Abstract
Background
Chronic cocaine use is associated with neurobiological and cognitive deficits that persist into abstinence, hindering success of behavioral treatment strategies and perhaps increasing likelihood of relapse. The effects of current cocaine use and abstinence on neurobiology and cognition are not well characterized.
Methods
Adult male rhesus monkeys with an extensive cocaine self-administration history (~ 5 years) and age-matched controls (n=4/group) performed cognitive tasks in morning sessions and self-administered cocaine or food in afternoon sessions. Positron emission tomography (PET) and [18F]-fluorodeoxyglucose (FDG) was employed to assess cerebral metabolic rates of glucose utilization (MRglu) during cognitive testing.
Results
Cocaine-experienced monkeys required significantly more trials and committed more errors on reversal learning and multi-dimensional discriminations, compared to controls. Cocaine-naive but not cocaine-experienced monkeys showed greater MRglu during a multi-dimensional discrimination task in the caudate nucleus, hippocampus, anterior and posterior cingulate, regions associated with attention, error-detection, memory, and reward. Using a delayed match-to-sample (DMS) task, there were no differences in baseline working memory performance between groups. High dose cocaine self-administration disrupted DMS performance, but tolerance developed. Acute abstinence from cocaine did not affect performance but by day 30 of abstinence, accuracy increased significantly while performance of cocaine-naive monkeys was unchanged.
Conclusions
These data document direct effects of cocaine self-administration on cognition and neurobiological sequelae underlying cognitive deficits. Improvements in working memory can occur in abstinence, albeit across an extended period critical for treatment-seekers, suggesting pharmacotherapies designed to enhance cognition may improve success of current behavioral modification strategies.
Keywords: [18F]-fluorodeoxyglucose (FDG), CANTAB, delayed match-to-sample, PET imaging, set shifting, cerebral metabolic rates of glucose utilization
INTRODUCTION
Chronic cocaine use is associated with structural and functional alterations within the central nervous system that underlie cognitive deficits in attention, memory, impulsivity, and behavioral flexibility (1-7). These deficits extend into periods of abstinence (e.g. 4, 8-10) and have been correlated with retention and success of behavioral treatment programs (7, 11-12). However, the effects of acute versus long-term abstinence on executive function are not well characterized. Identifying neuronal activity associated with cognitive deficits, as well as the effects of abstinence from cocaine during the first month, a critical period of treatment (e.g. 13-14), may lead to more successful treatment strategies employing cognitive enhancers (e.g. 15-16).
Cognitive deficits have been shown following approximately 30 days of cocaine abstinence (e.g. 17-19) although performance was compared to non-drug using control groups, not to within-subject measures obtained prior to cocaine abstinence. Recent studies reported that memory-related cognitive deficiencies dissipated over extended abstinence periods (e.g. 10, 20-21). For example, cocaine users that remained abstinent for 30 days performed worse on measures of attention and memory compared to drug-naive controls, but performed significantly better than current cocaine users (10). Similarly, abstinent cocaine users showed improved cognitive function, including verbal memory at 6 months compared to earlier assessments at 6 weeks of abstinence (20). However, human studies cannot control for factors such as cognitive predisposition, environmental stressors or polydrug use. Further, the criteria used to define abstinence are limited to urinalysis results and self-reports, hindering temporal refinement regarding recent cocaine use. Therefore, the current study sought to examine the effects of current cocaine self-administration on several cognitive domains shown to be impaired in human cocaine users, examine neurobiological substrates mediating cognition and examine the effects of abstinence on working memory in a rhesus monkey model of cocaine abuse.
Adult rhesus monkeys performed cognitive tasks in morning sessions. Monkeys with a ~5 year cocaine self-administration history continued to self-administer cocaine in afternoon sessions, while cocaine-naive monkeys performed similar operant behavioral sessions, except that responding was maintained by food reinforcement. First, to examine the influence of current cocaine self-administration on cognition, we examined associative learning via a simple discrimination task, and two aspects of behavioral flexibility that are mediated via different neurobiological substrates (e.g., 22-24), reversal learning and dimensional set-shifting. Secondly, we used [18F]-fluorodeoxyglucose (FDG) and PET imaging during an extra-dimensional shift to examine rates of glucose utilization, an indirect measure of neuronal activity. Lastly, monkeys were trained to perform a delayed match-to-sample (DMS) task to assess visual working memory. Following determination of a stable cognitive baseline, the effects of high-dose cocaine self-administration on DMS performance were examined for 10 days, followed by 30 days of experimenter-induced abstinence.
METHODS AND MATERIALS
Subjects
Nine singly housed adult male rhesus macaques (Macaca mulatta) served as subjects. Four monkeys (ages 12-13 years) had an extensive cocaine self-administration history (~5 yrs; mean 1395 mg/kg cumulative cocaine intake; Table 1) at the initiation of this study (25-26). Five age-matched (ages 11-12 years), experimentally naive monkeys served as controls. Each monkey was surgically implanted with an intravenous catheter (23), fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit in a primate restraint chair (Primate Products). Monkeys were weighed weekly and fed enough food daily (Purina Monkey Chow and fresh fruit) to maintain ~95% free-feeding body weight; water was available ad libitum in their homecage, which measured 0.71 × 0.84 × 0.84 m (Allentown Caging Inc., Allentown, NJ). Environmental enrichment was provided as outlined in the Institutional Animal Care and Use Committee's Non-Human Primate Environmental Enrichment Plan. All experimental procedures were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Table 1.
Subject information and drug history pertaining to Exps. 1 and 2
| TABLE 1 | CUMULATIVE COCAINE INTAKE (mg/kg) | DURATION BTWN ID/ED TASKS | |||
|---|---|---|---|---|---|
| ID | AGE | ID/ED 1 | ID/ED 2 | DAYS | |
| Coc-Exp | 1374 | 13 | 1770.9 | 1888.7 | 215 |
| 1375 | 14 | 785.0 | 905.1 | 239 | |
| 1377 | 14 | 1439.6 | 1560.1 | 209 | |
| 1381 | 13 | 1172.2 | 1224.9 | 54 | |
| Coc-Naïve | 1681 | 12 | 0 | 0 | 152 |
| 1682 | 11 | 0 | 0 | 224 | |
| 1683 | 13 | 0 | 0 | 198 | |
| 1696 | 12 | 0 | 0 | 174 | |
Operant Behavior
All animals underwent two daily operant behavioral sessions (5-7 days/week) consisting of cognitive testing between 0700-1000 using the Cambridge Neuropsychological Test Automated Battery (CANTAB) apparatus (Lafayette Instruments, Lafayette, IN) and either 1 g banana-flavored food (cocaine-naive) or 0.1 (Exps. 1-2) or 0.3 (Exp. 3) mg/kg/injection cocaine (cocaine-experienced) self-administration during afternoon sessions between 1300-1600, where responding was maintained under a fixed-ratio (FR) 30 schedule of reinforcement with a maximum of 15 reinforcers available per session. A minimum of 2 hours elapsed between morning and afternoon sessions during which monkeys were returned to their home cages.
Experiment 1
Reversal learning and set-shifting tasks
All monkeys were trained to respond on the CANTAB touch-sensitive computer screens (27). Following training, monkeys were exposed to an initial simple discrimination (SD) and reversal (SDR) task. Briefly, two shapes appeared on the left and right side of the screen. Responding on one shape resulted in delivery of a 190-mg food pellet (S+) while responding on the other shape resulted in trial termination (S-). Shapes were pseudo-randomly distributed on each side of the screen with a maximum of 200 trials/day. Following acquisition of the SD (defined as 6 correct responses in a row), the contingencies were reversed (SDR) such that responding on the previous S- now resulted in reinforcement and the previously reinforced shaped terminated the trial.
The day after completing the initial SD/SDR task, monkeys began a modified version of the intra/extra-dimensional (ID/ED) set-shifting task as previously described (22-23, 27). In brief, monkeys had to learn to focus their attention within one of two stimulus set (shapes or lines) and discriminate between two stimuli within this set, one resulting in reinforcement (S+) and one resulting in trial termination (S-). Monkeys were tested across four stages of this task; simple discrimination (SD), compound discrimination (CD), intradimensional shift (IDS), and extradimensional shift (EDS). The first three stages established one attentional set (e.g., shapes) and the last stage (EDS) required the monkeys to shift attention to the opposite set (e.g., lines) to obtain reinforcement. Since frequent exposure to this task may result in improved performance over time, possibly inducing a ceiling effect, a minimum of ~ 2 months passed between this first exposure and the second exposure to the ID/ED task, coinciding with FDG-PET (see below; Exp. 2, Table 1). During this period monkeys began training on a DMS task (Exp. 3).
Experiment 2
FDG-PET imaging and extra-dimensional set shifting
Each animal underwent two FDG-PET studies associated with cognition, each occurring ~18 hours after the last self-administration session: (1) a baseline (BL) session to control for visual-motor activity and (2) during an EDS, with the order counterbalanced within groups and separated by a minimum of 2 weeks. The BL consisted of each response on a purple square (3.8 × 3.8 cm) randomly located on the computer screen resulting in delivery of a sucrose pellet, followed by a 30 sec timeout. Prior to the FDG study during an EDS, monkeys completed the SD, CD, and IDS. However, before progressing to the EDS, contingencies associated with the IDS remained for ~150 additional trials over the course of 2-3 days to establish that the attentional set could be retained overnight (see Table S1 in Supplement 1). On the day of the PET study, approximately 1.0 ml [18F]FDG (dose range= 5.5-5.9 mCi) was injected into the vascular access port followed by a 3 ml flush of sterile saline and the cognitive session was immediately started. After 40 minutes, the session was terminated and the monkey was anesthetized with an intramuscular injection of 0.04 mg/kg Dexmedetomidine (Dexdomitor, Pfizer Pharmaceuticals) and 5.0 mg/kg ketamine HCl and transported to the Wake Forest University School of Medicine PET Center for imaging. PET scans were completed within 90 minutes of the initial FDG administration (range 70-90 minutes) and within 45 minutes of sedation (range 20-50 minutes). Afternoon self-administration sessions did not occur on the afternoon following a PET study.
Experiment 3
Effects of cocaine self-administration on working memory
Monkeys were trained to perform a DMS task where a “target” image appeared on the screen. Following a response on the “target” and a variable delay (0-120 sec), multiple images appeared and responding on the “target” image resulted in delivery of 2 sucrose pellets. Conditions remained unchanged until percent accuracy on the DMS task was above 80% for three consecutive days with a 1 sec delay and 2 distracter (incorrect) images, after which delays were slowly introduced in ascending order such that 3 delays were randomly distributed throughout each session (~33 trials/delay). R-1381 (cocaine-exposed) and R-1683 (cocaine-naive) did not acquire the DMS task, as a result R-1756 was added to the cocaine-naive group.
After baseline performance had been established, delays were individualized to produce similar delay-dependent reductions in percent accuracy (three delays per monkey: short delay, >75% accuracy; middle delay, 50-75%; long delay, <50%; see Table 3) and the total number of trials was reduced to 60 (20 trials/delay). For R-1682 the number of distracter images was increased to 3 to make baseline accuracy similar across all monkeys. Following determination of a 5-day stable baseline, where morning sessions followed days in which 0.1 mg/kg/injection cocaine was available, the dose of cocaine was increased to 0.3 mg/kg/injection for 10 consecutive days. For R-1377, total cocaine intake steadily decreased from days 1-4 (4.5 to 2.7 mg/kg), so the available cocaine dose was reduced to 0.2 mg/kg/injection for day 5-10. As a result, cocaine intake doubled in all monkeys during this 10-day exposure (Table 3). Following this period, afternoon sessions were discontinued for 30 consecutive days. Thirty days of DMS performance in the cocaine-naive monkeys were used for comparison with the 30 days of abstinence in the cocaine-experienced monkeys.
Table 3.
Individual delays generating high, middle, and low percent accuracies and cocaine intake (mg/kg/session) and response rates (resp/sec).
| Exp. 3 | Cocaine self-administration | |||||
|---|---|---|---|---|---|---|
| Starting delays (sec) | BL (0.1 mg/kg/inj) | Increased Access (0.3 mg/kg/inj) | ||||
| Day 1-5 | Day 1-5 | Day 6-10 | ||||
| short | mid | long | Intake/rate¶ | Intake/rate | Intake/rate | |
| Coc-Exp (Monkey) | ||||||
| R-1374 | 0 | 60 | 120 | 1.5/0.18 | 4.38/0.08 | 4.14/0.06 |
| R-1375 | 0 | 45 | 90 | 1.5/0.16 | 3.06/0.04 | 3.12/0.04 |
| R-1377 | 0 | 45 | 90 | 1.5/0.29 | 3.30/0.05^ | 3.00/0.12^ |
| AVE | 1.5/0.21 | 3.58/0.06 | 3.42/0.07 | |||
| Coc-naive (Monkey) | Pellets (total #) | |||||
| R-1681 | 5 | 60 | 120 | 15/2.14 | -- | -- |
| R-1682 | 0 | 60 | 120 | 15/1.56 | -- | -- |
| R-1696 | 0 | 45 | 90 | 15/2.48 | -- | -- |
| R-1756 | 5 | 30 | 60 | 15/0.35 | -- | -- |
Dose was decreased from 0.3 to 0.2 mg/kg on day 5; averages are for days 1-4 and 5-10
first number represents reinforcement frequency (mg/kg/session for coc-exp monkeys and # pellets for coc-naïve animals) and second number represent response rates (responses/second)
Data analysis
Experiment 1
The primary dependent variables were number of trials completed and number of errors committed to acquire each stage during the discrimination, reversal, and set-shifting tasks. Omitted trials were rare, and excluded from analysis. Individual-subject data were normalized by square-root transformation prior to statistical analysis (e.g. 27). Response and pellet retrieval latencies were also examined. Two-way analyses of variance (ANOVAs) were conducted using group (cocaine-experienced vs cocaine-naive) and stage (SD, SDR for reversal learning and SD, CD, ID, ED for set-shifting) as factors. Significant main effects were followed by post hoc Bonferonni t-tests. A pre-planned t-test was conducted between groups in the ED component of the set-shifting task.
Experiment 2
FDG-PET data were analyzed as described by Porrino et al. (28,29; see Supplement 1). Whole brain analyses of MRglu were performed for within-group, extra-dimensional shift versus baseline condition and between-group baseline performance. For each within-group comparison a statistical parametric map was created by applying a paired t-test factorial design matrix. A non-paired t-test factorial design matrix was applied for the baseline comparison between groups. An initial voxel height threshold of p<0.005 (uncorrected) and minimum cluster size of 25 contiguous voxels were set to establish clusters; of the identified clusters, only those with a p< 0.05 value (corrected for search volume) were considered significant. Areas of activation are displayed on a T1 MR template (30) and the associated brain regions were identified using a rhesus monkey brain atlas (31).
Experiment 3
The primary dependent variable was percent accuracy. To compare baseline delay-effect curves between groups, the 3-day means from the first exposure to each delay were used. A two-way repeated measures ANOVA was conducted using experimental group and delay (0-90 sec) to compare percent accuracy during the initial determination of the delay-effect curve. One-way repeated measures ANOVAs were conducted to compare number of omitted trials, response latencies for target and match phases, and pellet retrieval latencies between groups.
Changes in percent accuracy from the 5-day baseline period in which monkeys self-administered 0.1 mg/kg/injection cocaine were examined by conducting two-way repeated measures ANOVAs using delay (short, mid, long) and condition (baseline, 0.3 mg/kg/injection cocaine days 1-5 and 6-10, and six bins of 5 days each during abstinence) as factors. Significant main effects were followed by Bonferoni post-hoc tests comparing each 5-day bin to baseline. One-way repeated measure ANOVAs were conducted to examine number of omitted trials and response latencies within each group. R-1377 showed robust disruptions in responding during DMS sessions on days 6 and 7 following increased cocaine dose availability (<5 completed trials each day); data for these two days were excluded from statistical analyses. In all cases, p<0.05 was considered significant.
RESULTS
Experiment 1
Reversal learning and set-shifting tasks
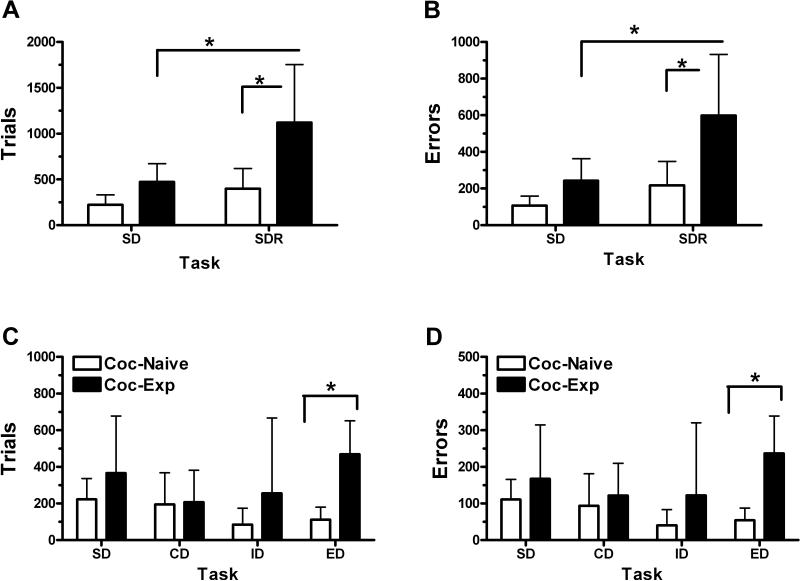
During morning cognitive sessions, all monkeys acquired the tasks and during the afternoon sessions, all monkeys reliably obtained the maximum 15 reinforcers during food or cocaine self-administration sessions. There was a main effect of group (F1,6= 6.11, p<0.05) and task (F1,6=13.67,p<0.05) on the number of trials to complete the stimulus discrimination and reversal task. Post hoc tests indicated that the cocaine-experienced group required a greater number of trials on the reversal component compared to the cocaine-naive group (t=1.66; p<0.05) and more trials during the reversal component compared to their respective simple discrimination component (t=3.30, p<0.05; Fig 1A). There was a main effect of task (F1,6=10.47, p<0.05) but not group (F1,6=5.90, p=0.051) such that the cocaine-experienced group committed more errors during the reversal component compared to the cocaine-naive group (t=2.64; p<0.05) and during the reversal component compared to their respective simple discrimination (t=3.06, p<0.05; Fig 1B).
Figure 1. Performance across two measures of behavioral flexibility, reversal learning and set shifting in cocaine-naive and cocaine-experienced monkeys.
Group data (mean ± SD) for the number of trials completed (A,C) and errors committed (B,D) to acquire each stage of the reversal learning (top) and set-shifting (bottom) tasks. Open bars represent cocaine-naive monkeys, filled bars represent cocaine-experienced monkeys; SD, simple discrimination; SDR, reversal of simple discrimination; CD, compound discrimination; ID, intra-dimensional shift; ED, extra-dimensional shift; * denotes significance at p<0.05.
Both groups acquired the SD, CD and ID tasks in a similar number of trials. Pre-planned comparisons showed the cocaine-experienced monkeys required significantly more trials (t6=3.76, p<0.01; Fig. 1C) to acquire the EDS. The number of errors committed was significantly different between groups (F1,24=4.42, p<0.05), with the cocaine-experienced monkeys making significantly more errors during the EDS compared to cocaine-naive monkeys (t6=2.38, p<0.05; Fig. 1D). Cocaine-experienced monkeys had quicker response latencies and pellet retrieval latencies across all stages of the set-shifting tasks compared to cocaine-naive monkeys (see Supplement 1).
Experiment 2
FDG-PET imaging and extra-dimensional set-shifting
There were no group differences between the total trials or % accuracy across any stages of the BL or ID/ED task associated with FDG-PET. This was expected since the EDS session was terminated after 40 minutes. During the BL condition, there were few differences between groups in baseline metabolic activity (Table S2 in Supplement 1). During the EDS, both groups showed higher glucose utilization in the right lateral orbital gyrus and right posterior cingulate/postcentral gyrus, compared to their respective BL metabolic activity (Table 2, Fig. 2). In contrast, only cocaine-naive monkeys showed increased glucose utilization in the anterior cingulate/frontal gyrus, midcingulate/prefrontal gyrus, right caudate, right globus pallidus, left inferior temporal, lingual gyrus, hippocampus, and the precuneus, while cocaine-experienced monkeys showed greater glucose utilization in the left superior occipital gyrus (Table 2, Fig. 2). Relative to the BL condition, cocaine-naive monkeys showed less glucose utilization during the EDS in the left fronto-orbital gyrus and left precentral gyrus whereas the cocaine-experienced monkeys showed lower glucose utilization in the right thalamus, right inferior occipital gyrus, and right cuneus (Table 2).
Table 2.
Glucose utilization between a baseline motor task (BL) and an extradimensional shift (EDS) in cocaine-naive and cocaine-experienced monkeys (n=4/group).
| Condition | Brain Region | Hemisphere | Standard z-score1 | Cluster size (# of voxels) |
|---|---|---|---|---|
| ED shift > BL | ||||
| Cocaine-naive | ||||
| lateral orbital gyrus | RT | 3.63 | 34 | |
| ant. cingulate/ sup. frontal gyrus | Both | 3.94 | 359 | |
| mid-cingulate/precentral gyrus | Both | 4.27 | 111 | |
| post. cingulate/postcentral gyrus | RT | 3.32 | 27 | |
| caudate nucleus/globus pallidus | RT | 3.30 | 53 | |
| inf. temporal gyrus | LF | 3.72 | 67 | |
| Hippocampus/lingual gyrus | LF | 4.43 | 183 | |
| Precuneus | Both | 4.12 | 151 | |
| Cocaine SA | ||||
| lateral orbital gyrus | RT | 4.05 | 31 | |
| post. cingulate/postcentral gyrus | RT | 3.07 | 27 | |
| sup. occipital gyrus | LF | 3.91 | 26 | |
| BL > ED shift | ||||
| Cocaine-naive | ||||
| fronto-orbital gyrus | LF | 3.83 | 50 | |
| precentral/postcentral gyrus | LF | 4.52 | 78 | |
| Cocaine SA | ||||
| thalamus | RT | 3.89 | 74 | |
| inf. occipital gyrus | RT | 3.72 | 37 | |
| cuneus | RT | 3.67 | 30 | |
p< 0.05; corrected for search volume
Figure 2. Areas of increased glucose utilization during an extradimensional shift.
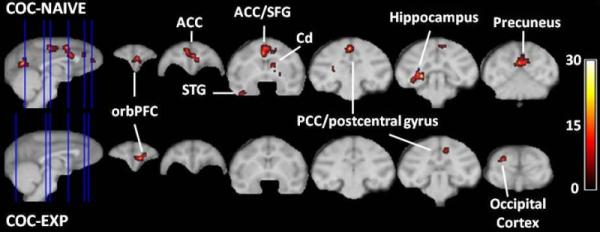
Group data from whole-brain analyses showing areas where cocaine-naive monkeys (top) and cocaine-experienced monkeys (bottom) showed significantly greater glucose utilization during the initial response to an EDS compared to their respective baseline measures. The saggital slices (far left) show the location (blue lines) of respective coronal slices in a rostrocaudal direction. Cluster threshold p<0.05, corrected for search volume; scale bar represents t score. orbPFC- orbital prefrontal cortex; Cd- caudate nucleus; STG- superior temporal gyrus; ACC-anterior cingulate gyrus; MCC-mid-cingulate/precentral gyrus; PCC- posterior cingulate gyrus.
Experiment 3
Effects of cocaine self-administration on working memory
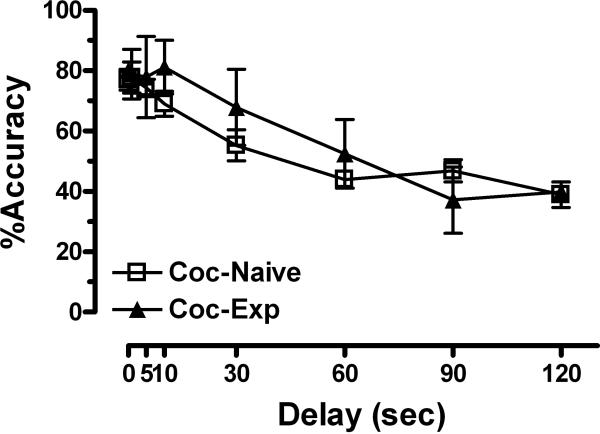
When 0.1 mg/kg/injection cocaine (baseline) was available in the afternoon sessions, all monkeys consistently received all 15 injections for a cumulative daily intake of 1.5 mg/kg. During acquisition of DMS performance there was a significant effect of delay (F6,29=24.89, p<0.001) but not group (Figure 3). There were no differences between groups in response or pellet retrieval latencies (data not shown).
Figure 3. Baseline delayed match-to-sample performance in monkeys.
Group delay-effect curves (mean ± SEM) for cocaine-naive monkeys (n=4, except t=90, 120, n=3) and monkeys with a cocaine self-administration history (n=3, except t=120, n=1).
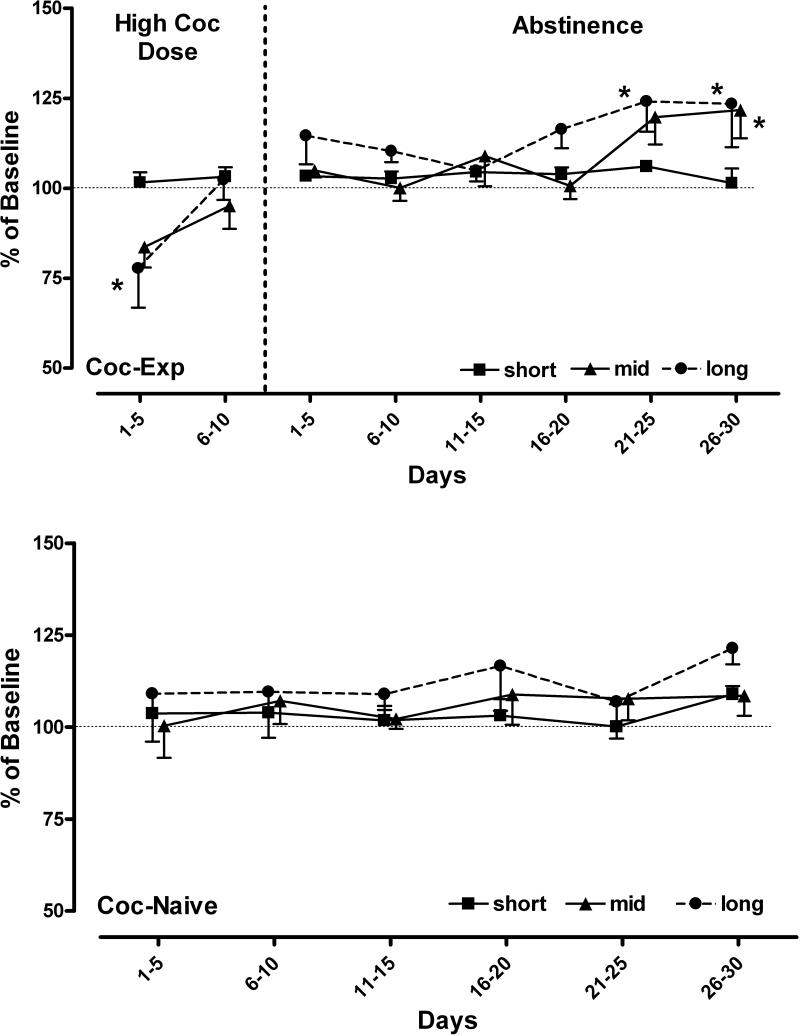
Three delays were then chosen for each monkey (short, mid, long) that engendered similar delay-effect curves between groups. When the cocaine dose was increased to 0.3 mg/kg/injection, the average cumulative intake over the 10 days was 3.5 mg/kg cocaine (Table 3). There was a significant effect of condition (increased cocaine dose or abstinence) on percent change in accuracy from baseline (F8,32=6.92, p<0.001) and an interaction between condition and delay (F16, 32=2.29, p<0.022). Following increases in cocaine dose, percent change in accuracy during days 1-5 was significantly different from baseline at the long delay only (t=2.90, p<0.05; Figure 4, top). Tolerance developed to this effect, such that days 6-10 were not significantly different from baseline.
Figure 4. Effects of high-dose cocaine self-administration and abstinence on delayed match-to-sample performance.
Group data (mean ± SEM) for monkeys with a cocaine self-administration history (top) and cocaine-naive control monkeys (bottom). Data are expressed in 5-day bins as a percent of baseline performance for each delay (short, mid, long) when 0.1 mg/kg/injection cocaine was self-administered each previous afternoon (cocaine-experienced) or the equivalent 5-day period when food pellets were self-administered (cocaine-naive); * denotes significant difference from 5 day baseline average (p<0.05).
Following discontinuation of daily cocaine self-administration sessions, DMS performance was significantly affected (Figure 4, top). On days 21-25, performance during the longest delay (t=3.19, p<0.05) and at the middle and long delays during days 26-30 of abstinence were significantly higher than baseline (t=2.87; p<0.05; t=3.10, p<0.05, respectively). There were no significant changes in the number of omitted trials, response or pellet retrieval latencies compared to baseline across either group or any condition (data not shown). For comparison, there were no significant differences in the percent change from baseline over 30 sessions in the cocaine-naive group (Figure 4, bottom).
DISCUSSION
The present study used a monkey model of cocaine abuse to assess multiple cognitive domains associated with cocaine-related impairments in humans. Monkeys with an extensive history of cocaine self-administration did not show impairments in simple discrimination, a measure of associative learning but performed worse on multi-dimensional discriminations and reversal learning compared to cocaine-naive monkeys. Cognitive deficits were associated with differences in glucose utilization assessed via FDG-PET between cocaine-experienced and cocaine-naive monkeys. Delayed-match-to-sample (DMS) performance was significantly disrupted by increasing the amount of cocaine self-administered, although tolerance developed to this effect. Acute abstinence did not adversely affect working memory, while continued abstinence resulted in significant increases in memory. This within-subject assessment suggests cognitive performance is malleable during this critical period when likelihood to relapse is high (13-14). The current behavioral data add to studies demonstrating cocaine-associated cognitive impairments in rodents (32-34) and monkeys (35-38) and the PET data extend lesioning studies (e.g. 22, 27, 39) to implicate multiple brain regions mediating cognitive function and cocaine-associated deficits.
Both reversal learning and set-shifting tasks examine aspects of behavioral flexibility but are mediated by orbitofrontal (orbPFC) and dorsolateral PFC, respectively (see 40 for review). Cocaine use has been associated with disruptions in reversal learning in animals and humans (e.g. 35, 38, 41, 42, current data) and deficits in set-shifting are common in cocaine users (e.g. 10, 43). Reversal learning requires the ability to inhibit a previously established response based on an acquired stimulus-reinforcement association. Set-shifting requires the formation of an attentional set based on relevant stimuli and the disregard to irrelevant stimuli prior to shifting focus between different modalities to establish a new stimulus-reinforcement association. Typically a within-group difference between EDS and IDS verifies an established attentional set that must be broken prior to EDS acquisition (e.g. 22-23, 39), which we did not observe in either group, perhaps due to large variability. Therefore, we cannot assume an attentional set was formed. However, the IDS and EDS components of this task functioned as multi-dimensional discrimination tasks, and a different behavioral and metabolic profile was apparent between groups. We did observe deficits on compound discrimination tasks, in that monkeys with an extensive cocaine history required more trials than the cocaine-naïve monkeys, a difference that was significant in the EDS component. Importantly, there were no differences in simple discrimination between groups, suggesting that increasing cognitive demand revealed attentional deficits, possibly through an inability to disregard irrelevant stimuli.
Although a set-shift was not apparent, there were group differences in the EDS component, which makes measures of brain function an important group comparison. In cocaine-naive monkeys, there were increases in glucose utilization during the EDS compared to baseline in the caudate nucleus, hippocampus and cortical regions including the orbPFC, medial PFC, anterior and posterior cingulate (ACC and PCC, respectively), temporal cortex and precuneus. Although FDG-PET does not allow for network analyses, these areas are implicated in frontal-cingulate, parietal-frontal, and striato-cortical circuits that are involved in processes important for executive function including attentional processes, sensory integration, memory formation, behavioral flexibility, stimulus-reward associations, and error-detection (see 44-47 for reviews). Importantly, the PET data in the cocaine-naive monkeys showed similar activation patterns as seen in drug-naive human fMRI studies examining attentional tasks (e.g. 43, 47, 48), supporting this model for further translational research.
In contrast to what was observed in control monkeys, cocaine-experienced monkeys did not show increased activity in regions integral for attentional processing, error-detection and stimulus-reward feedback (49, 50). ACC hypoactivity is frequently associated with cocaine use regardless of the cognitive domain examined (e.g. 2-4, 48, 51). Although fewer increases in activity in the cocaine-experienced monkeys could be alternately interpreted as a lack of “motivation” to perform the task, quicker response and pellet retrieval latencies suggest that the food pellets were reinforcing in both groups (see Supplement 1).
Strikingly, both groups of monkeys showed increased activity in the orbPFC a region often associated with dysfunction during abstinence from cocaine exposure (55). Similar to the current assessment, orbPFC was unaltered in rodents with recent cocaine exposure (56), suggesting dysfunction may occur in extended but not acute periods of abstinence. Lesion studies in rodents, monkeys and humans have classically associated orbPFC activity with reversal learning although recent evidence supports a larger role regarding feedback from stimulus-reinforcement events (see 55 for review). FDG-PET is thought to provide an indirect measure of synaptic activity (for reviews see 57, 58) relative to a specific baseline condition. Perhaps, the orbPFC has intact inputs but inadequate output activity to other brain regions such as the ACC.
In the present study, a chronic cocaine self-administration history resulted in impairments on measures of behavioral flexibility but not working memory performance. We did not assess DMS performance in cocaine-naive monkeys that subsequently self-administered cocaine, but in a similar study tolerance developed to the initial cognitive disrupting effects of cocaine in rhesus monkeys trained on a DMS task prior to cocaine self-administration (38). The development of tolerance may account for the lack of initial deficits in working memory given the extensive training necessary for this task. Increasing the dose of cocaine disrupted percent accuracy at the longest delay, when cognitive demand is high and most sensitive to disruption, and tolerance developed to these effects. In humans, cocaine intake is interspersed between high-intensity binges and subsequent lesser use or abstinence (e.g. 13-14) that may be more disruptive to cognitive performance than stable daily intake, whereby animals may adjust to these conditions over time (see 59 for review). Importantly, disrupted cognitive performance was not accompanied by effects on response latencies or number of trials omitted, suggesting that the impairments were specific to working memory and not attention or reaction time.
To our knowledge, this is the first study in monkeys to utilize a within-subjects design to assess both cocaine-induced deficits and changes across abstinence periods during daily cognitive assessment in monkeys. In human studies measuring working memory (4, 60), acute abstinence from cocaine was associated with deficits, when compared to a cocaine-naive group. In the present study, no deficits were observed following acute abstinence, and working memory at the middle and long delays significantly improved compared to baseline, for days 21-30 of abstinence. No changes occurred at the shortest delay as cognitive demand was low and baseline performance was already at a high percent accuracy. Improvements following repeated task performance are common in both animals and humans (e.g. 27, 61). However, similar improvements did not occur in the cocaine-naive group suggesting the rapid improvement was attributed to abstinence from chronic cocaine use.
In summary, the current monkey model demonstrated cognitive impairments across two primary cognitive components of executive function (62), “inhibition” and “updating” (reversal learning and working memory, respectively) as well as metabolic dysregulation as a result of cocaine self-administration. Cognitive impairments in behavioral flexibility, linked to aspects of impulsivity (e.g. 24, 63), may contribute to poor treatment success and shortened abstinence. Studies in human cocaine abusers have shown improvements in memory following extended durations of cocaine abstinence, although improvements typically occurred following 3-6 months of abstinence with intermittent testing (20, 64). Due to the high rate of relapse within the first month of abstinence (e.g. 13-14), the likelihood for most treatment-seeking cocaine users to remain abstinent long enough for cognitive improvements to occur is poor. Thus, this nonhuman primate model can be utilized to evaluate pharmacological and behavioral approaches to improve cognitive performance, potentially leading to greater rates of treatment success. The current study focused on cognition in monkeys with ~5 year cocaine self-administration histories, modeling chronic cocaine users. Future studies can parse the effects of initial cocaine exposure, long-term effects and re-exposure following abstinence, modeling initiation, maintenance and relapse, respectively.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Michael R. Weed, Ph.D. and Nancy Ator, Ph.D. for the generous donation of primate chairs and sound-attenuating chambers. The authors wish to acknowledge the technical assistance of Tonya Calhoun, Kimberly Black, Holly Smith, Mack Miller, Linda Porrino, Ph.D. and James Daunais, Ph.D. for assistance with MR/PET data acquisition and analysis, and Brandi Blaylock, Paul Czoty, Ph.D. and Michelle Nicolle, Ph.D. for insightful comments on earlier versions of this manuscript.
This research was supported by National Institute on Drug Abuse DA10584 and DA06630.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 2.Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neurosci. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang G, Volkow ND. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Nat Acad Sci. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;30:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 9.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 10.Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacol (Berl) 2011;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Dependence. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J Subst Abuse Treat. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Gawin FH, Kleber HD. Cocaine use in a treatment population: patterns and diagnostic distinctions. National Institute on Drug Abuse; Washington, DC: 1985. pp. 185–193. National Institute on Drug Abuse Research monograph Series 61. 1985. [PubMed] [Google Scholar]
- 14.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Arch Gen Psych. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 15.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: Translation to prevention and treatment interventions. Brain Research Reviews. 2011;65:124–49. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- 18.Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- 19.Gillen RW, Kranzler HR, Bauer LO, Burleson JA, Samarel D, Morrison DJ. Neuropyschologic findings in cocaine-dependent outpatients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1061–1076. doi: 10.1016/s0278-5846(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 20.Di Sclafani V, Tolou-Shamas M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. Am J Drug Alcohol Abuse. 2008;34:109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- 22.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 23.Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Task: effects of excitotoxic lesions in the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–76. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- 24.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacol. 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czoty PC, Reboussin BA, Calhoun TL, Nader SH, Nader MA. Long-term cocaine self-administration under fixed-ratio and second-order schedules in monkeys. Psychopharmacol. 2007;191:287–95. doi: 10.1007/s00213-006-0665-z. [DOI] [PubMed] [Google Scholar]
- 26.Blaylock BL, Gould RW, Banala A, Grundt P, Luedtke RR, Newman AH, Nader MA. Influence of cocaine history on the behavioral effects of dopamine D(3) receptor-selective compounds in monkeys. Neuropsychopharmacol. 2011;36:1104–1113. doi: 10.1038/npp.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LE. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res. Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 28.Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black KJ, Koller JM, Snyder AZ, Parlmutter JS. Atlas template images for nonhuman primate neuroimaging: baboon and macaque. Methods Enzymol. 2004;385:91–102. doi: 10.1016/S0076-6879(04)85006-7. [DOI] [PubMed] [Google Scholar]
- 31.Saleem K, Logothetis N. A combined MRI and Histology: Atlas of the Rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego: 2007. p. 326. [Google Scholar]
- 32.Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 33.Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacol. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacol. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacol. 2002;26:183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Heitz RP, Sampson AR, Zhang W, Bradberry CW. Evidence of temporal cortical dysfunction in rhesus monkeys following chronic cocaine self-administration. Cereb Cortex. 2008;18:2109–2116. doi: 10.1093/cercor/bhm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Heitz RP, Bradberry CW. A touch screen based stop signal response task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J Neurosci Methods. 2009;15:67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psych. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacol. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- 44.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:21–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 45.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 46.van Schouwenburg M, Aarts E, Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Design. 2010;16:2026–2032. doi: 10.2174/138161210791293097. [DOI] [PubMed] [Google Scholar]
- 47.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 48.Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23:670–679. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102(Suppl. 1):33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions, or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 53.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 54.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “On-Line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum H. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl.) 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- 57.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Phil Trans R Soc Lond B. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2004;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 60.Kelley BJ, Yeager KR, Pepper TH, Beversdorf DQ. Cognitive impairment in acute cocaine withdrawal. Cog Behav Neurol. 18:108–112. doi: 10.1097/01.wnn.0000160823.61201.20. 20f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Backman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Neely AS, Virta J, Laine M, Rinne JO. Effects of working-memory training on striatal dopamine release. Science. 2011;333:718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- 62.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Serrano MJ, Perales JC, Moreno-Lopez L, Perez-Garcia M, Verdejo-Garcia A. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology. 2012;219:673–683. doi: 10.1007/s00213-011-2485-z. [DOI] [PubMed] [Google Scholar]
- 64.van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, Plotkin D. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.