells develop chronic infections and ultimately die from an otherwise sublethal infection [99]. Most intracellular pathogens, and particularly for those that gain access to the cytosol, require activated CTL for immune protection and this is indeed the case for Lm [75,100-102]. By contrast, antibody responses are typically not a significant means of immune protection during natural infection [75,102], presumably because cytosolic Lm spreads directly from infected cells to establish secondary infection to neighboring cells via actin-based motility. In mice, clearance of Lm is associated with long-lived protective cellular immune responses. Although T cell responses are crucial for sterilizing immunity to Lm, certain innate and inflammatory events are important for initial containment of the infection. Moreover, inflammatory responses contribute to antigen presentation by appropriately activated APCs and subsequent initiation of T cell responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC 08-Dec. [01-Mar.-2012];Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado. 2011 http://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/120811/index.html. [Online].
- 2.Waite JC, et al. Dynamic imaging of the effector immune response to listeria infection in vivo. PLoS Pathog. 2011;7:e1001326. doi: 10.1371/journal.ppat.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlan JW. Early pathogenesis of Listeria monocytogenes infection in the mouse spleen. J. Med. Microbiol. 1996;44:295–302. doi: 10.1099/00222615-44-4-295. [DOI] [PubMed] [Google Scholar]
- 4.Neuenhahn M, et al. CD8α+ Dendritic Cells Are Required for Efficient Entry of Listeria monocytogenes into the Spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Aoshi T, et al. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur. J. Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 7.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr KD, et al. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur. J. Immunol. 2011;41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelson BT, et al. CD8α+ Dendritic Cells Are an Obligate Cellular Entry Point for Productive Infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, et al. Ly6G(+) neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. The Journal of Immunology. 2011;187:5293–5298. doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muraille E, et al. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. Eur. J. Immunol. 2005;35:1463–1471. doi: 10.1002/eji.200526024. [DOI] [PubMed] [Google Scholar]
- 12.Probst HC, et al. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin. Exp. Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashayekhi M, et al. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell LM, et al. Distinct responses of splenic dendritic cell subsets to infection with Listeria monocytogenes: maturation phenotype, level of infection, and T cell priming capacity ex vivo. Cell. Immunol. 2011;268:79–86. doi: 10.1016/j.cellimm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verschoor A, et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nature Immunology. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 17.Kang S-J, et al. Regulation of Hierarchical Clustering and Activation of Innate Immune Cells by Dendritic Cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humann J, et al. Expression of the p60 autolysin enhances NK cell activation and is required for listeria monocytogenes expansion in IFN-gamma-responsive mice. J. Immunol. 2007;178:2407–2414. doi: 10.4049/jimmunol.178.4.2407. [DOI] [PubMed] [Google Scholar]
- 19.Lucas M, et al. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humann J, Lenz LL. Activation of Naive NK Cells in Response to Listeria monocytogenes Requires IL-18 and Contact with Infected Dendritic Cells. The Journal of Immunology. 2010;184:5172–5178. doi: 10.4049/jimmunol.0903759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt RL, et al. A LysM and SH3-Domain Containing Region of the Listeria monocytogenes p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells. PLoS Pathog. 2011;7:e1002368. doi: 10.1371/journal.ppat.1002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira HC, Kaufmann SH. Role of NK1.1+ cells in experimental listeriosis. NK1+ cells are early IFN-gamma producers but impair resistance to Listeria monocytogenes infection. J. Immunol. 1994;152:1873–1882. [PubMed] [Google Scholar]
- 23.Hao J, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. Journal of Experimental Medicine. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayamajhi M, et al. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J. Exp. Med. 2010;207:327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connell RM, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrero JA, et al. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auerbuch V, et al. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayamajhi M, et al. Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Virulence. 2010;1:418–422. doi: 10.4161/viru.1.5.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji NM, et al. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 30.Sauer J-D, et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proceedings of the National Academy of Sciences. 2011;108:12419–12424. doi: 10.1073/pnas.1019041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 32.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 33.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011 doi: 10.1038/nature10558. DOI: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 34.Havell EA, et al. Type I IL-1 receptor blockade exacerbates murine listeriosis. J. Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 35.Rogers HW, et al. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 1994;153:2093–101. [PubMed] [Google Scholar]
- 36.Labow M, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 37.Hirsch E, et al. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaccum MB, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 39.Neighbors M, et al. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J. Exp. Med. 2001;194:343–354. doi: 10.1084/jem.194.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seki E, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 41.Lochner M, et al. Decreased Susceptibility of Mice to Infection with Listeria monocytogenes in the Absence of Interleukin-18. Infect. Immun. 2008;76:3881–3890. doi: 10.1128/IAI.01651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahjat KS, et al. Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect. Immun. 2006;74:6387–6397. doi: 10.1128/IAI.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–1398. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh R, et al. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leber JH, et al. Distinct TLR- and NLR-Mediated Transcriptional Responses to an Intracellular Pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaffrey RL, et al. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci U S A. 2004;101:11386–11391. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahjat KS, et al. Suppression of Cell-Mediated Immunity following Recognition of Phagosome-Confined Bacteria. PLoS Pathog. 2009;5:e1000568. doi: 10.1371/journal.ppat.1000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 49.Eisenbarth SC, et al. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ichinohe T, et al. Inflammasome recognition of influenza virus is essential for adaptive immune responses. Journal of Experimental Medicine. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar H, et al. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. The Journal of Immunology. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 52.Shio MT, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauvau G, et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–9. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 54.Datta SK, et al. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity. 2006;25:143–152. doi: 10.1016/j.immuni.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Lenz LL, et al. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc Natl Acad Sci U S A. 2003;100:12432–12437. doi: 10.1073/pnas.2133653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenz LL, Portnoy DA. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol. Microbiol. 2002;45:1043–1056. doi: 10.1046/j.1365-2958.2002.03072.x. [DOI] [PubMed] [Google Scholar]
- 57.Rahmoun M, et al. Priming of protective anti-Listeria monocytogenes memory CD8+ T cells requires a functional SecA2 secretion system. Infect. Immun. 2011;79:2396–2403. doi: 10.1128/IAI.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muraille E, et al. Cytosolic expression of SecA2 is a prerequisite for long-term protective immunity. Cell. Microbiol. 2007;9:1445–1454. doi: 10.1111/j.1462-5822.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 59.Narni-Mancinelli E, et al. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. Journal of Experimental Medicine. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zammit DJ, et al. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belz GT, et al. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reinicke AT, et al. Dendritic cell cross-priming is essential for immune responses to Listeria monocytogenes. PLoS ONE. 2009;4:e7210. doi: 10.1371/journal.pone.0007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 65.Way SS, et al. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J. Immunol. 2003;171:533–537. doi: 10.4049/jimmunol.171.2.533. [DOI] [PubMed] [Google Scholar]
- 66.Tam MA, Wick MJ. MyD88 and interferon-alpha/beta are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria. Immunology. 2009;128:429–438. doi: 10.1111/j.1365-2567.2009.03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapadia D, et al. Interplay between CD8α+ dendritic cells and monocytes in response to Listeria monocytogenes infection attenuates T cell responses. PLoS ONE. 2011;6:e19376. doi: 10.1371/journal.pone.0019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meeks KD, et al. IL-23 is required for protection against systemic infection with Listeria monocytogenes. The Journal of Immunology. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 69.Xu S, et al. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. The Journal of Immunology. 2010;185:5879–5887. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 70.Cho H, et al. Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3. The Journal of Immunology. 2011;186:489–498. doi: 10.4049/jimmunol.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colmone A, Wang C-R. H2-M3-restricted T cell response to infection. Microbes Infect. 2006;8:2277–2283. doi: 10.1016/j.micinf.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 72.Chow MT, Teh H-S. H2-M3-restricted CD8+ T cells augment CD4+ T-cell responses by promoting DC maturation. Eur. J. Immunol. 2010;40:1408–1417. doi: 10.1002/eji.200939934. [DOI] [PubMed] [Google Scholar]
- 73.Zhan Y, et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8+ spleen dendritic cells. Eur. J. Immunol. 2011;41:2585–2595. doi: 10.1002/eji.201141540. [DOI] [PubMed] [Google Scholar]
- 74.Nakayama Y, et al. C3 promotes expansion of CD8+ and CD4+ T cells in a Listeria monocytogenes infection. The Journal of Immunology. 2009;183:2921–2931. doi: 10.4049/jimmunol.0801191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pamer EG. Immune responses to Listeria monocytogenes. Nature Reviews Immunology. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 76.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pepper M, et al. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rowe JH, et al. Foxp3(+) Regulatory T cells, Immune Stimulation and Host Defense against Infection. Immunology. 2011 doi: 10.1111/j.1365-2567.2011.03551.x. DOI: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ertelt JM, et al. Selective Priming and Expansion of Antigen-Specific Foxp3-CD4+ T Cells during Listeria monocytogenes Infection. The Journal of Immunology. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ertelt JM, et al. Foxp3+ regulatory T cells impede the priming of protective CD8+ T cells. The Journal of Immunology. 2011;187:2569–2577. doi: 10.4049/jimmunol.1100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams MA, et al. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. The Journal of Immunology. 2009;183:2382–2389. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 85.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 86.Sun JC, et al. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui W, et al. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Obar JJ, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. The Journal of Immunology. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitchell DM, et al. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. The Journal of Immunology. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bachmann MF, et al. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 93.Feau S, et al. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nature Publishing Group. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams MA, et al. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mackaness GB. Cellular resistance to infection. J. Exp. Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 96.Edelson BT, Unanue ER. Immunity to Listeria infection. Curr. Opin. Immunol. 2000;12:425–31. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 97.Stavru F, et al. Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunol. Rev. 2011;240:160–184. doi: 10.1111/j.1600-065X.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 98.Ladel CH, et al. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- 99.Bhardwaj V, et al. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J. Immunol. 1998;160:376–84. [PubMed] [Google Scholar]
- 100.Prlic M, et al. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr. Opin. Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 101.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual Review Of Immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 102.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr. Opin. Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 104.Warren SE, et al. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J. Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim S, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur. J. Immunol. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsuchiya K, et al. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. The Journal of Immunology. 2010;185:1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 107.Warren SE, et al. Generation of a Listeria vaccine strain by enhanced caspase-1 activation. Eur. J. Immunol. 2011;41:1934–1940. doi: 10.1002/eji.201041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sauer J-D, et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Groß O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 110.Hara H, et al. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J. Immunol. 2008;180:7859–7868. doi: 10.4049/jimmunol.180.12.7859. [DOI] [PubMed] [Google Scholar]