Abstract
Rapamycin is an FDA approved drug for the prevention of immunorejection following organ transplantation. Pharmacological studies suggest a potential new application of rapamycin in attenuating cardiomyopathy, but the potential for this application is not yet supported by genetic studies of genes in target of rapamycin (TOR) signaling in rodents. Recently, supporting genetic evidence was presented in zebrafish using two adult cardiomyopathy models. By characterizing a heterozygous zebrafish target of rapamycin (ztor) mutant, the therapeutic effect of long-term TOR signaling inhibition was demonstrated. Dose- and stage-dependent functions of TOR signaling provide an explanation for the seemingly contradictory results obtained in genetic studies of TOR components in rodents. The results from the zebrafish studies, together with the supporting preliminary clinical studies, suggested that TOR signaling inhibition should be further pursued as a novel therapeutic strategy for cardiomyopathy. Future directions for developing TOR-based therapy include assessing the long-term benefits of rapamycin as a candidate drug for heart failure patients, defining the dynamic activity of TOR, exploring the impacts of TOR signaling manipulation in different models of cardiomyopathies, and elucidating the downstream signaling branches that confer the therapeutic effects of TOR signaling inhibition.
1. Pharmacological studies from rodents prompted a consideration of TOR-based therapy for cardiomyopathy
Congestive heart failure is one of the leading causes of mortality and morbidity in the USA, affecting an increasingly large part of the population. In 2010, 6.6 million American adults suffered from this condition, and the prevalence of congestive heart failure is estimated to increase by 25% through 2030 (Roger, et al. 2012). Left ventricular hypertrophy (LVH) is a common pathological marker of cardiomyopathies and cardiac disease secondary to other conditions such as hypertension, coronary artery disease, hypercholesterolemia, and diabetes (Artham, et al. 2009). Cardiac hypertrophy is the central adaptive mechanism of the adult heart in response to an increased hemodynamic overload, which, in the short term, helps to preserve ventricular function. However, long-term continuous ventricular remodeling becomes pathological and accelerates the onset of congestive heart failure. In this context, the development of new therapeutic approaches to regulate myocardial growth and delay or halt pathological remodeling remains a major goal of current cardiovascular research.
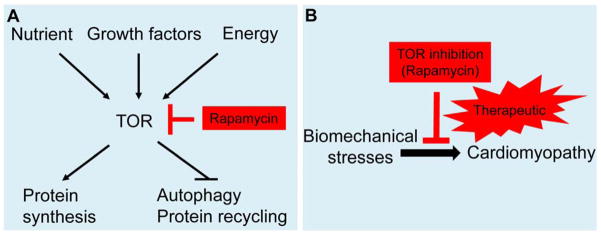
TOR (mammalian target of rapamycin) is a serine/threonine kinase that functions as a sensor to integrate a variety of inputs, including growth factor signaling, nutritional status, or energy stress, and functions to regulate downstream cellular responses (Figure 1A) (Laplante, et al. 2009, Zoncu, et al. 2011). TOR is the catalytic core of two multiprotein complexes, TORC1 and TORC2, which regulate different downstream cellular events. While TORC1 regulates cell growth, proliferation, and survival by sensing mitogen, energy, and nutrient signals, TORC2 is known to regulate the actin cytoskeleton. Rapamycin (Sirolimus) is a macrocyclic lactone produced by Streptomyces hygroscopicus that was first isolated from a soil sample from Easter Island (Rapa Nui). Rapamycin specifically inhibits TORC1, but not TORC2, and because of its powerful anti-proliferative effects, rapamycin has been used clinically to prevent renal/heart transplant rejection, prevent coronary restenosis after stent placement, and develop anti-cancer drugs (Hausleiter, et al. 2004, Kahan 2000, Keogh, et al. 2004, Raichlin, et al. 2007). In addition, TOR is the key kinase that controls autophagy, a cellular degradation process responsible for the turnover of unnecessary or dysfunctional organelles and cytoplasmic proteins (Gustafsson, et al. 2008). TOR signaling has also been suggested to be an important pathway in mammalian aging, as TOR suppression has been shown to prolong the life span of mice (Harrison, et al. 2009). The multiple effects of TOR in many disease states have stimulated active research but have also raised concerns about the specificity of TOR-based therapeutic strategies.
Figure 1. TOR signaling and cardiomyopathy.
A) Schematic of TOR signaling. TOR responds to growth factors via the PI3K-TSC-RheB signaling pathway, to nutrients via RAG GTPase, and to energy via AMPK. TOR regulates downstream cellular events including protein synthesis and autophagy. B) Partial inhibition of TOR signaling via rapamycin attenuates cardiomyopathy, which can be pursued as a new therapeutic strategy.
A beneficial effect of TOR signaling inhibition in the heart was first suggested by pharmacological studies using rapamycin. In a pressure overload mice model of cardiac hypertrophy, Shioi et al. demonstrated that rapamycin administration, prior to the induction of pressure overload, significantly reduced hypertrophic cardiac growth without compromising cardiac function (Shioi, et al. 2003). More important, the same group later reported that rapamycin administration reversed the established hypertrophy (McMullen, et al. 2004). The therapeutic effects of either rapamycin or everolimus (RAD), another TOR inhibitor, were also reported in thyroid hormone-induced cardiomyopathy (Kuzman, et al. 2007), ischemia/reperfusion injury (Khan, et al. 2006), left ventricular remodeling after myocardial infarction (Buss, et al. 2009), and LEOPARD syndrome (Marin, et al. 2011). However, other studies reported that the cardiac-specific knock-out of either Mtor or raptor, a gene that encodes a key protein in the TORC1 pathway, in a pressure overload mouse model accelerated the progression of heart failure (Shende, et al. 2011, Zhang, et al. 2010). A recent mouse transgenic study using both a constitutively active and a kinase dead version of TOR also failed to find a function of TOR in cardiomyopathy (Shen, et al. 2008). Thus, while pharmacological studies using rapamycin have suggested that a TOR-based therapy for cardiomyopathy may be effective (Figure 1B), this concept has not been supported by the aforementioned genetic analyses in rodents.
2. Adult zebrafish as a new model for studying cardiomyopathy
The zebrafish is a new vertebrate model for studying cardiomyopathies. Compared to the widely used mouse model, zebrafish possess advantageous embryology and accessible genetic tools, such as genome-wide mutagenesis screening. A large collection of embryonic lethal mutants have been generated, some of which affect the heart (Stainier, et al. 1996). The molecular and functional characterization of these mutants has uncovered genes that are critical during cardiogenesis, including causative genes for cardiomyopathies, such as titin and tnnt2 (Sehnert, et al. 2002, Xu, et al. 2002). Genetic studies utilizing zebrafish ilk and nexilin mutants led to the discovery of their human orthologues, which were found to be novel causative genes of human cardiomyopathies (Hassel, et al. 2009, Knoll, et al. 2007). These embryonic models of cardiomyopathies are being exploited to elucidate the underlying molecular mechanism and to seek therapeutic interventions (Becker, et al. 2012).
One shortcoming of using the embryonic zebrafish to model cardiomyopathy is the intrinsic difficulty in representing the full spectrum of the pathogenesis of cardiomyopathy, which is mainly a progressive disease in adults. Therefore, the adult zebrafish have been recently explored to model cardiomyopathy. The first cardiomyopathy-like remodeling process was reported in tr265, an anemic mutant fish line originally identified from an ENU-based mutagenesis screen (Sun, et al. 2009). A nonsense mutation in Band 3 in homozygous tr265/tr265 mutants disrupts most of the erythrocytes and results in severe and persistent anemia, which imposes chronic stress on the heart. In response to this high-output stress, the hearts from the surviving adult fish undergo a significant ventricular chamber enlargement that consists of both cardiomyocyte hypertrophy and hyperplasia. Similar to human and rodent models, the hallmarks of pathological cardiomyopathy including muscular disarray, reduced cardiac function, and activated fetal gene expression are observed (Sun, et al. 2009). Another cardiomyopathy model in adult zebrafish has been generated in fish injected with Doxorubucin (DOX) (Ding, et al. 2011). DOX is an FDA-approved anti-tumor drug that has been widely used to treat human patients. However, an overdose of DOX leads to irreversible cardiomyopathy, which often occurs years after DOX treatment. Similar to the rodent, an intraperitoneal injection of a single bolus of DOX into the adult zebrafish leads to a heart remodeling process with hallmarks of cardiomyopathy (Ding, et al. 2011). The heart remodeling process in the DOX model exhibits a different pathology from the anemia model. The anemia model shows increased cardiomyocyte hyperplasia, but the DOX model does not, and there is increased apoptosis in the DOX model, but not the anemia model.
The strengths of using adult zebrafish models include the ease and low cost associated with the generation and handling of a large numbers of mutants. The survival curve analyses can be conducted routinely for multiple lines, which is crucial for assessing the long-term effects of a progressive disease such as cardiomyopathy. Most of the cellular and molecular analyses of cardiomyopathy can be conducted in this model (Sun, et al. 2009). The potential to conduct mutagenesis screens and/or chemical genetic screens may provide new research opportunities in the field of cardiomyopathy that are prohibitive in other animal models. To quantify cardiac function, high-frequency ultrasound has been employed (Sun, et al. 2008), as well as a casper;Tg(cmlc2:DsRed) fish line that highlighted the beating heart via both the reduction of pigmentation and the enhancement of the cardiac signal by a fluorescence reporter (Hoage, et al. 2012). Taking advantage of the transparency of the tail fin of the adult fish, an indirect non-invasive method that measures blood flow velocity has also been developed (Hoage, et al. 2012). Single-channel EKG technology has been utilized to quantify heart rate in adult fish (Milan, et al. 2006). Despite these efforts, the non-invasive cardiac functional assessment of an adult zebrafish heart remains a weakness of this vertebrate model. More sophisticated cardiac physiological techniques are needed, and these methods will be important to discern the different forms of adult heart diseases in an adult zebrafish.
3. Genetic evidence from zebrafish for TOR-based therapy of cardiomyopathy
Both the anemia and the DOX models of cardiomyopathy have been utilized to assess the effects of rapamycin on cardiac remodeling in adult zebrafish. Adult fish have been treated by bathing them in solutions containing rapamycin. Similar to the anti-hypertrophic effects of rapamycin in rodent models, a 1-week treatment program is sufficient to attenuate the increased ventricle size in both cardiomyopathy models (Ding, et al. 2011).
To genetically test the concept of a TOR-based therapy for cardiomyopathy, a ztor mutant generated from an insertional mutagenesis screen was used (Ding, et al. 2011). Similar to homozygous TOR mutants in C. elegans, Drosophila, and mice, the homozygous ztor mutant results in embryonic lethality. However, heterozygous ztor fish with partially reduced TOR activity can survive into adulthood without any obvious phenotypes. A therapeutic effect of TOR signaling inhibition was documented in both the anemia and the DOX models, which manifested as rescued cardiac function and attenuated pathological hallmarks (Ding, et al. 2011). Importantly, the survival rates are increased by TOR signaling inhibition in both models. Thus, studies in adult fish provide the first genetic evidence to support a therapeutic effect of TOR signaling inhibition on cardiomyopathy. Because of the different etiologies of the two models, TOR may be a common signaling pathway for different forms of cardiomyopathy.
The consistent results from the pharmacological studies in zebrafish and rodents underscore the conservation of these vertebrate models in studying the molecular mechanisms of cardiomyopathies. The different conclusions derived from genetic studies in zebrafish compared to rodents might be due to different levels of TOR signal reduction. In contrast to the rather mild reduction of TOR activity observed in the zebrafish ztor heterozygous mutants, the TOR signaling in the mouse conditional knockout models is more severely depleted, and the mice only survive for eight days after the induction of TOR depletion (Zhang, et al. 2010). Because of the crucial role of TOR in regulating cellular metabolism during physiological conditions, severely depleting TOR signaling may be detrimental to the homeostasis of the cardiomyocytes. In contrast, zebrafish studies demonstrated that partially attenuated TOR signaling renders the cardiomyocytes more capable of adapting to the stresses that induce cardiomyopathy. Why the transgenic rodent models with the heart specific kinase-dead TOR domain (TOR-KD) failed to exert the cardiac protective effects is unclear (Shen, et al. 2008). One possibility is that this version of the TOR mutation may not affect the downstream signaling branch responsible for conferring the therapeutic effect of TOR signaling inhibition. Further studies are needed to clarify these issues.
An important finding from the adult fish studies is the dynamic activity of TOR signaling during pathogenesis of the disease in both the anemia and the DOX models. For example, post-DOX injection autophagy is activated at the initial acute phase but is suppressed at later stages when the pathological cardiomyopathy phenotypes are apparent (Ding, et al. 2011). This observation provides a possible explanation for the seemingly opposite effect of TOR signaling on DOX-induced cardiac responses, i.e., the beneficial effect of long-term TOR signaling inhibition on DOX-induced cardiomyopathy in adult zebrafish (Ding, et al. 2011) as compared with the beneficial effect of TOR signaling activation on DOX-induced acute cardiotoxicity in mice (Zhu, et al. 2009). Consistent with the latter report, TOR signaling inhibition also exerts a detrimental effect on DOX-induced acute cardiotoxicity in the zebrafish model (Ding, et al. 2011). Thus, the dynamic TOR signaling activity in the different types and/or stages of cardiomyopathy needs to be further investigated, and this information will be critical for developing TOR-based therapeutic strategies.
4. Current status of TOR-based therapy of cardiomyopathy in humans
Echo studies in animal models and data on the use of TOR inhibitors in the clinical setting are now emerging and suggest that these agents may have a role in the treatment of cardiomyopathy. Most of the clinical evidence on the use of TOR inhibitors in LVH arose from studies in renal transplant patients. LVH is a strong predictor of both all-cause mortality and the de novo onset of congestive heart failure; therefore, minimization of LVH is a key therapeutic goal in these patients (Paoletti, et al. 2010). In a retrospective study, over the course of a year, 83 cardiac transplant recipients treated with a calcineurin inhibitor (CNI) as the primary immunosuppressant were compared with 58 patients for whom the CNI was replaced with rapamycin (sirolimus) because of nephrotoxicity and/or allograft vasculopathy. In the patients treated with rapamycin, the left ventricular mass and left atrial volume indexes significantly decreased, while an increase was observed in the patients who continued treatment with the CNI (Kushwaha, et al. 2008). Notably, the left ventricular mass index reduction was almost completely restricted to the 13 rapamycin-treated patients with LVH at baseline. Moreover, this study also demonstrated that the activity of cardiomyocyte p27Kip1 was significantly increased in rapamycin-treated patients, and this observation supports the notion that LVH reduction was mediated by TOR inhibition of cardiac cell growth. In a study by Paoletti et al., when CNI therapy was replaced with rapamycin, the 1-year (Paoletti, et al. 2008) and 3-year (Paoletti, et al. 2011) LVH regression was significantly reduced in 13 recipients with biopsy-proven chronic allograft injury, whereas no left ventricular mass changes were observed in the 26 controls who continued on the CNI-based immunosuppression. Recent results from this study showed that rapamycin was the strongest predictor of LVM reduction by multivariate regression analysis (Paoletti, et al. 2011). The evolution of LVH in cardiac transplant patients, after switching from a CNI to rapamycin, was also assessed by Raichin et al. in another Mayo Clinic study. This study demonstrated that left ventricular mass was significantly decreased in patients who were converted to the rapamycin treatment, and no changes were observed when the patients were treated with the CNIs (Raichlin, et al. 2007). The LVH regression observed in the studies by both Paoletti et al. and Raichin et al. depended on the interventricular septum thickness reduction without changes in the left ventricle internal size.
In addition to the studies in the renal transplant patients, a case report on the management of a pediatric liver transplant patients (Turska-Kmiec, et al. 2007) provided one of the earliest clinical indications of the potential of TOR inhibition in the reduction of cardiac hypertrophy by showing that the replacement of tacrolimus with rapamycin reversed tacrolimus-induced hypertrophic cardiomyopathy.
Overall, the current clinical data are consistent in demonstrating the therapeutic benefit of TOR signaling inhibition for the reduction of LVH. However, several challenges need to be addressed in the clinical setting. Taking into account the pivotal physiological role of TOR, it is not surprising that the administration of TOR inhibitors has been associated with numerous adverse effects, including hyperlipidemia, defective wound healing, edema, bacterial infections, acne, pneumonitis, and proteinuria (Morrisett, et al. 2002, Zuckermann, et al. 2008). Randomized, controlled clinical trials are necessary to establish the efficacy and safety of the TOR inhibitors in renal transplant patients or other clinical scenarios where TOR inhibition may contribute to the reversal of LVH.
5. Remarks for future studies
Mounting evidence from both animal models and clinical studies suggests that partial TOR signaling inhibition is a viable avenue for therapeutic intervention in cardiomyopathic states. Some of the key areas that require further research are summarized below.
First, rapamycin and related rapalogues should be further assessed as the first-generation candidate drugs for TOR-based therapy. The efficacy and safety of long-term rapamycin treatment on cardiac function and survival need to be established initially in large mammal models and then in human patients. The therapeutic dose range needs to be determined, as suggested by zebrafish studies.
Second, the dynamic activity of TOR signaling in different types of cardiomyopathies and/or in different pathological stages of a particular form of cardiomyopathy need to be carefully defined so that TOR signaling can be modulated properly. At certain stages of cardiomyopathy or for particular cardiomyopathic responses such as inflammation, the activation of TOR signaling, rather than the inhibition of TOR, might be beneficial (Song, et al. 2010). Research in this direction will provide useful guidelines for inhibiting TOR signaling at the proper stage and for the proper duration in different types of cardiomyopathies, which are both crucial for maximizing the therapeutic benefit.
Third, to develop next-generation compounds for TOR-based therapeutics, basic research is needed to identify the downstream signaling branches that confer the therapeutic effects of TOR signaling inhibition. One candidate signaling branch is autophagy, which is a cellular recycling program. Accumulating evidence suggests that autophagy is an effective mechanism that eliminates toxic peptides, which may contribute to the pathogenesis of many cardiomyopathies (Rothermel, et al. 2008). It is anticipated that next-generation therapies, which will be based on the downstream targets of TOR, will eliminate the side effects associated with the pleiotropic consequences of targeting the TOR itself. Studies with powerful genetic tools in lower animal models, such as zebrafish, will facilitate our understanding in this area of research.
In summary, further investigation of the TOR-signaling pathway will contribute to the development of a pathway-based therapeutic approach for a broad spectrum of cardiomyopathies of different etiologies. Close collaboration between basic researchers using a variety of animal models and clinicians will be essential to ultimately bring TOR-based therapy from the bench to the bedside.
Acknowledgments
We apologize to colleagues whose work could not be cited due to the scope and space limitations of the manuscript. This work is supported by RO1HL107304, AHA10GRNT4130009, and Mayo Foundation to Xu, and internal Mayo CR20 grant to Kushwaha.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artham SM, Lavie CJ, Milani RV, et al. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–167. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Becker JR, Robinson TY, Sachidanandan C, et al. In vivo natriuretic peptide reporter assay identifies chemical modifiers of hypertrophic cardiomyopathy signalling. Cardiovasc Res. 2012;93:463–470. doi: 10.1093/cvr/cvr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss SJ, Muenz S, Riffel JH, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Ding Y, Sun X, Huang W, et al. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circulation research. 2011;109:658–669. doi: 10.1161/CIRCRESAHA.111.248260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44:654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel D, Dahme T, Erdmann J, et al. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat Med. 2009;15:1281–1288. doi: 10.1038/nm.2037. [DOI] [PubMed] [Google Scholar]
- Hausleiter J, Kastrati A, Mehilli J, et al. Randomized, double-blind, placebo-controlled trial of oral sirolimus for restenosis prevention in patients with in-stent restenosis: the Oral Sirolimus to Inhibit Recurrent In-stent Stenosis (OSIRIS) trial. Circulation. 2004;110:790–795. doi: 10.1161/01.CIR.0000138935.17503.35. [DOI] [PubMed] [Google Scholar]
- Hoage T, Ding Y, Xu X. Quantifying cardiac functions in embryonic and adult zebrafish. Methods Mol Biol. 2012;843:11–20. doi: 10.1007/978-1-61779-523-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–2700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- Khan S, Salloum F, Das A, et al. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Knoll R, Postel R, Wang J, et al. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116:515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- Kushwaha SS, Raichlin E, Sheinin Y, et al. Sirolimus affects cardiomyocytes to reduce left ventricular mass in heart transplant recipients. Eur Heart J. 2008;29:2742–2750. doi: 10.1093/eurheartj/ehn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzman JA, O’Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology. 2007;148:3477–3484. doi: 10.1210/en.2007-0099. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of cell science. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin TM, Keith K, Davies B, et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011;121:1026–1043. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen JR, Sherwood MC, Tarnavski O, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol. 2006;291:H269–273. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- Morrisett JD, Abdel-Fattah G, Hoogeveen R, et al. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- Paoletti E, Amidone M, Cassottana P, et al. Effect of sirolimus on left ventricular hypertrophy in kidney transplant recipients: a 1-year nonrandomized controlled trial. Am J Kidney Dis. 2008;52:324–330. doi: 10.1053/j.ajkd.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Paoletti E, Cannella G. Regression of left ventricular hypertrophy in kidney transplant recipients: the potential role for inhibition of mammalian target of rapamycin. Transplant Proc. 2010;42:S41–43. doi: 10.1016/j.transproceed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Paoletti E, Ratto E, Bellino D, et al. Effect of early conversion from CNI to sirolimus on outcomes in kidney transplant recipients with allograft dysfunction. J Nephrol. 2011:0. doi: 10.5301/jn.5000044. [DOI] [PubMed] [Google Scholar]
- Raichlin E, Bae JH, Khalpey Z, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, et al. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Shen WH, Chen Z, Shi S, et al. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mTOR) mutant impairs the mTOR-mediated signaling and cardiac function. J Biol Chem. 2008;283:13842–13849. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende P, Plaisance I, Morandi C, et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- Shioi T, McMullen JR, Tarnavski O, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- Song X, Kusakari Y, Xiao CY, et al. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen JN, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Sun L, Lien CL, Xu X, Shung KK. In vivo cardiac imaging of adult zebrafish using high frequency ultrasound (45–75 MHz) Ultrasound Med Biol. 2008;34:31–39. doi: 10.1016/j.ultrasmedbio.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Hoage T, Bai P, et al. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One. 2009;4:e6596. doi: 10.1371/journal.pone.0006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turska-Kmiec A, Jankowska I, Pawlowska J, et al. Reversal of tacrolimus-related hypertrophic cardiomyopathy after conversion to rapamycin in a pediatric liver transplant recipient. Pediatr Transplant. 2007;11:319–323. doi: 10.1111/j.1399-3046.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Meiler SE, Zhong TP, et al. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet. 2002;30:205–209. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Soonpaa MH, Chen H, et al. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119:99–106. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann A, Manito N, Epailly E, et al. Multidisciplinary insights on clinical guidance for the use of proliferation signal inhibitors in heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27:141–149. doi: 10.1016/j.healun.2007.08.014. [DOI] [PubMed] [Google Scholar]