Abstract
Background
Despite the wealth of studies demonstrating the impairing effects of alcohol on behavioral inhibition, less is known regarding effects of the drug on attentional inhibition (i.e., the ability to ignore distracting stimuli in the environment in order to focus attention on relevant information). The current study examined alcohol impairment of both behavioral and attentional inhibition, as well as potential associations between the two mechanisms of inhibitory control.
Methods
Men (n = 27) and women (n = 21) performed a measure of behavioral inhibition (cued go/no-go task) and a measure of attentional inhibition (delayed ocular return task) following three doses of alcohol: 0.65 g/kg, 0.45 g/kg, and 0.0 g/kg (placebo).
Results
Alcohol impaired both behavioral and attentional inhibition relative to placebo; however, correlational analyses revealed no associations between measures of behavioral and attentional inhibition following any dose. Additionally, men committed more inhibitory failures on the behavioral inhibition task, whereas women committed more inhibitory failures on the attentional inhibition task.
Conclusions
These findings suggest that behavioral and attentional inhibition are equally sensitive to the impairing effects of alcohol, yet represent distinct components of inhibitory control. Additionally, the observed gender differences in control of behavior and attention could have important implications regarding negative consequences associated with alcohol-induced disinhibition in men and women.
Keywords: alcohol, behavioral inhibition, attentional inhibition, gender
I. Introduction
Alcohol intoxication is associated with acute disinhibition, and this is thought to underlie the impulsive and risky behavior often observed in intoxicated individuals. As disinhibited behavior (e.g., driving while intoxicated, provoking fights, risky sexual behavior) is likely to put an individual at risk for personal harm, understanding the effects of alcohol on basic inhibitory mechanisms is an important question for researchers. To date, the majority of this research has concentrated on the impairing effects of alcohol on behavioral inhibition (i.e., the ability to inhibit or suppress behavioral impulses in order to control inappropriate actions). It also has been recognized for some time that inhibitory control mechanisms exert an influence over attentional mechanisms as well, allowing an individual to ignore distracting stimuli in the environment in order to focus attention on relevant information (Houghton and Tipper, 1994). However, little research attention has been given to examination of alcohol effects on attentional inhibition, and as such much less is known regarding the degree to which alcohol might acutely disrupt attentional control. Alcohol-induced impairment of inhibitory control of attention could independently contribute to risk for injury, particularly during any act that requires a substantial degree of focus (e.g., operating a vehicle). As such, it is important to gain a better understanding of how alcohol might impair mechanisms of attentional inhibition, and the extent to which such impairment might relate to impairment of behavioral inhibition.
Both behavioral and attentional control mechanisms are thought to be governed by two independent processes: an activational process and an inhibitory process (Fowles, 1987; Gray, 1976; Logan and Cowan, 1984). These two processes act in opposition, and the outcome (i.e., activation or inhibition) is assumed to occur based on the relative strength of each. In terms of behavior, the activational process is responsible for executing a behavioral response, whereas the inhibitory process is responsible for inhibiting inappropriate responses. Laboratory measures of behavioral inhibition, including the stop signal and go/no-go tasks, present a reaction time scenario in which a participant must make a response (i.e., a key press) as quickly as possible to go signals, and inhibit that response when a stop signal or no-go target occasionally appears. In regard to attention, the inhibitory process suppresses the direction of attention towards irrelevant stimuli and focuses attention on relevant information, thus facilitating selective attention (Godijn and Theeuwes, 2003; Houghton and Tipper, 1994). Laboratory measures of attentional inhibition, including antisaccade and delayed ocular return (DOR) tasks, involve the inhibition of a reflexive saccade to the sudden appearance of a distracter object. As such, individuals must utilize inhibitory mechanisms of attention to overcome the prepotent impulse to look at the distracter stimulus. In both behavioral and attentional inhibition tasks, fast responding is encouraged, thus increasing response pre-potency and making inhibition more difficult.
A large number of studies have investigated the acute effects of alcohol on tasks of behavioral inhibition, and results show a reliable disinhibiting effect of the drug. For instance, alcohol has been shown to increase commission errors on go/no-go and continuous performance tasks in a dose dependent manner (e.g., Dougherty et al., 1999; Marczinski and Fillmore, 2003; Weafer and Fillmore, 2008). Stop-signal tasks also show that alcohol produces acute impairments of inhibitory control as evidenced by slower response inhibition and by increased failures to inhibit responses (de Wit et al., 2000; Fillmore and Vogel-Sprott, 1999). Further, greater sensitivity to alcohol-induced disinhibition has been associated with heavy alcohol consumption, implicating alcohol impairment of behavioral inhibition in risk for alcohol abuse (Fillmore, 2003; Marczinski et al., 2007; Weafer and Fillmore, 2008).
In regard to the acute impairing effects of alcohol on attentional inhibition, the evidence is mixed. Abroms et al. (2006) reported a significant dose-dependent increase in premature saccades on the DOR task in response to placebo and two doses of alcohol. Alcohol has also been shown to increase inhibition errors on the antisaccade task, but only in individuals with no family history of alcoholism (Ramchandani et al., 1999). By contrast, several studies have found no effect of alcohol on antisaccade errors (e.g., Blekher et al., 2002; Vorstius et al., 2008), and some studies have reported that alcohol actually decreases inhibitory errors on these tasks (Khan et al., 2003; Roche and King, 2010; Vassallo and Abel, 2002). Thus, in contrast to the reliably disinhibiting effects of alcohol on behavioral control, the acute effects of alcohol on attentional control are less well understood. It is unclear why these studies have produced such mixed results. However, this lack of consistency highlights the importance of comparing the effects of alcohol on behavioral and attentional inhibition in the same individuals, as well as the importance of employing tests that have been sensitive to the disruptive effects of alcohol.
It is possible that magnitude of alcohol impairment might differ between behavioral and attentional inhibition. Indeed, there is some evidence to suggest a dissociation between the two control mechanisms in sober individuals. For instance, fMRI work has demonstrated distinctions between neuroanatomical control of behavioral and attentional inhibition (Aron et al., 2004; Leung and Cai, 2007). Additionally, Logan and Irwin (2000) compared performance on a visual and manual stop signal task and found that stop signal reaction time was faster for eye movements, indicating greater inhibitory control of attention compared to behavior. Studies from our group have shown that individuals characterized by attentional impairments (i.e., children and adults with ADHD) display greater deficits in attentional control compared to behavioral control (Adams et al., 2010; Roberts et al., 2011; Weafer et al., 2011). Finally, gender differences have been reported in opposite directions for behavioral and attentional inhibition. Specifically, men have been shown to display deficits of behavioral inhibition compared to women (Hansen, 2011; Yuan et al., 2008), whereas studies of patients with schizophrenia and their relatives suggest that women might demonstrate deficits of attentional inhibition compared to men (Crawford et al., 1998; Radant et al., 2007).
Given such initial evidence of independence between behavioral and attentional control mechanisms, it is possible that sensitivity to the impairing effects of alcohol might differ depending on the specific type of inhibition examined. However, no studies to date have compared alcohol-induced impairment of inhibitory control in behavior and in attention. The aim of the current study was to compare alcohol impairment of both behavioral and attentional inhibition within the same individuals in response to the same doses of alcohol. Participants performed a measure of behavioral inhibition (cued go/no-go task) and a measure of attentional inhibition (DOR task) in response to placebo and two active doses of alcohol (0.45 g/kg and 0.65 g/kg). This allowed for a comparison of dose-related alcohol impairment of both mechanisms within the same individuals at similar breath alcohol concentrations (BrACs) at time of testing. In addition to comparing alcohol effects, we conducted exploratory correlational analyses to examine associations between behavioral and attentional mechanisms of inhibition. Finally, based on previous reports of gender differences in both behavioral and attention inhibition (Crawford et al., 1998; Hansen, 2011; Radant et al., 2007; Yuan et al., 2008), we also examined potential gender differences in alcohol impairment of both mechanisms of control.
2. Methods
2.1 Participants
Forty-eight adult drinkers (27 men and 21 women) between the ages of 21 and 29 (mean age = 23.3, SD = 2.5) were recruited to participate in this study. Screening measures were conducted to determine medical history and current and past drug and alcohol use. Any volunteers who self-reported head trauma, psychiatric disorder, or substance abuse disorder were excluded from participation. Volunteers who reported alcohol dependence, as determined by a score of 5 or higher on the Short-Michigan Alcoholism Screening Test (S-MAST; Selzer et al., 1975), were also excluded. Volunteers were recruited via notices placed on community bulletin boards and by university newspaper advertisements. The University of Kentucky Medical Institutional Review Board approved the study, and participants received $160 for their participation.
2.2 Materials and Measures
The tasks were operated using E-prime Experiment Generation Software (Psychology Software Tools, Pittsburgh, PA) and performed on a PC. A Model 504 Eye Tracking System (Applied Science Laboratory, Boston, MA) was used to measure eye movements during the DOR task.
2.2.1 Cued Go/No-go Task
Behavioral inhibition was measured by a cued go/no-go reaction time task used in other research to measure inhibitory control (e.g., Fillmore et al., 2005; Marczinski and Fillmore, 2003). The task requires finger presses on a keyboard, and measures the ability to inhibit the prepotent behavioral response of executing the key press. Cues provide preliminary information regarding the type of imperative target stimulus (i.e., go or no-go) that is likely to follow, and the cues have a high probability of signaling the correct target. Participants were instructed to press the forward slash (/) key on the keyboard as soon as a go (green) target appeared and to suppress the response when a no-go (blue) target was presented. Key presses were made with the right index finger. To encourage quick and accurate responding, feedback was presented to the participant during the inter-trial interval by displaying the words correct or incorrect along with the reaction time (RT) in milliseconds. A test required approximately 15 minutes to complete. The primary measure of this task was participants’ proportions of inhibitory failures (p-inhibitory failures), measured as the proportion of no-go targets in the go cue condition in which a participant failed to inhibit a response. Mean response RT to go targets in the go cue condition was also recorded.
2.2.2 Delayed Ocular Return (DOR) Task
Attentional inhibition was measured by the DOR task, which has been used in previous studies to measure inhibitory control (Abroms et al., 2006; Weafer et al., 2011). This task involves eye movements that are indicative of shifts of visual attention (e.g., Godijn and Theeuwes, 2003), and measures the ability to intentionally inhibit the tendency to make a reflexive saccade toward the sudden appearance of a visual stimulus on a computer screen (Ross et al., 2000; 2005; 1994). Participants were seated in a darkened room and instructed to maintain focus on a fixation point. While participants attended to the fixation point, a bright target stimulus was briefly presented in the periphery. The onset of the stimulus in this context normally causes a saccade to be reflexively executed toward the stimulus (Peterson et al., 2004; Theeuwes et al., 1999). However, in the DOR task, subjects are instructed to “delay” looking at this stimulus (i.e., intentionally inhibit the reflexive saccade), and instead maintain their gaze on the fixation point until it disappears. The disappearance of the fixation point was the signal for participants to then make a saccade as quickly as possible to the location in which the target stimulus had appeared. A test consisted of 96 trials and required 7 min to complete. The primary measure of this task was the number of trials in which a participant failed to inhibit the reflexive saccade (i.e., premature saccades), indicating failure of attentional inhibition. A saccade was considered premature if it covered at least half the distance to the target location before the disappearance of the fixation point. Saccadic RT was also measured for all valid trials.
2.2.3 Barratt Impulsiveness Scale (BIS-10; Patton et al.,1995)
Participants completed the BIS to provide a self-report measure of trait impulsivity. Participants indicate how typical each of 30 statements (e.g., “I am self controlled”) is for them on a 4-point Likert scale. A total score and six factors scores are obtained, with higher scores indicating greater total levels of impulsiveness. This measure was included in order to examine correlations between both behavioral and attentional inhibition and trait impulsivity.
2.2.4 Time Line Follow-Back (TLFB; Sobell and Sobell, 1992)
Participants completed a retrospective time line calendar of their alcohol consumption for the past 90 days to assess daily patterns of drinking. The measure uses “anchor points” to structure and facilitate participants’ recall of past drinking episodes. For each day, participants estimated the number of standard drinks they consumed and the number of hours spent drinking. This information, along with gender and body weight, was used to estimate the resultant BrAC obtained for each drinking day using well established, valid anthropometric-based BrAC estimation formulae that assume an average clearance rate of 15 mg/dl per hour (McKim, 2007; Watson et al., 1981). Days in which the estimated resultant BrAC was 80 mg/100 ml or higher were classified as binge days (National Institute on Alcohol Abuse and Alcoholism, 2004). The TLFB provided four measures of drinking habits over the past 90 days: (a) binge days; (b) drunk days (number of days participants reported feeling drunk); (c) drinking days (number of days alcohol was consumed); (d) total drinks.
2.2.5 Beverage Rating Scale
Participants completed a beverage rating scale to report the perceived alcoholic content of their beverages in terms of bottles of beer containing 5% alcohol. The scale ranged from 0 to 10 bottles of beer, in 0.5 bottle increments. The scale is useful in determining whether participants who receive a placebo beverage are able to detect that no alcohol has been received (e.g., Fillmore and Blackburn, 2002; Fillmore and Vogel-Sprott, 2000).
2.3 Procedure
Interested volunteers responded to study advertisements by calling the laboratory to participate in an intake-screening interview conducted by a research assistant. At that time, they were informed that the purpose of the study was to examine the effects of alcohol on behavioral tasks. All sessions were conducted in the Behavioral Pharmacology Laboratory of the Department of Psychology and testing began between 10 a.m. and 6 p.m. All participants were tested individually. Sessions were scheduled at least 24 hours apart and were completed within four weeks. Participants were instructed to fast for four hours prior to each alcohol session, as well as to refrain from consuming alcohol or any psychoactive drugs or medications for 24 hours before all sessions. Prior to each session, participants provided urine samples that were tested for drug metabolites, including amphetamine, barbiturates, benzodiazepines, cocaine, opiates, and tetrahydrocannabinol (ON trak TesTstiks, Roche Diagnostics Corporation, Indianapolis, IN, USA) and, in women, HCG, in order to verify that they were not pregnant (Mainline Confirms HGL, Mainline Technology, Ann Arbor, MI, USA). Breath samples were also provided and analyzed by an Intoxilyzer, Model 400 (CMI, Inc., Owensboro, KY, USA) at the beginning of each session to verify a zero BrAC.
2.3.1 Intake Session
All participants completed an intake session in order to become acquainted with laboratory procedures. During this session, informed consent for participation was provided. Participants’ heights and weights were measured, and the questionnaire measures were completed. Participants also performed practice tests to become familiar with the cued go/no-go and DOR tasks.
2.3.2 Test Sessions
Performance was tested under three doses of alcohol: 0.0 g/kg (placebo), 0.45 g/kg, and 0.65 g/kg. Doses were reduced to 87% for women to achieve equivalent BrACs for men and women (Fillmore, 2001; Mulvihill et al., 1997). Each dose was administered on a separate test session, and dose order was counter-balanced across genders. The 0.65 g/kg dose produces an average peak BrAC of 80 mg/100 ml, and the 0.45 g/kg dose produces an average peak BrAC of 50 mg/100 ml. The alcohol beverage was served as one part alcohol and three parts carbonated mix, and was consumed in six min. The placebo beverage consisted of four parts carbonated mix and was served in the same manner. Alcohol (3 ml) was floated on top, and the glass was sprayed with an alcoholic mist, which resembled condensation and provided a strong alcoholic odor. Previous research has shown that individuals report that this beverage contains alcohol (e.g., Fillmore and Blackburn, 2002).
Participants performed the cued go/no-go task 35 minutes after drinking, followed by the DOR task at 50 minutes after drinking. Participants’ BrACs were measured immediately preceding both tasks. Breath samples were also obtained at these times during the placebo session, ostensibly to measure participants’ BrACs. Once the testing was finished, participants remained at leisure in the lounge area until their BrACs reached 20 mg/100 ml or below. Upon completing the final session, participants were paid and debriefed.
2.4 Data Analyses
All dependent measures were analyzed by 3 (dose) X 2 (gender) mixed-design analyses of variance (ANOVAs) in which dose was the within-subjects factor and gender was the between-subjects factor. All analyses were first conducted with dose order as a between-subjects factor. There were no significant main effects or interactions involving dose order for any dependent measures, and therefore all analyses are presented collapsed across dose order. Correlational analyses were also conducted to compare measures of behavioral and attentional inhibition and RT both in the placebo condition and following the two active doses of alcohol.
3. Results
3.1 Demographic, Trait Impulsivity, and Drinking Habits Measures
Table 1 summarizes the drinking habits, trait impulsivity scores, and demographics for men and women. In regard to alcohol consumption, the table shows that the men and women were comparable in terms of number of drinking days, binge days, and drunk days. Men consumed a greater total number of drinks, t(46) = 2.5, p = .02, d = .73. There were no gender differences in self-reported impulsivity as measured by total BIS score or on any of the subscales, ps > .17. Men and women did not differ in terms of age, but men were both taller, t(44) = 6.3, p < .001, d = 1.7, and heavier, t(46) = 6.0, p < .001, d = 1.89, than women.
Table 1.
Mean Drinking Habit, Self-report Impulsivity, and Demographic Measures by Gender
| Group | Contrasts (p value) | ||||
|---|---|---|---|---|---|
|
| |||||
| Men
|
Women
|
||||
| M | SD | M | SD | ||
| TLFB | |||||
| Binge Days | 15.4 | 13.1 | 13.0 | 9.5 | .49 |
| Drunk Days | 12.6 | 11.1 | 8.7 | 6.5 | .15 |
| Drinking Days | 31.4 | 16.0 | 28.2 | 12.6 | .46 |
| Total Drinks | 213.4 | 160.3 | 118.5 | 74.1 | .02 |
| BIS | |||||
| Total | 61.1 | 7.6 | 59.7 | 6.8 | .51 |
| Attention | 9.9 | 2.6 | 9.7 | 2.8 | .78 |
| Motor | 15.1 | 2.7 | 14 | 2.5 | .17 |
| Self-control | 11.6 | 2.7 | 11.8 | 2.3 | .77 |
| Cognitive complexity | 10.8 | 2.3 | 10.8 | 2.6 | .94 |
| Perseveration | 8.0 | 1.4 | 7.5 | 1.9 | .33 |
| Cognitive instability | 5.7 | 1.5 | 5.9 | 1.8 | .67 |
| Demographics | |||||
| Age | 22.8 | 2.3 | 23.9 | 2.7 | .13 |
| Height (cm) | 180.5 | 7.7 | 167.7 | 5.0 | <.001 |
| Weight (kg) | 79.4 | 9.8 | 62.8 | 9.4 | <.001 |
Note. Group contrasts were tested by one-way between subjects ANOVAs.
3.2 Breath Alcohol Concentrations
Mean BrAC following the 0.65 g/kg dose was significantly higher than mean BrAC following the 0.45 g/kg dose preceding both the cued go/no-go, t(47) = 8.0, p < .001, d = 1.16, and the DOR tasks, t(47) = 14.6, p < .001, d = 2.11. Between-groups t tests revealed no gender differences in BrAC at either time point under either dose (ps > .37). Based on the entire sample, mean BrACs preceding the cued go/no-go and DOR tasks under the 0.45 g/kg dose were 59.3 mg/100 ml (SD = 13.5) and 55.0 mg/100 ml (SD = 11.1), and mean BrACs under the 0.65 g/kg dose were 80.8 mg/100 ml (SD = 17.6) and 84.4 mg/100 ml (SD = 15.3).
No detectable BrACs were observed in the placebo condition. The beverage rating scale showed that 44 of the 48 participants (92%) reported some alcohol in the placebo beverage. Therefore, the placebo appeared credible for establishing the expectation that alcohol was received. Mean beverage ratings showed that participants estimated the alcohol content of the placebo beverage to be equivalent to 1.45 (.92) bottles of 5% beer. Participants’ mean ratings of the alcohol content of the 0.45 g/kg and the 0.65 g/kg dose were equivalent to 3.9 (1.8) and 4.6 (1.5) bottles of 5% beer, respectively.
3.3 Inhibitory Control
3.3.1 Behavioral Inhibition
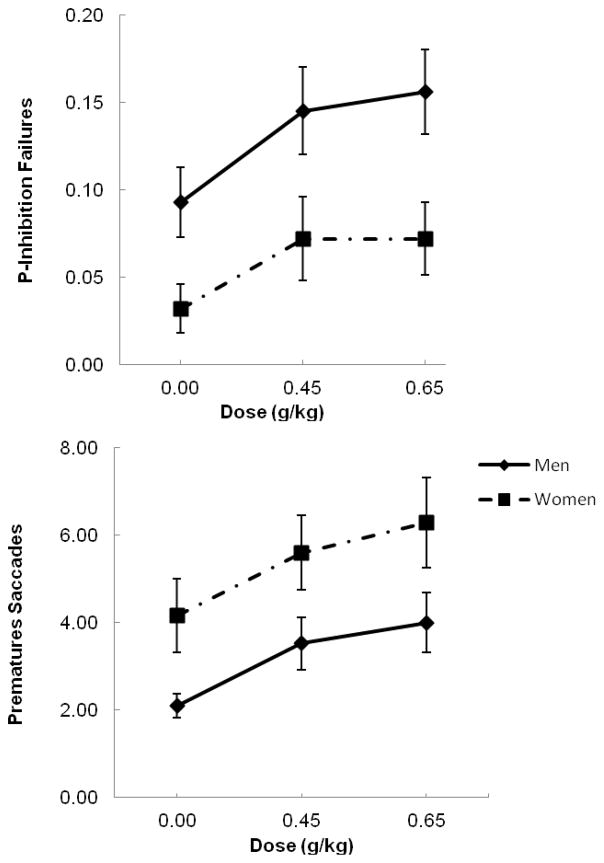
Analyses of p-inhibitory failures to no-go targets on the cued go/no-go task revealed a significant main effect of dose, F(2, 92) = 8.7, p < .001, partial η2 = .16, and a significant main effect of gender, F(1, 46) = 6.9, p = .01, partial η2 = .13, but no dose X gender interaction (p = .71). Mean p-inhibitory failures are presented in Figure 1, separately for men and women (left panel). The figure shows that the main effect of dose is due to an increase in p-inhibitory failures in response to alcohol. This was confirmed by paired t tests of dose effects within the entire sample. Compared to placebo, p-inhibitory failures were significantly increased following the 0.45 g/kg dose, t(47) = 3.7, p = .001, d = .53 and the 0.65 g/kg dose, t(47) = 3.8, p < .001, d = .54. The figure also shows that the main effect of gender is due to greater mean inhibitory failures overall in men than in women. Between-groups t tests confirmed that men committed significantly more inhibitory failures than women in response to all three doses: placebo, t(46) = 2.4, p = .02, d = 0.68; 0.45 g/kg, t(46) = 2.1, p = .04, d = .61; and 0.65 g/kg, t(46) = 2.5, p = .02, d = .73.
Fig. 1.
Mean p-inhibitory failures (left panel) and premature saccades (right panel) for men and women under three alcohol doses: 0.0 g/kg (placebo), 0.45 g/kg, and 0.65 g/kg. Capped vertical lines show standard errors of the mean.
3.3.2 Attentional Inhibition
Analyses of premature saccades on the DOR task revealed a significant main effect of dose, F(2, 92) = 11.1, p < .001, partial η2 = .19, and a main effect of gender, F(1, 46) = 5.9, p = .02, partial η2 = .11, but no gender X dose interaction (p = .96). Mean premature saccades are presented in Figure 1, separately for men and women (right panel). The figure shows that the main effect of dose is due to an increase in premature saccades in response to alcohol. This was confirmed by paired t tests of dose effects within the entire sample. Compared to placebo, premature saccades were significantly increased following the 0.45 g/kg dose, t(47) = 3.4, p = .001, d = .50, and the 0.65 g/kg dose, t(47) = 4.3, p < .001, d = .61. The figure also shows that the main effect of gender is due to more premature saccades in women than in men. Between-groups t tests confirmed that women committed significantly more premature saccades than did men in response to placebo, t(46) = 2.6, p = .01, d = .75, and 0.45 g/kg alcohol, t(46) = 2.0, p < .05, d = .59, and there was a trend toward more premature saccades in women following the 0.65 g/kg dose, t(46) = 1.9, p = .06, d = .56.
3.3.3. Associations between Behavioral and Attentional Inhibition
Correlational analyses were conducted to test for associations between behavioral and attentional inhibition. No significant correlations were observed between the measures under any dose (ps > .08). Similarly, no significant correlations were observed between measures of inhibition and trait impulsivity (ps > .24).
3.4 Reaction Time
3.4.1 Response RT
There was a main effect of gender for response RT on the cued go/no-go task, F(1, 46) = 6.3, p = .02, partial η2 = .12. No significant main effect or interaction involving dose was found (ps > .18). Mean response RT is presented in Table 2. The table shows the main effect of gender is due to overall faster RT in men compared to women. Between-groups t tests confirmed that men responded significantly faster than women following each dose: placebo, t(46) = 2.4, p = .02, d = .69; 0.45 g/kg, t(46) = 2.4, p = .02, d = .70; and 0.65 g/kg, t(46) = 2.4, p = .02, d = .70.
Table 2.
Mean (SD) Response and Saccadic RT by Gender
| Dose
|
|||
|---|---|---|---|
| 0.0 g/kg | 0.45 g/kg | 0.65 g/kg | |
| Response RT (ms) | |||
| Men | 284.1 (27.5) | 285.9 (31.2) | 287.4 (31.9) |
| Women | 303.7 (29.4) | 307.3 (30.0) | 309.6 (32.0) |
| Saccadic RT (ms) | |||
| Men | 353.3 (60.8) | 375.5 (63.3) | 402.8 (70.2) |
| Women | 379.3 (79.9) | 393.3 (76.3) | 404.8 (82.5) |
3.4.2 Saccadic RT
Analyses of saccadic RT on the DOR task revealed a main effect of dose, F(2, 92) = 13.4, p < .001, partial η2 = .23. No main effect or interaction involving gender was found (ps > .25). Mean saccadic RT is presented in Table 2. The table shows that the main effect of dose is due to a slowing of RT in response to alcohol in both men and women, and this was confirmed by paired t tests within the entire sample. Compared to placebo, saccadic RT was significantly slowed following the 0.45 g/kg dose, t(47) = 2.5, p = .02, d = .36, and the 0.65 g/kg dose, t(47) = 5.4, p < .001, d = .79.
3.4.3. Associations between Response and Saccadic RT
Correlational analyses were conducted to test for associations between response RT and saccadic RT. The two RT measures were positively correlated following both 0.45 g/kg alcohol (r = .31, p = .03) and 0.65 g/kg alcohol (r = .32, p = .03), but not following placebo (p = .09).
4. Discussion
The current study investigated alcohol impairment of behavioral and attentional control in men and women. Results showed that the drug significantly impaired both behavioral and attentional inhibition relative to placebo. However, correlational analyses showed that individual differences in behavioral and attentional inhibition bore no relation to one another in the placebo or active dose conditions, despite positive associations between response RT and saccadic RT following alcohol. Finally, the study showed significant gender differences on both measures of inhibition. These gender differences were in opposite directions, with men exhibiting poorer behavioral inhibition compared to women, and women exhibiting poorer attentional inhibition.
These findings demonstrate that both behavioral and attentional inhibition are sensitive to the acute effects of alcohol, in that alcohol increased inhibitory errors on both tasks. Moreover, both behavioral and attentional control were significantly impaired even at the lower dose of alcohol, indicating sensitivity to alcohol impairment at BrACs lower than the binge level of intoxication (National Institute on Alcohol Abuse and Alcoholism, 2004). The current findings also suggest that the magnitude of alcohol impairment is similar for both behavioral and attentional inhibition. Indeed, effect sizes for impairment in response to both doses on both tasks were all within the medium range (.50–.61). Finally, alcohol effects on inhibitory control could not be attributed to any speeding of RT for either the behavioral or attentional measures. Alcohol had no effect on behavioral RT, and actually slowed saccadic RT, yet inhibitory errors were increased under the drug for both tasks.
These findings also demonstrate important distinctions between behavioral and attentional inhibition. Specifically, individual differences in the two inhibitory control measures were not related under any dose administered in this study. This is especially noteworthy given the significant associations between individual differences in RT on the behavioral and attentional tasks following both active doses of alcohol. Thus, despite some degree of association between speed of activation of behavioral and attentional mechanisms, inhibitory mechanisms of behavior and attention appear to operate somewhat independently. Taken together, these findings suggest that attentional and behavioral control might be equally sensitive to alcohol, yet might represent two distinct neural systems. Indeed, this is consistent with neuroimaging studies that suggest some segregation of neuroanatomical control of behavioral and attentional inhibition (Aron et al., 2004; Leung and Cai, 2007).
In addition to the lack of association between behavioral and attentional inhibition, no significant associations were observed between either measure of inhibitory control and self-reported trait impulsivity. This finding is generally consistent with the majority of studies that have failed to observe significant associations between behavioral tasks and self-report measures of impulsivity (e.g., Enticott et al., 2006; Horn et al., 2003; Reynolds et al., 2006, but see also Castellanos-Ryan et al., 2011; Swann et al., 2002). The general lack of consistency between measures of inhibition obtained from behavioral tasks and trait measures of impulsivity could potentially suggest low task validity. However, it is important to note that trait measures examine impulsivity as patterns of under-controlled behaviors that are evident by social behaviors, interpersonal interactions, and affective responses to situations. By contrast, behavioral tasks provide a microanalysis of inhibitory control as the ability to exert brief, momentary suppression over a simple action (e.g., key press, eye movement). As such, when considering the divergence in the scope of analysis between these approaches, the lack of correlation between laboratory assessments of inhibition and self-report measures of trait impulsivity is not so surprising (Fillmore and Weafer, in press).
Observation of gender differences in behavioral and attentional inhibition in opposite directions provides additional support for the independence of these two mechanisms. It is important to note that the gender differences cannot be attributed to differences in BrACs or drinking habits between men and women. We adjusted the dose administered to women to 87% of that administered to men, and BrAC analyses verified that there were no gender differences throughout time of testing. Additionally, detailed reports of participants’ recent drinking habits showed that men and women in the current study were comparable in terms of number of drinking days, binge days, and drunk days. Men reported more total drinks consumed; however, considering that men are generally larger than women, it is likely that both genders consumed comparable mean weight-adjusted doses of alcohol.
The observed gender differences could have important implications regarding negative consequences associated with alcohol-induced disinhibition. For instance, the deficits in behavioral inhibition observed in men compared to women suggests that men might be at increased risk for negative outcomes associated with alcohol-induced behavioral disinhibition (e.g., driving while intoxicated, provoking fights). Moreover, increased sensitivity to the behaviorally disinhibiting effects of alcohol has also been implicated in greater risk for abuse (Fillmore, 2003; Marczinski et al., 2007; Weafer and Fillmore, 2008). That is, impairment of behavioral control mechanisms from an initial dose of alcohol is thought to impair the ability to suppress or control additional alcohol consumption, and as such, individuals who exhibit greater disinhibition under the drug might experience greater difficulty in controlling excessive alcohol consumption. Consistent with this hypothesis and the current finding of more pronounced deficits of behavioral inhibition in men, epidemiological research has shown that men are more likely than women to binge drink (e.g., Courtney and Polich, 2009), and they are also more likely to engage in risky behaviors and experience a greater number of adverse consequences while drinking (Wilsnack et al., 2000). As such, alcohol-induced behavioral disinhibition could be an important contributing factor to increased risk for abuse and alcohol-related problems observed in men.
Similarly, impairment of attentional inhibition could also potentially confer increased risk for alcohol abuse, and this might be especially true for women. A growing body of research has shown that alcohol abusers display a biased attention towards drug-related stimuli, and that such stimuli could elicit craving and drug self-administration in these individuals (Field and Cox, 2008; Field et al., 2009; Robinson and Berridge, 1993, 2000). Thus, it is important for individuals attempting to control substance use to exercise inhibitory control of attention and ignore drug-related stimuli when they are encountered. In support of this hypothesis, initial studies have begun to link poor attentional control and increased risk for abuse. For instance, our group showed that poor inhibition of attention predicted greater self-reported alcohol consumption in individuals with ADHD (Weafer et al., 2011). Additionally, children with a family history of alcoholism have also shown deficits in attentional control (e.g., Habeych et al., 2006; Iacono et al., 1999). These studies, along with the current observation of deficits of attentional inhibition in women, suggest that poor attentional control might be an important predictor of excessive alcohol use for women. Indeed, women have been shown to be more easily distracted by novel stimuli, and to be more sensitive to the influence of attentional cues (Bayliss et al., 2005; Garcia-Garcia et al., 2008; Stoet, 2010). As such, it is possible that alcohol-related cues might be more difficult for female drinkers to ignore, and this might be an important contributing factor for excessive alcohol consumption in women. Although speculative, these are intriguing questions to be addressed in future studies.
Acknowledgments
Role of Funding Source This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA018274, R01 AA012895, and F31 AA018584. The NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA018274, R01 AA012895, and F31 AA018584.
Footnotes
Contributors Jessica Weafer and Mark T. Fillmore designed the study and wrote the protocol. Jessica Weafer oversaw all data collection and coding and undertook the statistical analysis. Both authors contributed to preparation of the manuscript and have approved the final manuscript.
Conflict of Interest The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abroms BD, Gottlob LR, Fillmore MT. Alcohol effects on inhibitory control of attention: distinguishing between intentional and automatic mechanisms. Psychopharmacology. 2006;188:324–334. doi: 10.1007/s00213-006-0524-y. [DOI] [PubMed] [Google Scholar]
- Adams ZW, Milich R, Fillmore MT. Examining manual and visual response inhibition among ADHD subtypes. J Abnorm Child Psychol. 2010;38:971–983. doi: 10.1007/s10802-010-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, di Pellegrino G, Tipper SP. Sex differences in eye gaze and symbolic cueing of attention. Q J Exp Psychol A. 2005;58:631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Blekher T, Beard JD, O’Connor S, Orr WE, Ramchandani VA, Miller K, Yee RD, Li TK. Response of saccadic eye movements to alcohol in African American and non-Hispanic white college students. Alcohol Clin Exp Res. 2002;26:232–238. [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, Conrod PJ. Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcohol Clin Exp Res. 2011;35:140–155. doi: 10.1111/j.1530-0277.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW. Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley Family Study. Am J Psychiatry. 1998;155:1703–1710. doi: 10.1176/ajp.155.12.1703. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- Enticott P, Ogloff J, Bradshaw J. Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Pers Individ Dif. 2006;41:285–294. [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol Addict Behav. 2001;15:325–332. [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J Stud Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and itstreatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Behavioral inhibition and addiction. In: MacKillop J, de Wit H, editors. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. John Wiley and Sons Limited; West Sussex UK: in press. [Google Scholar]
- Fowles DC. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. J Res Pers. 1987;21:417–435. [Google Scholar]
- Garcia-Garcia M, Dominguez-Borras J, SanMiguel I, Escera C. Electrophysiological and behavioral evidence of gender differences in the modulation of distraction by the emotional context. Biol Psychol. 2008;79:307–316. doi: 10.1016/j.biopsycho.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Parallel allocation of attention prior to the execution of saccade sequences. J Exp Psychol Hum Percept Perform. 2003;29:882–896. doi: 10.1037/0096-1523.29.5.882. [DOI] [PubMed] [Google Scholar]
- Gray JA. The behavioral inhibition system: a possible substrate for anxiety. In: Feldman MP, Broadhurst A, editors. Theoretical and Experimental Bases of the Behavior Therapies. Wiley; London: 1976. pp. 3–41. [Google Scholar]
- Habeych ME, Folan MM, Luna B, Tarter RE. Impaired oculomotor response inhibition in children of alcoholics: the role of attention deficit hyperactivity disorder. Drug Alcohol Depend. 2006;82:11–17. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hansen S. Inhibitory control and empathy-related personality traits: sex-linked associations. Brain Cogn. 2011;76:364–368. doi: 10.1016/j.bandc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Houghton G, Tipper SP. A model of inhibitory mechanisms in selective attention. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. Academic Press; San Diego, CA: 1994. pp. 53–112. [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Khan SA, Ford K, Timney B, Everling S. Effects of ethanol on anti-saccade task performance. Exp Brain Res. 2003;150:68–74. doi: 10.1007/s00221-003-1400-1. [DOI] [PubMed] [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Irwin DE. Don’t look! Don’t touch! Inhibitory control of eye and hand movements. Psychol Bull Rev. 2000;7:107–112. doi: 10.3758/bf03210728. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- McKim WA. Drugs and Behavior: An Introduction to Behavioral Pharmacology. Pearson Prentice Hall; New Jersey: 2007. [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Newsletter. 2004. NIAAA Council Approves Definition of Binge Drinking; p. 3. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peterson MS, Kramer AF, Irwin DE. Covert shifts of attention precede involuntary eye movements. Percept Psychophys. 2004;66:398–405. doi: 10.3758/bf03194888. [DOI] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, Freedman R, Green MF, Greenwood TA, Gur RE, Light GA, Meichle SP, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang MT, Turetsky BI, Tsuang DW. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23:1320–1330. [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards J, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Linking impulsivity and inhibitory control using manual and oculomotor response inhibition tasks. Acta Psychol (Amst) 2011;138:419–428. doi: 10.1016/j.actpsy.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Roche DJ, King AC. Alcohol impairment of saccadic and smooth pursuit eye movements: impact of risk factors for alcohol dependence. Psychopharmacology. 2010;212:33–44. doi: 10.1007/s00213-010-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Harris JG, Olincy A, Radant A. Eye movement task measures inhibition and spatial working memory in adults with schizophrenia, ADHD, and a normal comparison group. Psychiatry Res. 2000;95:35–42. doi: 10.1016/s0165-1781(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Ross RG, Heinlein S, Zerbe GO, Radant A. Saccadic eye movement task identifies cognitive deficits in children with schizophrenia, but not in unaffected child relatives. J Child Psychol Psychiatry. 2005;46:1354–1362. doi: 10.1111/j.1469-7610.2005.01437.x. [DOI] [PubMed] [Google Scholar]
- Ross RG, Hommer D, Breiger D, Varley C, Radant A. Eye movement task related to frontal lobe functioning in children with attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1994;33:869–874. doi: 10.1097/00004583-199407000-00013. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stoet G. Sex differences in the processing of flankers. Q J Exp Psychol. 2010;63:633–638. doi: 10.1080/17470210903464253. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE, Zelinsky GJ. Influence of attentional capture on oculomotor control. J Exp Psychol Hum Percept Perform. 1999;25:1595–1608. doi: 10.1037//0096-1523.25.6.1595. [DOI] [PubMed] [Google Scholar]
- Vassallo S, Abel LA. Ethanol effects on volitional versus reflexive saccades. Clin Exp Ophthalmol. 2002;30:208–212. doi: 10.1046/j.1442-9071.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- Vorstius C, Radach R, Lang AR, Riccardi CJ. Specific visuomotor deficits due to alcohol intoxication: evidence from the pro- and antisaccade paradigms. Psychopharmacology. 2008;196:201–210. doi: 10.1007/s00213-007-0954-1. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects: updating the Widmark Equation. J Stud Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology. 2008;201:315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Milich R, Fillmore MT. Behavioral components of impulsivity predict alcohol consumption in adults with ADHD and healthy controls. Drug Alcohol Depend. 2011;113:139–146. doi: 10.1016/j.drugalcdep.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlstrom S, Bondy S, Csemy L, Ferrence R, Ferris J, Fleming J, Graham K, Greenfield T, Guyon L, Haavio-Mannila E, Kellner F, Knibbe R, Kubicka L, Loukomskaia M, Mustonen H, Nadeau L, Narusk A, Neve R, Rahav G, Spak F, Teichman M, Trocki K, Webster I, Weiss S. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction. 2000;95:251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, He Y, Qinglin Z, Chen A, Li H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. 2008;45:986–993. doi: 10.1111/j.1469-8986.2008.00693.x. [DOI] [PubMed] [Google Scholar]