Abstract
Background
Marijuana use by teenagers often predates the use of harder drugs, but the neurobiological underpinnings of such vulnerability are unknown. Animal studies suggest enhanced heroin self-administration (SA) and dysregulation of the endogenous opioid system in the nucleus accumbens shell (NAcsh) of adults following adolescent Δ9-tetrahydrocannabinol (THC) exposure. However, a causal link between Penk expression and vulnerability to heroin has yet to be established.
Methods
To investigate the functional significance of NAcsh Penk tone, selective viral-mediated knockdown and overexpression of Penk was performed, followed by analysis of subsequent heroin SA behavior. To determine whether adolescent THC exposure was associated with chromatin alteration, we analyzed levels of histone H3 methylation in the NAcsh via ChIP at five sites flanking the Penk gene transcription start site.
Results
Here, we show that regulation of the proenkephalin (Penk) opioid neuropeptide gene in NAcsh directly regulates heroin SA behavior. Selective viral-mediated knockdown of Penk in striatopallidal neurons attenuates heroin SA in adolescent THC-exposed rats, whereas Penk overexpression potentiates heroin SA in THC-naïve rats. Furthermore, we report that adolescent THC exposure mediates Penk upregulation through reduction of histone H3 lysine 9 (H3K9) methylation in the NAcsh, thereby disrupting the normal developmental pattern of H3K9 methylation.
Conclusions
These data establish a direct association between THC-induced NAcsh Penk upregulation and heroin SA and indicate that epigenetic dysregulation of Penk underlies the long-term effects of THC.
Keywords: drug addiction, marijuana, rat, nucleus accumbens, striatopallidal, epigenetics
INTRODUCTION
Drug addiction is a chronic and relapsing disease that often begins during adolescence. Epidemiological evidence documents an association between marijuana use during adolescence and subsequent abuse of drugs such as heroin and cocaine (1, 2). While many factors including societal pressures, family, culture, and drug availability can contribute to this apparent `gateway' association, little is known about the neurobiological basis underlying such potential vulnerability. Of the neural substrates that have been investigated, the enkephalinergic opioid system is consistently altered by developmental marijuana exposure (3–5), perhaps reflecting neuroanatomical interactions between cannabinoid receptor type 1 and the enkephalinergic system (6, 7). Debates exist, however, regarding the relationship between proenkephalin (Penk) dysregulation and opiate susceptibility. We previously reported that adult rats exposed to Δ9-tetrahydrocannabinol (THC; primary psychoactive component of marijuana) during adolescence exhibit increased heroin self-administration (SA) as well as increased expression of Penk, the gene encoding the opioid neuropeptide enkephalin, in the nucleus accumbens shell (NAcsh), a mesolimbic structure critically involved in reward-related behaviors (3). Although these data suggest that increased NAcsh Penk expression and heroin SA behavior are independent consequences of adolescent THC exposure, they do not address a possible causal relationship between THC-induced Penk upregulation in NAcsh and enhanced behavioral susceptibility to opiates. Moreover, insights regarding the neurobiological mechanisms by which adolescent THC exposure maintains upregulation of Penk into adulthood remain unknown.
Here, we take advantage of viral-mediated gene transfer strategies to show that adulthood addiction-like behaviors induced by adolescent THC exposure are dependent on discrete regulation of NAcsh Penk gene expression. A number of recent studies have demonstrated an important role for histone methylation in the regulation of drug-induced behaviors and transcriptional plasticity, particularly alteration of repressive histone H3 lysine 9 (H3K9) methylation at NAc gene promotors (8, 9). We report here that one mechanism by which adolescent THC exposure may mediate Penk upregulation in adult NAcsh is through reduction of H3K9 di- and trimethylation, a functional consequence of which may be decreased transcriptional repression of Penk.
MATERIALS AND METHODS
Animals and THC Treatment
Male 21-day old Long Evans rats (Taconic) were used; procedures conducted in accordance with approved protocols. Rats received intraperitoneal injections of THC (1.5mg/kg) (RTI International, USA) or vehicle (0.9% NaCl with 0.3% Tween 80) every 3rd day (8 injections) during adolescence (PND 28–49)(3). For SA experiments, Penk- and GFP-infused rats were treated with vehicle during adolescence and GFP-, miR ctrl-, and miR Penk-infused rats were treated with THC during adolescence.
Lentiviral Vectors
Lentiviral vectors were constructed as described. (Supplemental Information). In all cases, in vivo transgene expression was validated and NAcsh-specific expression confirmed via in situ hybridization histochemistry.
Surgeries
Two weeks after final THC treatment, rats were anesthetized with isoflurane/O2 (2.5–4.5%), and bilaterally stereotaxically infused with 0.5μl virus (Penk or GFP) or 1.0μl virus (GFP, miR ctrl, miR Penk) into NAcsh (from bregma: AP +1.7mm; ML +2.3mm; DV −6.8mm (from dura), 10° from midline) at 0.1μl/min. Two weeks subsequently, rats underwent surgery for jugular catheters (Brian Fromant, Cambridge, UK) for future SA experiments.
Heroin Self-Administration and Locomotor Activity
Heroin SA was conducted according to published protocols (3, 5)(Supplemental Information). Briefly, rats self-administered heroin (30μg/kg/injection) 3hr daily under a fixed-ratio 1 (FR1) reinforcement-schedule until stable baseline responding was established. Following stabilization, a between-session dose response was conducted (30/7.5/100/15/60 μg/kg/injection; 1 dose/day). After a 3wk abstinence period, cue-induced drug-seeking behavior was evaluated (depression of the drug-paired lever had no programmed consequence) followed 1wk later by mild stress (24hr food deprivation)-induced drug-seeking. Both tests were 1hr. Activity was measured by infrared beams during the SA sessions.
In situ Hybridization Histochemistry (ISHH)
ISHH was conducted according to published protocols (3, 10) (Supplemental Information). [35S]-labeled Penk riboprobe (generated from a PCR-derived cDNA fragment: bases 585–1140; Genbank accession: NM_017139) was applied to duplicate brain sections (2×103 cpm/mm2), overnight 55°C hybridization, and sections exposed (imaging plate; FUJIFILM) with 14C standards for 28hr. Disintegrations-per-minute (dpm/mg) autogradiographic measurements were obtained for the NAc and averaged/animal.
Gene Expression Analysis
RNA was prepared from fresh-frozen bilateral NAcsh punches. cDNA was obtained using a first-strand synthesis kit (Quanta Biosciences). Quantitative real-time PCR analysis was performed using Taqman-based probes (Applied Biosystems; Penk, Rn00567566_m1; 18 S, 4319413E; Pdyn, Rn00571351_m1.); reactions run in triplicate, each gene run separately. Data normalized to eukaryotic 18S rRNA and analyzed via the ΔΔCT method (11).
Chromatin Immunoprecipitation (ChIP)
Fresh tissue was prepared for ChIP as previously described (8, 12) with minor modifications (Supplemental Information). Briefly, two bilateral NAcsh punches/rat (three rats pooled per sample) were collected and processed. Immunoprecipitated (IP'd) samples (antibodies: H3K9me2, ab1220; H3K9me3, ab8898; H3K36me3, ab9050; H3K4me3, ab8580) abcam, MA, USA) were subject to qPCR (SYBR Green (Roche)) and normalized to their non-IP'd input controls. Each reaction was run in triplicate and analyzed via the ΔΔCT method (11).
Statistics
We used two-tailed unpaired Student's t-tests (for comparison of two groups), one-way ANOVAs followed by followed by Tukey's HSD test or two-tailed Student's t-tests when appropriate (for three groups), and two-way repeated measures ANOVAs followed by one-way ANOVAs (to examine significant repeated-measure effects). All values represent mean±SEM (*p<0.05; **p<0.01; ***p<0.001). Statistical calculations performed using JMP software (SAS, NC, USA).
RESULTS
Penk overexpression in NAcsh increases heroin self-administration
To investigate the direct role of NAcsh Penk tone in the regulation of heroin SA behavior, we first verified the effects of local overexpression of Penk by infusing a lentiviral vector encoding Penk and green fluorescent protein (GFP) into NAcsh of adult rats (Fig. 1a,b). Viral spread was 1 mm3 from the needle tip.
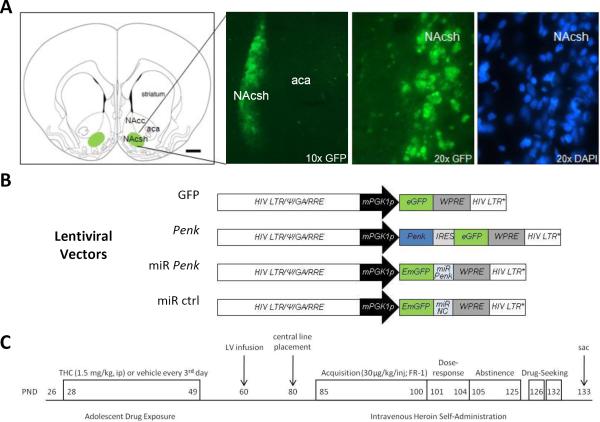
Figure 1. Lentivirus-mediated Penk gene manipulation and heroin self-administration.
(A) GFP expression is restricted to NAcsh (Adapted from Paxinos and Watson, 2007). (B) Lentiviral vectors (C) Behavioral research design. NAcsh, nucleus accumbens shell; NAcc, nucleus accumbens core; aca, anterior commissure; GFP, green fluorescent protein; DAPI, 4',6-diamidino-2-phenylindole; mPGK1p, mouse phosphoglycerate kinase-1 promoter; eGFP, enhanced green fluorescent protein; EmGFP, emerald green fluorescent protein; WPRE, woodchuck post-transcriptional regulatory element; Ψ, encapsidation signal including the 5′ portion of the gag gene (GA); RRE, Rev-responsive element; LTR, long terminal repeat; LTR*, LTR with deletion in the U3 region; Penk, 956 nucleotide fragment containing the coding region of the rat Penk cDNA; IRES, encephalomyocarditis virus internal ribosome entry site; miR NC, miRNA targeting non-vertebrate gene (Invitrogen); miR Penk, miRNA targeting nucleotides 709–729 of rat Penk coding region; PND, postnatal day; LV, lentiviral vector; sac, sacrifice. See also Table S1 and Fig. S2 in the Supplement.
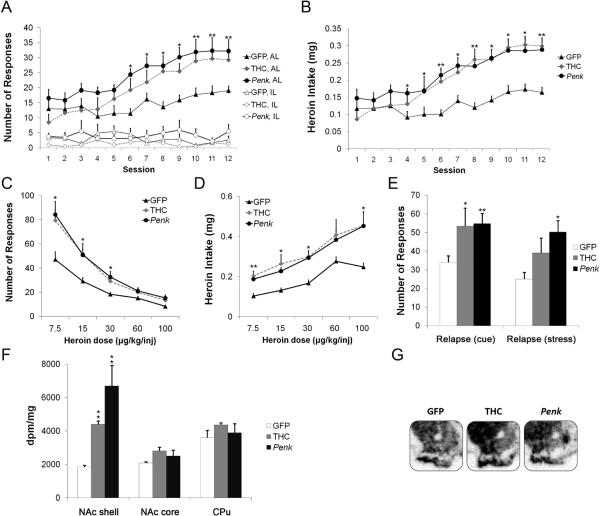
To investigate the functional significance of enhanced NAcsh Penk tone, we tested the involvement of increased NAcsh Penk expression on a FR1 schedule of heroin SA. Two cohorts of animals were treated with vehicle during adolescence and then given bilateral NAcsh infusions of lentivirus vectors expressing Penk or GFP in young adulthood. A third cohort of rats was treated with THC during adolescence (Fig. 1c; Table S1 in the Supplement). Bilateral NAcsh infusions of Penk enhanced responding for heroin (treatment by session interaction, F22,213=2.036, P<0.01, Fig. 2a) and mean heroin intake (treatment by session interaction, F22,213=2.589, P<0.001, Fig. 2b), without modifying inactive lever pressing or locomotor activity (Fig. S1A in the Supplement), compared with GFP-infused controls. Consistent with previous data (3), animals treated with THC during adolescence also exhibited increased heroin SA compared to control conditions (GFP-infusion), an effect similar in magnitude to that resulting from NAcsh Penk overexpression (Fig. 2a,b). Animals treated with THC during adolescence also exhibited increased heroin SA compared with vehicle-exposed “sham” animals that had undergone bilateral NAcsh infusion with saline (Fig. S2 in the Supplement). Following stabilization of heroin SA behavior, we next examined whether NAcsh Penk overexpression affected dose-dependent responding for heroin in a between-session dose response test. Penk overexpression and adolescent THC treatment led to upward vertical shifts in the heroin dose-response function, including higher peak SA rates on the descending limb of the dose-response curve (treatment by dose interaction, F8,74=3.859, P<0.001, Fig. 2c) and higher drug intake across the range of doses studied (Fig. 2d).
Figure 2. Penk overexpression in NAcsh potentiates heroin self-administration.
(A) Acquisition of heroin SA (FR-1, 30 μg/kg/injection). (B) Mean heroin intake. (C) Between-session dose-response (7.5, 15, 30, 60, 100 μg/kg/infusion; randomized order). (D) Mean heroin intake. (E) Heroin-seeking behavior (cue- and stress-induced) in Penk-infused, GFP-infused, or THC-exposed rats. (F) Penk mRNA levels in NAcsh, NAc core, and caudate-putamen (CPu) following heroin SA. (G) Representative in situ hybridization autoradiograms demonstrating striatal Penk mRNA expression following heroin SA. For all figures, n=5–9/group. Data shown as mean±SEM. *P<0.05; **P<0.01 compared to GFP-expressing controls for each session. AL, active lever; IL, inactive lever, dpm, disintegrations per minute. See also Fig. S1A in the Supplement.
Penk overexpression in NAcsh promotes enhanced heroin-seeking
To determine whether NAcsh Penk infusion facilitated subsequent behavioral susceptibility to relapse, we measured heroin-seeking behavior following abstinence. In light of self-reports by drug-dependent individuals that exposure to drug-associated stimuli and stress precipitate drug craving and relapse (13–15), we first assessed heroin-seeking behavior under cue-induced reinstatement conditions. The magnitude of heroin-seeking behavior in the absence of reinforcement was assessed by the amount of responding at the previously drug-paired lever. Adolescent THC exposure and NAcsh Penk overexpression led to an increase in drug-paired lever responding (F2,20=3.859, P<0.05, Fig. 2e) when compared to GFP-infused controls, indicating that prior NAcsh Penk infusion enhanced the ability of the drug-associated environment to elicit drug-seeking behavior. We next examined if NAcsh Penk infusion enhanced drug-seeking behavior triggered by exposure to a stressor previously shown to increase heroin-seeking (4). One week after the first drug-seeking test, we evaluated drug-seeking behavior following 24hr food deprivation. NAcsh Penk overexpression, but not adolescent THC-exposure, potentiated stress-induced heroin-seeking compared to GFP-infused controls (F2,19=4.829, P<0.05; Fig. 2e). Thus, increased Penk tone in NAcsh increased heroin-seeking behavior triggered by a mildly stressful event, indicating an enhanced propensity for relapse in these animals. Importantly, autoradiographic ISHH image analysis revealed significant upregulation of Penk mRNA expression in NAcsh of Penk-infused and adolescent THC-exposed animals compared to GFP-infused control animals (F2,23=11.026, P<0.05; Fig. 2f,g) following the completion of behavioral experiments. In contrast, Pdyn mRNA levels in NAcsh were unchanged in vehicle-exposed, GFP-infused, and Penk-infused animals (Fig. S3 in the Supplement). These data demonstrate that specific Penk upregulation in NAcsh promotes drug-seeking after prolonged abstinence from heroin, indicating an important role for increased NAcsh Penk tone in the propensity for both cue- and stress- induced heroin-seeking behavior.
NAcsh Penk knockdown attenuates adolescent THC-induced heroin self-administration
The pronounced increase in heroin-taking and -seeking behavior resulting from NAcsh Penk overexpression prompted us to assess whether Penk-mediated regulation of these behaviors were selective to striatopallidal neurons that preferentially express the Penk gene. To this end, we used a microRNA (miR) targeting the Penk mRNA, allowing for miR-mediated mRNA cleavage specific to Penk-expressing neurons. After confirming the specific activity of the Penk miR in vitro (Fig. S4 in the Supplement), we verified the effects of local overexpression of a lentiviral vector containing the Penk miR tagged with GFP (miR Penk) into NAcsh (Fig. 1b).
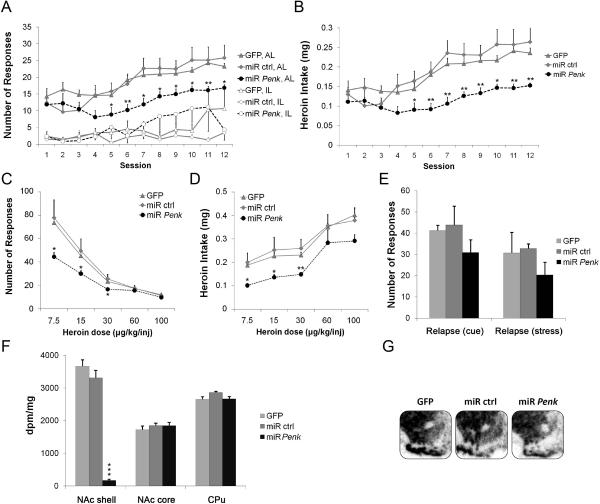
To investigate whether miR-mediated Penk knockdown could reverse the behavioral phenotype that was induced by adolescent THC exposure and NAcsh Penk infusion, three cohorts of animals were treated with THC during adolescence and then given bilateral NAcsh infusions of the miR Penk lentivirus, or one of two lentiviral control vectors, one of which contained no targeting miRNA sequence (GFP) and the other a miR known to target a sequence not found in vertebrate DNA (miR ctrl), in young adulthood (Fig. 1c; Table S1 in the Supplement). Despite the marked downregulation of Penk mRNA expression that resulted from miR Penk infusion, animals readily learned the SA paradigm. MiR Penk in NAcsh reduced both overall responding for heroin (treatment by session interaction, F22,213=1.759, P<0.05, Fig. 3a) and mean heroin intake (treatment by session interaction, F22,211=2.136, P<0.01, Fig. 3b) compared to GFP- and miR ctrl-infused control animals. Interestingly, the heroin-taking behavior exhibited by miR Penk animals was similar to the behavior displayed by GFP animals unexposed to THC during adolescence (Fig. 2a,b). NAcsh miR Penk also increased locomotor activity (F2,15=11.441, P<0.001; Fig. S1B in the Supplement) in line with the inhibitory role of the striatopallidal pathway in regulating motor behavior. Overall, these data provide evidence that NAcsh miR Penk blocks the behavioral phenotype induced by adolescent THC, and further implicate a role for NAcsh Penk as a key mediator of heroin susceptibility.
Figure 3. Penk knockdown in NAcsh attenuates heroin self-administration.
(A) Acquisition of heroin SA (FR-1, 30 μg/kg/injection). (B) Mean heroin intake. (C) Between-session dose-response (7.5, 15, 30, 60, 100 μg/kg/infusion; randomized order). (D) Mean heroin intake. (E) Heroin-seeking behavior (cue- and stress-induced) in miR Penk-infused, miR ctrl-infused, or GFP-infused rats exposed to adolescent THC. (F) Penk mRNA levels in NAcsh, NAc core, and caudate-putamen (CPu) following heroin SA. (G) Representative in situ hybridization autoradiograms demonstrating striatal Penk mRNA expression following heroin SA. For all figures, n=5–9/group. Data shown as mean±SEM. *P<0.05; **P<0.01 compared to GFP-expressing controls for each session. AL, active lever; IL, inactive lever, dpm, disintegrations per minute. See also Fig. S1B in the Supplement.
In contrast to Penk overexpression, miR Penk in NAcsh led to a downward shift in the dose-response function, including both lower maximal SA rates (treatment by dose interaction, F8,77=4.120, P<0.001, Fig. 3c) and heroin intake (Fig. 3d) on the lower end of the dose-response curve compared to GFP and miR ctrl-infused control animals. As a downward shift in the dose-response curve opposes alterations thought to be associated with the transition to more addicted states, these data indicate that reduced shell Penk tone decreases apparent behavioral susceptibility to heroin reinforcement. Given that NAcsh miR Penk attenuated the behavioral phenotype induced by adolescent THC exposure, we next investigated whether NAcsh miR Penk affected behavioral susceptibility to drug-seeking. Mir Penk did not affect cue- or stress-induced heroin-seeking when compared to GFP and miR ctrl controls (Fig. 3e). There was a significant downregulation of NAcsh Penk mRNA expression in miR Penk animals (F2,24=222.929, P<0.001; Fig. 3f,g) compared to GFP and miR ctrl control animals following the completion of behavioral experiments. The viral manipulation was specific to the Penk gene as Pdyn mRNA levels in NAcsh were unchanged as a result of miR Penk infusion (Fig. S3 in the Supplement). Taken together, these data establish a causal link between adolescent THC-mediated Penk dysregulation and the subsequent expression of behavioral susceptibility to heroin.
Adolescent THC regulates repressive histone H3 methylation at the Penk gene in NAcsh
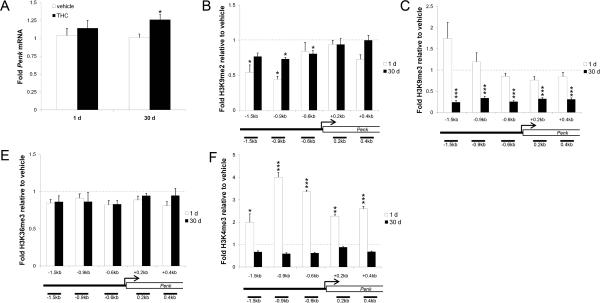
Given the protracted behavioral consequences of THC exposure during adolescence (3), we aimed to identify whether alterations at the level of chromatin regulation were associated with the transcriptional dysregulation of Penk that follows adolescent THC exposure. One day after the last THC treatment, NAcsh Penk mRNA levels were not significantly altered (Fig. 4a), however, consistent with previous data, Penk mRNA expression was significantly increased in NAcsh 30 days after cessation of adolescent THC administration (t15=2.78, P<0.05; Fig. 4a) compared to control animals. As a first step towards characterizing the potential epigenetic regulation of Penk, we investigated whether adolescent THC exposure was associated with altered levels of histone H3 methylation. In light of recent reports describing cocaine-induced alteration of repressive histone H3 lysine 9 (H3K9) methylation at gene promotors in the NAc (8, 9), we studied di- and trimethylation of H3K9 (H3K9me2, H3K9me3) at the Penk gene, as well as trimethylation of histone H3 lysine 36 (H3K36me3) and lysine 4 (H3K4me3), marks that have been associated with transcriptional activation (16, 17). Animals were treated with THC during adolescence and levels of H3K9me2, H3K9me3, H3K4me3, and H3K36me3 were analyzed in NAcsh via ChIP followed by qPCR analysis of five sites flanking the Penk gene transcription start site (TSS), three spanning regulatory elements in the 5'UTR, and two in the coding region (Figure S5 and Table S2 in the Supplement). One day following the final THC treatment, H3K9me2 was decreased at two sites in the Penk promoter region in the most upstream regions evaluated (−1.5-t11=−2.417, P<0.05; −0.9-t11=−2.738, P<0.05; point-wise comparison Fig. 4b) compared to vehicle-treated adolescent control animals. H3K9me3 did not differ statistically between the groups, but did tend to be increased at the same promoter regions where H3K9me2 was decreased (Fig. 4c). No change was observed in H3K36me3 (Fig. 4d). H3K4me3 levels were increased at each region evaluated (−1.5-t10=2.545, P<0.05; −0.9-t9=7.109, P<0.0001; −0.6-t10=5.621, P<0.001; +0.2-t10=3.550, P<0.01; +0.4-t10=8.144, P<0.0001; Fig. 4e).
Figure 4. Adolescent THC regulates Penk gene expression and histone H3 methylation in NAcsh.
(A) NAcsh Penk mRNA levels 1 day (adolescent) and 30 days (adult) after adolescent THC or vehicle (n=9–10/group). (B)–(E) NAcsh histone H3 methylation fold changes at the Penk gene 1 day and 30 days after the last adolescent exposure to THC relative to vehicle treated animals (n=6–8/group (3 animals pooled/n)). (B) H3K9me2. (C) H3K9me3. (D) H3K36me3. (E) H3K4me3. Data shown as mean±SEM.*P<0.05; **P<0.01; ***P<0.001 compared to vehicle-exposed animals at the same time point. kb, kilobases; TSS, transcription start site. See also Fig. S3 and Table S2 in the Supplement.
One month following cessation of adolescent THC treatment, H3K9me2 remained decreased at Penk in adult NAcsh, but significant effects were observed at promoter sites 0.9 kb and 0.6 kb upstream of the Penk TSS (−0.9-t9=−2.260, P<0.05; −0.6-t8=−2.480, P<0.05; Fig. 4b). In contrast to the pattern of H3K9me3 observed in adolescent NAcsh, however, H3K9me3 was decreased at all regions of the Penk gene in adult NAcsh (−1.5-t10=−4.698, P<0.001; −0.9-t10=−7.172, P<0.0001; −0.6-t10=−6.959, P<0.0001; +0.2-t10=−5.681, P<0.001; +0.4-t10=−6.451, P<0.0001; Fig. 4c), a finding consistent with the increased Penk gene expression (Fig. 4a) in these animals. No alterations were observed in H3K36me3 or H3K4me3 in adult animals (Fig. 4d,e). Taken together, these data suggest that decreases in H3K9me2 and H3K9me3 binding at the Penk promoter in adult NAcsh may mediate the upregulation of Penk transcription characteristic of adult animals with adolescent THC exposure.
Developmental regulation of histone H3 methylation at the Penk gene in NAcsh
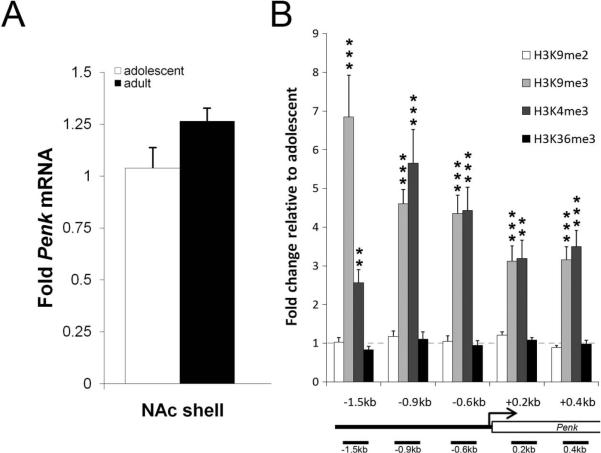
Given that few studies have investigated the ontogeny of the enkephalinergic system, we were interested to study potential developmental differences in the regulation of Penk gene expression and histone H3 methylation at the Penk gene. Evaluation of NAcsh Penk mRNA levels in THC-naïve adolescent and adult animals revealed no significant difference between developmental periods (Fig. 5a). We next examined H3K9me2, H3K9me3, H3K36me3, and H3K4me3 levels at the Penk gene of THC-naïve adolescent and adult animals. While H3K9me2 and H3K36me3 levels were similar between adolescent and adult animals, levels of H3K9me3 were increased in adult NAcsh at all regions studied (−1.5-t9=4.941, P<0.0001; −0.9-t9=8.589, P<0.0001; −0.6-t10=7.160, P<0.0001; +0.2-t10=5.473, P<0.0001; +0.4-t10=6.249, P<0.0001; Fig. 5b). H3K4me3 was also elevated in adulthood compared to the adolescent period at all regions (−1.5-t9=4.211, P<0.01; −0.9-t9=5.172, P<0.001; −0.6-t9=5.586, P<0.001; +0.2-t9=4.179, P<0.01; +0.4-t9=5.434, P<0.001; Fig. 5b). The concomitant enrichment of both H3K9me3 and H3K4me3 at the Penk gene could account for the lack of significant difference observed in Penk mRNA levels between adolescence and adulthood. These data provide evidence that specific histone H3 methyl marks in NAcsh are developmentally regulated.
Figure 5. Histone H3 methylation at the Penk gene in NAcsh is dynamic during normal development.
(A) Adolescent and adult NAcsh Penk mRNA levels (n=9–10/group). (B) NAcsh histone H3 methylation fold changes at the Penk gene (n=6–8/group (3 animals pooled/n)). Data shown as mean±SEM. *P<0.05; **P<0.01; ***P<0.001 compared to adolescent animals. kb, kilobases; TSS, transcription start site.
DISCUSSION
The present studies reveal a direct link between NAcsh Penk gene expression and enhanced behavioral susceptibility to heroin SA that mimics that seen in adult animals exposed to THC during adolescence. Such findings lend strong support to the hypothesis that adolescent THC exposure contributes to an opiate-vulnerable phenotype in adulthood. Here, we show that overexpression of NAcsh Penk in THC-naïve animals potentiates heroin SA, a behavioral effect that is attenuated by striatopallidal Penk knockdown in THC-exposed animals. Together, these data indicate a direct relationship between adolescent THC-induced Penk upregulation and heightened heroin-taking in adulthood. Furthermore, we suggest that adolescent THC exposure may mediate adult NAcsh Penk upregulation through regulation of repressive histone H3K9 methylation, an epigenetic effect that represents a profound pathologic departure from the distinct developmental pattern of histone H3 methylation that normally occurs at Penk in NAcsh across the transition from adolescence to adulthood.
Of the opioid neuropeptides, enkephalin is consistently associated with regulating hedonic state (18, 19). Although our SA paradigm was not designed to dissociate between reward and incentive motivational state, heroin SA behavior did not differ between groups during the early stages of acquisition, arguing against a Penk-mediated generalized impairment of basal hedonic tone. Moreover, although selective knockdown of Penk expression reduced overall heroin intake over time, it did not affect acquisition of SA behavior. Instead, Penk-overexpressing and THC-exposed animals continued to increase their heroin intake, ultimately stabilizing at a higher drug intake level during the maintenance phase, suggesting that these animals have different hedonic set points compared to controls. (3). Additionally, the present data demonstrate that animals with elevated NAcsh Penk expression exhibit potentiated drug-seeking behavior induced by drug-associated environmental cues and mild stress. Interestingly, stress-induced sensitivity to heroin drug-seeking was also apparent in adults following prenatal THC exposure (4). While the animals' affective state underlying sensitivity to heroin is not yet fully understood, the present experiments implicate a direct role for NAcsh Penk in the opiate-susceptible behavioral phenotype similar to the consequence of adolescent THC exposure.
In the NAc, Penk is predominantly expressed in striatopallidal medium spiny neurons that project to ventral pallidum (20). Viral overexpression of Penk was not localized to a specific striatal subpopulation in the present study, but it nevertheless resulted in the same behavioral pattern of heroin SA demonstrated by rats exposed to adolescent THC, suggesting that an increase in NAcsh enkephalinergic tone may be sufficient to impact opioid susceptibility. In contrast, miR knockdown of Penk is inherently specific to striatopallidal cells, and such manipulations attenuated the enhancement of heroin SA induced by adolescent THC exposure. Importantly, NAcsh Pdyn levels were unaffected by any of the manipulations, indicating specificity of the behavioral alterations to selective NAcsh Penk alteration. Together, these findings emphasize the important role of Penk in mediating long-term effects of THC that contribute to opiate susceptibility. How regulation of Penk striatopallidal regulation contributes to specific components of addiction-related behavior in the non-drug state remains to be established.
Given the protracted behavioral effects of adolescent THC exposure, alterations at the level of chromatin regulation are prime candidates for investigation. While several studies have suggested an important role for transient histone modifications in the regulation of drug-induced behaviors, only recently has histone methylation, a more stable modification, been demonstrated as a potential mediator of drug-induced transcriptional plasticity (8, 9). Histone methylation is highly complex, as N-terminal histone lysine residues can be mono, di, or trimethylated, with each valence state differentially regulating the recruitment of proteins that activate or repress transcription (21, 22). While increased H3K9me2 binding has been demonstrated at promoters of repressed eukaryotic genes, the present findings confirm that reduced H3K9me2 binding plays a role at promoters of activated eukaryotic genes. Of the histone marks quantified in the present study, dimethylation of H3K9 at upstream regions of the Penk gene in NAcsh was reduced both 1 day and 30 days after THC administration. In contrast, the pronounced enrichment across the Penk promoter of the activating mark H3K4me3 seen 1 day after THC exposure was normalized by adulthood.
In addition to modulation of H3K9me2, adolescent THC exposure also had significant effects on H3K9me3, an unexpected finding given that H3K9me3 is typically enriched at peri-centromeric heterochromatin and sites of repressed chromatin (23–26). However, several groups have reported the presence of H3K9me3 in transcribed regions of active mammalian genes (27–30). The current finding that H3K9me3 was decreased long-term (30 days) in the transcribed regions of Penk in the adult NAcsh as a consequence of adolescent THC exposure raises the possibility that reduced H3K9me3 in the coding regions of active genes may also contribute to transcriptional plasticity. Current technologies cannot establish causal regulation of histone methylation at a single gene level, but accumulating evidence suggests that H3K9me3 may play a significant role in regulating active genes (30, 31). Given the low levels of H3K9me3 at most expressed genes (26), however, the magnitude of the fold changes seen with adolescent THC in the adult may be artificially enhanced. Though presently impossible to know the absolute neurobiological consequences of small relative changes in histone marks, the differential profile of H3K9me3 at the Penk gene 1 day as compared to 30 days after THC exposure, coupled with the potentiated SA behavior evident in adult animals, support a functional role for even small perturbations in H3K9me3 at the Penk gene and thus requires further investigation.
To date, no studies have examined histone methylation during normal development. Adolescence is a critical phase of brain maturation, and the current results demonstrate distinct development-specific patterns of histone H3 modifications at the NAcsh Penk gene. While stable levels of H3K9me2 and H3K36me3 were observed in NAcsh of adolescent and adult animals, the profiles of H3K9 and H3K4 trimethylation varied across this developmental period. The chromatin landscape is highly complex, but trimethylation of H3K4 (transcriptional activation) concomitant with trimethylation of H3K9 (transcriptional repression) may account for the developmental transcriptional stability of NAcsh Penk as there was no difference in Penk mRNA levels in adolescent vs. adult. Furthermore, that H3K9me3 and H3K4me3 displayed similar magnitudes of induction and distribution across the Penk gene in adolescent NAcsh suggests that trimethylation of these marks may be coordinated (30) during normal development. Though the functional consequences on NAcsh Penk gene expression did not differ between adolescence and adulthood, the distinct epigenetic profiles during these ontogenetically disparate periods may allow the Penk gene to be “primed” to respond differentially to similar environmental cues. Limited studies have investigated the differential neurobiologic effects of THC exposure in adolescence versus adulthood, but mounting evidence documents differential responsivity to drugs of abuse in the adolescent as compared to adult brain (32, 33). Overall, our study emphasizes that adolescent THC exposure leads to a departure of the normal trajectory of the transcriptional and epigenetic state of the Penk gene, a disruption which may mediate the expression of enhanced behavioral vulnerability to opiates in adulthood.
In conclusion, our findings indicate that marijuana exposure in and of itself can serve as a risk factor that acts `above the genome' and can imprint upon the existing epigenetic landscape of adolescent neurodevelopment. Thus, the epigenetic effects of adolescent THC exposure may act in concert to augment future behavioral responses to drugs of abuse via stable and long-term regulation of genes at the transcriptional level. The results also support a novel role for the Penk gene as an emergent endogenous risk factor resulting from adolescent THC exposure, the dysregulation of repressive histone H3 methylation of which may underlie the long-term behavioral consequences of adolescent THC.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Ian Maze for comments on the manuscript and Dr. Yanhua Ren for technical advice.
This work was funded by grants from The National Institute on Drug Abuse DA024929 (HCT), T32 DA007135 (MMJ), DA08227 (EJN), DA030359 (YLH) and DA19350 (YLH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE OF BIOMEDICAL FINANCIAL INTERESTS AND POTENTIAL CONFLICTS OF INTEREST The authors report no biomedical financial interest or potential conflicts of interest.
REFERENCES
- 1.Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- 2.Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- 3.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 4.Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 5.Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008 doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurd YL. In situ hybridization with isotopic riboprobes for detection of striatal neuropeptide mRNA expression after dopamine stimulant administration. Methods Mol Med. 2003;79:119–135. doi: 10.1385/1-59259-358-5:119. [DOI] [PubMed] [Google Scholar]
- 11.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 15.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Shilatifard A. Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol. 2008;20:341–348. doi: 10.1016/j.ceb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 19.Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120:783–798. doi: 10.1016/s0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 21.Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 22.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 23.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 24.Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, et al. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 25.Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, et al. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maze I, Feng J, Wilkinson MB, Sun H, Shen L, Nestler EJ. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proc Natl Acad Sci U S A. 2011;108:3035–3040. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkman AB, Roelofsen T, Pennings SW, Martens JH, Jenuwein T, Stunnenberg HG. Histone modification patterns associated with the human X chromosome. EMBO Rep. 2006;7:628–634. doi: 10.1038/sj.embor.7400686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rougeulle C, Chaumeil J, Sarma K, Allis CD, Reinberg D, Avner P, et al. Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Mol Cell Biol. 2004;24:5475–5484. doi: 10.1128/MCB.24.12.5475-5484.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Squazzo SL, O'Geen H, Komashko VM, Krig SR, Jin VX, Jang SW, et al. Suz12 binds to silenced regions of the genome in a cell-type-specific manner. Genome Res. 2006;16:890–900. doi: 10.1101/gr.5306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Vakoc CR, Sachdeva MM, Wang H, Blobel GA. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006;26:9185–9195. doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- 33.Izenwasser S. Differential effects of psychoactive drugs in adolescents and adults. Crit Rev Neurobiol. 2005;17:51–67. doi: 10.1615/critrevneurobiol.v17.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.