Abstract
Objective
This study was performed to evaluate the radiological features of and therapeutic responses to pulmonary disease caused by nontuberculous mycobacteria (NTM) in the setting of biological therapy for rheumatoid arthritis (RA).
Methods
We conducted a retrospective chart review of 13 patients from multiple centers who had developed pulmonary NTM disease during biological therapy for RA, including infliximab, etanercept, adalimumab, and tocilizumab.
Results
Most cases were asymptomatic or resulted in only common-cold-like symptoms. Abnormalities in computed tomography (CT) imaging were protean and frequently overlapped. The most predominant pattern was nodular/bronchiectatic disease (six cases), followed by alveolar infiltrate (three cases), cavitary disease (two cases), and pulmonary nodules (two cases). In most cases, pulmonary NTM disease had spread from a preexisting lesion; in particular, bronchial/bronchiolar abnormalities. In three cases, one or more nodular lesions with or without calcification were a focus of disease. Following the discontinuation of biological agents, most patients responded to anti-NTM therapy. Two patients showed no exacerbation in the absence of any anti-NTM therapy. In one patient, restarting tocilizumab therapy while continuing to receive adequate anti-NTM therapy produced a favorable outcome. In two other patients with a previous history of pulmonary NTM disease, introducing biological therapy led to recurrence, but anti-NTM therapy was effective in these patients.
Conclusion
CT abnormalities of pulmonary NTM disease in RA patients receiving biological therapy were variable, but were not unique to this clinical setting. NTM disease can spread from preexisting structural abnormalities, even if they are minute. Contrary to our expectations, the therapeutic outcomes of pulmonary NTM disease were favorable in these patients.
Keywords: Computed tomography, Nontuberculous mycobacteria, Pulmonary disease, Rheumatoid arthritis, Biological therapy
Introduction
Nontuberculous mycobacteria (NTM) are typically opportunistic pathogens. Factors that may contribute to host susceptibility to pulmonary NTM disease include immunosuppressive conditions and pulmonary disease [1–3]. Anti-tumor necrosis factor-α (TNFα) therapy appears to be an important predisposing factor for NTM disease. A recent survey of practicing infectious disease physicians in the United States has suggested that NTM diseases are more common than tuberculosis (TB) in patients undergoing anti-TNFα therapy [4]; in that survey, the majority of patients undergoing anti-TNFα therapy had rheumatoid arthritis (RA). Unlike TB infection, there is no evidence of a latent phase in NTM infection; therefore, screening for and preventive treatments against NTM infection in patients scheduled to begin anti-TNFα therapy might not be feasible [5]. Thus, the timely diagnosis of pulmonary NTM disease is critical during biological therapy for RA. Diagnosis relies upon careful monitoring of patients’ chest radiography and computed tomography (CT) scans, together with mycobacterial cultures of respiratory specimens [6]. At present, our experience with the diagnosis and management of pulmonary NTM disease in this clinical setting is still scanty, and the evidence-based data that might guide therapy for pulmonary NTM disease are insufficient [7].
To evaluate the clinical, microbiological, and radiological features of pulmonary NTM disease, as well as its responses to anti-NTM therapy in the clinical setting of biological therapy for RA, we conducted a retrospective multicenter study in Japan. The subjects were RA patients receiving currently approved anti-TNFα agents including infliximab, etanercept, adalimumab, and the anti-interleukin-6 (IL-6) receptor antibody tocilizumab. To explore a possible association between susceptibility to NTM infection and preexisting abnormal lesions in lungs, we compared CT findings prior to therapy with those obtained at the onset of NTM disease.
Patients and methods
Patients
In May 2010, we invited several expert rheumatologists and pulmonologists to participate in a retrospective multicenter study of pulmonary NTM disease occurring in RA patients being treated with biological agents. A total of 25 cases were collected from 20 institutes in Japan. We defined pulmonary NTM disease according to both the 2008 diagnostic criteria of pulmonary NTM disease proposed by the Japanese Society for Tuberculosis (JST) and the Japanese Respiratory Society (JRS) and the 2007 diagnostic criteria for NTM lung disease proposed by the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA): namely, patients must have positive culture results from two or more sputum samples obtained separately (or at least one isolation of NTM in the case of bronchoscopy specimens) plus one or more of the following radiological findings: nodular opacities, dissemination of small nodular or branching opacities, homogeneous opacities, cavitary opacities, and bronchiectasis or bronchiolectasis [6, 8]. Patients who were considered by consensus of all experts participating in this study to meet these criteria were given a definitive diagnosis of pulmonary NTM disease. Three of these cases had been reported elsewhere as case reports [9–11].
Data collection
By reviewing the patients’ medical records, we collected demographic characteristics, body mass index (BMI), and RA data including stage, disease duration, disease activity, and past and current treatment regimens for RA, together with data on the presence or absence of pulmonary disease. We also extracted data on respiratory symptoms at the onset of pulmonary NTM disease, etiologic organisms, anti-NTM treatment, and outcome.
Evaluation of chest CT findings
CT images were reviewed and scored independently by two board-certified radiologists (FS and TJ), both of whom have a great deal of experience in reading chest CT scans. In the event of disagreement, final decisions were made by consensus. CT abnormalities included the following findings: (a) nodules including centrilobular small nodules (5 mm or less in diameter), subpleural small nodules (5 mm or less in diameter), and large nodules (greater than 5 mm in diameter); (b) ground-glass attenuation; (c) airspace consolidation; (d) tree-in-bud sign (centrilobular small nodules connected to branching linear opacities); (e) bronchiectasis; (f) bronchiolectasis; and (g) cavities. In each case, the most likely pattern was identified based on predominant CT findings and their distribution.
Preexisting CT abnormalities in the areas from which NTM infection later spread were identified by comparing CT scans obtained prior to the introduction of biological therapy with those obtained at the time of development of pulmonary NTM disease.
Results
Clinical and demographic characteristics of patients who developed pulmonary NTM disease during biological therapy for RA
Of the 25 RA patients enrolled in this study, 13 met the 2008 JST/JRS criteria for diagnosis of pulmonary NTM disease. Table 1 shows the clinical and demographic characteristics of these patients as well as their data on lung disease. The majority of pulmonary NTM diseases occurred in elderly women, and six out of the 13 patients had a low BMI. All patients except case 9 had long-standing and advanced-stage RA. These patients had been treated with at least one biological agent (five patients with infliximab, seven with etanercept, two with adalimumab, and four with tocilizumab) prior to the onset of pulmonary NTM disease. The median interval between the introduction of biological therapy and the development of NTM disease was 10 months (range 6 weeks–8.5 years). Most patients developed pulmonary NTM disease within 12 months. Eleven patients had been receiving low-dose prednisolone at the time of development of NTM disease, and one patient was a former user of this medication. Lung diseases of the following types were diagnosed through reviewing medical records including CT scans taken prior to the introduction of biological therapy: bronchiectasis (five patients) and interstitial lung disease (four patients) were observed. Five patients had no pulmonary disease, though they had abnormal CT findings of which the distribution and extent were insufficient to warrant a diagnosis of pulmonary disease. Two patients had a history of pulmonary NTM infection that was proven by the patients’ radiological findings and mycobacterial cultures of respiratory specimens; both patients had received anti-NTM treatment at the time of the initial infection. Biological therapy was introduced more than 2 years after the first of a series of negative mycobacterial cultures.
Table 1.
Clinical and demographic characteristics of patients who developed pulmonary NTM disease during biological therapy for RA
| Case no. | Age (years)/sex | BMI | RA duration (years) | RA activity | Stage | Treatment for RA (duration) a | Lung disease |
|---|---|---|---|---|---|---|---|
| 1 | 70/F | 21.2 | 21 | Low | IV | TCZ (4 m), IFX (6 m), MTX (3 years), PSL (18 m) | IP, BE |
| 2 | 62/F | 16.7 | 26 | >Moderate | III | ADM (10 m), PSL (8 years), TAC (2.5 years), SASP (8 years) | IP |
| 3 | 71/F | 21.1 | 19 | Remission | II | TCZ (8.5 years), PSL (>10 years) | None |
| 4 | 72/F | 16.4 | 14 | >Moderate | IV | ETN (11 m), PSL (9 years), SASP (11 years) | BE |
| 5 | 66/F | 21.1 | 16 | >Moderate | III | ETN (31 m), PSL (5 years), MTX (3 years), BUC (1 year), SASP (1 year) | None |
| 6 | 66/F | 21.5 | 24 | >Moderate | IV | IFX (6 w), PSL (10 m), MTX (18 m) | None |
| 7 | 62/F | 21.5 | 22 | Remission | IV | ETN (23 m), IFX (5 m), PSL (>10 years), SASP (>10 years) | IP |
| 8 | 81/F | 16.1 | 8 | Low | IV | ETN (10 m) | BE |
| 9 | 68/F | 23.7 | 2 | ND | II | ETN (11 m), PSL (22 m), MTX (1 m), SASP (2 m) | IP, BE |
| 10 | 58/F | 19.4 | 25 | >Moderate | IV | ADM (3 m), IFX (30 m), PSL, MTX, SASP | None |
| 11 | 63/F | 20.8 | 17 | Low | IV | ETN (2 m), PSL (9 years), MTX (4 years), SASP | None |
| 12 | 65/F | 15.2 | 30 | >Moderate | III | TCZ (3 m), PSL (14 years), BUC (7 years) | NTMb |
| 13 | 78/M | 17.2 | 10 | Low | III | TCZ (3 m), IFX (9 m), ETN (6 m), PSL (7 years), MTX (7 years), TAC (16 m), BUC (20 m), SASP (4 years), LEF (2 m) | BE, NTMb |
RA rheumatoid arthritis, NTM nontuberculous mycobacteria, BMI body mass index, TCZ tocilizumab, IFX infliximab, ADM adalimumab, ETN etanercept, MTX methotrexate, SASP salazosulfapyridine, BUC bucillamine, TAC tacrolimus, LEF leflunomide, PSL prednisolone, IP interstitial pneumonia, BE bronchiectasis, ND no data, m months, w weeks
aUnderlines indicate anti-RA drugs that were being used at the time of development of NTM disease, and the duration of therapy with these drugs represents the interval between the development of NTM disease and the introduction of the anti-RA drug(s) being used at that time. The drugs without underlines were no longer used at the time of development of NTM disease, and the duration for these agents represents the duration of their previous use
bThese patients had received appropriate anti-NTM therapy for the previous occurrences of NTM disease
Clinical and microbiological characteristics of pulmonary NTM disease occurring during biological therapy for RA
Clinical symptoms at the onset of pulmonary NTM disease and NTM species isolated from cultures are shown in Table 2. Five patients had no respiratory symptoms when NTM disease was first suspected through chest imaging as part of a regular checkup during biological therapy for RA. Seven patients complained of common-cold-like symptoms such as cough, sputum production, and fever. Most cases were caused by Mycobacterium avium-intracellulare complex (MAC), but M. abscessus, a rapidly-growing NTM, was isolated from one patient. No patients had extrapulmonary or disseminated NTM disease.
Table 2.
Clinical, microbiological, and radiological characteristics of pulmonary NTM disease occurring in patients receiving biological therapy for RA
| Case no. | Clinical symptoms | NTM species isolated | Preexisting CT abnormalitiesa | CT patterns |
|---|---|---|---|---|
| 1 | Asymptomatic | M. intracellulare | Bronchiolitis + bronchiectasis | Nodular/bronchiectatic form |
| 2 | Asymptomatic | M. avium | Bronchiolitis | Pulmonary nodules |
| 3 | Common-cold-like | M. avium | NA | Cavitary form |
| 4 | Sputum production | M. abscessus | Bronchiolitis + bronchiectasis | Nodular/bronchiectatic form |
| 5 | Cough | M. avium | Bronchiolitis | Nodular/bronchiectatic form |
| 6 | Asymptomatic | M. avium | Bronchiolitis | Pulmonary nodules |
| 7 | Asymptomatic | M. avium | Nodule | Cavitary form |
| 8 | Cough, hemoptysis | M.intracellulare | Bronchiolitis + bronchiectasis | Nodular/bronchiectatic form |
| 9 | Cough | M. avium | Bronchiolitis + bronchiectasis | Nodular/bronchiectatic form |
| 10 | Fever | M. avium | Calcified nodule | Alveolar infiltrate |
| 11 | Asymptomatic | M. avium | Bronchiolitis + calcified nodule | Alveolar infiltrate |
| 12 | Cough, sputum production | M. avium | Bronchiolitis | Alveolar infiltrate |
| 13 | Cough, sputum production | M. intracellulare | Bronchiolitis + bronchiectasis | Nodular/bronchiectatic form |
NTM nontuberculous mycobacteria, RA rheumatoid arthritis, CT computed tomography, NA not available
aPreexisting CT abnormalities represent those in the area from which NTM disease had spread
CT abnormalities of pulmonary NTM disease occurring during biological therapy for RA
Abnormalities in CT images were variable and frequently overlapped. Pulmonary involvement could be found in any lobe or any segment, although the upper lobes, the right middle lobe, and the lingula were most frequently affected. Centrilobular small nodules were observed in all patients and tree-in-bud opacities appeared in most patients (92%). In addition, consolidation was seen in all patients. Diffuse ground-glass attenuation was fairly common (85%). Bronchiectasis and bronchiolectasis were also noted (77 and 54%, respectively). Large nodules were observed in 77% of patients. Cavities were less common (31%).
In the following sections, we present chest CT scans of case 2 (Fig. 1), case 7 (Fig. 2), case 8 (Fig. 3), case 10 (Fig. 4), case 11 (Fig. 5), and case 13 (Fig. 6). The order of the panels in each Figure reflects the course of events in the patient.
Fig. 1.
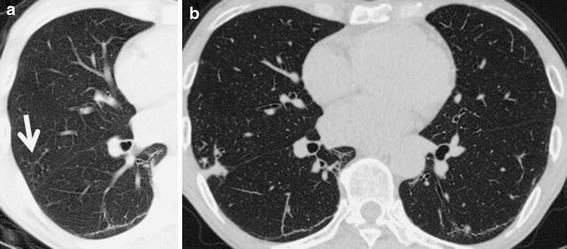
a A computed tomography (CT) scan of case 2 before the development of nontuberculous mycobacteria (NTM) disease. Centrilobular small nodules and tree-in-bud sign are seen in the right middle lobe (S4, arrow). b After 12 months of adalimumab therapy, nodules and consolidation have appeared in the same segment (S4)
Fig. 2.
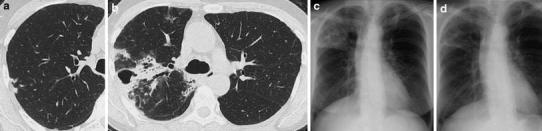
a A CT scan of case 7 before the development of pulmonary NTM disease. Several nodules were observed in the right upper lobe (S2). b, c After 28 months of anti-tumor necrosis factor-α (TNFα) therapy (etanercept for 23 months and infliximab for 5 months), a thick-walled cavity has appeared in the right upper lobe (S1). Bronchiolectasis and tree-in-bud opacities are observed in the same segment (S1). Consolidation is also prominent in this lobe (S1 and S2). In addition, diffuse ground-glass opacities have appeared in both lungs, especially in the right upper lobe. These findings may represent additional foci of infection. d A chest radiograph after 5 months of anti-NTM treatment shows a good response
Fig. 3.
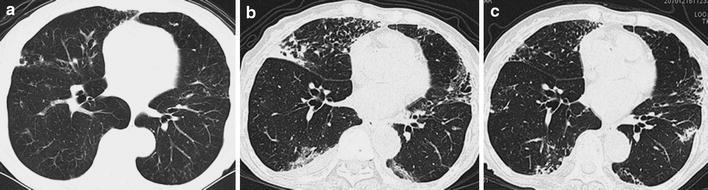
a A CT scan of case 8 before the development of NTM disease. Centrilobular small nodules, tree-in-bud sign, and bronchiectasis are seen in the right middle lobe and the lingula. A thick-walled cavity is present in the right middle lobe (S5). b After 10 months of etanercept therapy, bilateral centrilobular small nodules, tree-in-bud structures, and multifocal bronchiectasis are prominent in the right middle and lower lobes and the lingula. Ground-glass opacities and mild consolidation are seen in the whole right lung. The cavitary lesions are increased in size. c CT imaging taken after 18 months of anti-NTM therapy shows disappearance of consolidation
Fig. 4.
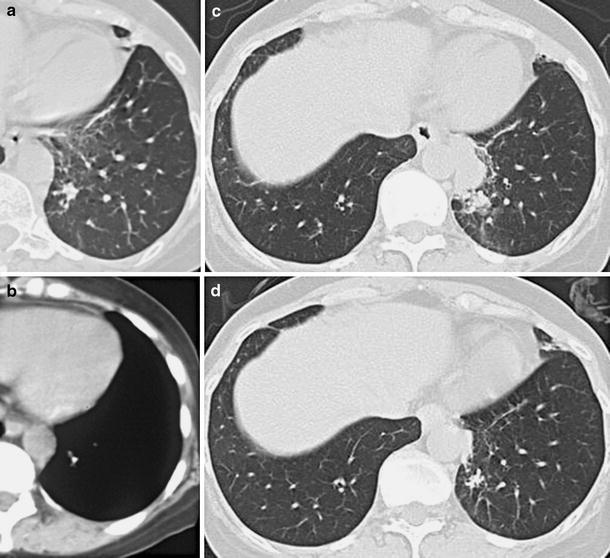
a, b CT scans of case 10 before the development of pulmonary NTM disease (the soft tissue window and the mediastinal window, respectively). A calcified large nodule is observed in the left lower lobe (S10). c After infliximab therapy (30 months) followed by adalimumab therapy (3 months), consolidation and patchy ground-glass infiltrates have appeared in the left lower lobe (S10). d CT imaging taken after 14 months of anti-NTM therapy shows complete resolution
Fig. 5.
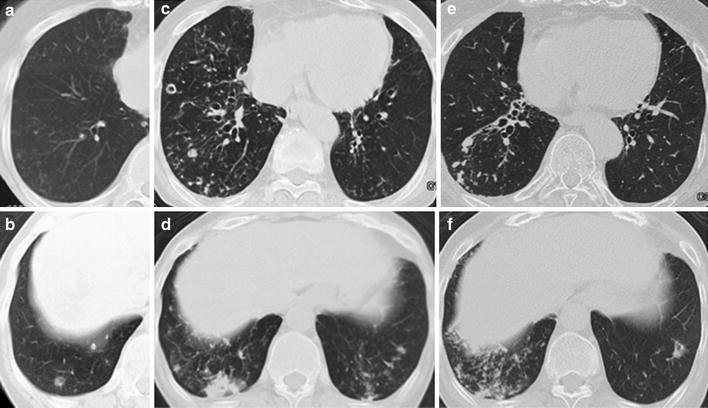
a, b CT scans of case 11 before the development of pulmonary NTM disease. Ground-glass opacities, centrilobular small nodules, and tree-in-bud sign are observed in the right middle lobe. In the right lower lobe, centrilobular small nodules and a calcified nodule are noted (S8). c, d After 2 months of etanercept therapy, diffuse ground-glass attenuation and centrilobular small nodules have appeared in the right middle and lower lobes, the lingula, and the left lower lobe. Consolidation is expanded in the right lower lobe. Thick-walled cavities have appeared in the right lower lobe (S8 and S9). e, f CT imaging after 2 years of anti-NTM treatment shows a satisfactory response
Fig. 6.
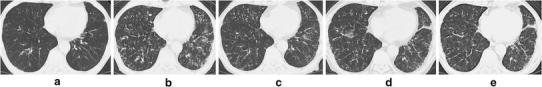
a CT imaging of case 13 before the introduction of anti-TNFα therapy. Bronchiectasis is prominent. b After 15 months of anti-TNFα therapy (infliximab for 9 months and etanercept for 6 months), centrilobular small nodules, tree-in-bud sign, bronchiectasis/bronchiolectasis, and ground-glass opacities have developed in both lungs. c A CT scan taken after 10 months of anti-NTM therapy. d Abnormal findings indicative of the nodular/bronchiectatic form have appeared after tocilizumab was introduced for 3 months. e A CT scan taken after 7 months of anti-NTM therapy. Architectural distortion was not evident at any point in the clinical course
Preexisting CT abnormalities in the areas from which NTM infection later spread
CT scans taken prior to the introduction of biological therapy were available in 12 patients; these scans were compared with those obtained at the time of development of pulmonary NTM disease. As shown in Table 2, we found that bronchiolar abnormalities, including centrilobular small nodules and branching centrilobular nodules, had frequently preexisted in the areas from which NTM infection later spread (10 cases out of 12) (Figs. 1a, 3a, 5a, b, 6a). Among these patients with preexisting CT abnormalities, five patients also had preexisting bronchiectasis in the corresponding areas (Figs. 3a, 6a). In case 7, NTM disease appeared to spread from two nodular lesions (Fig. 2a). In cases 10 and 11, pulmonary NTM infection spread from a calcified nodule (Figs. 4a, b, 5b). Taken together, the data indicate that local invasion of NTM started from preexisting lesions; in particular, from bronchial and bronchiolar abnormalities.
CT patterns of pulmonary NTM disease occurring during biological therapy for RA
According to their most predominant CT abnormalities, patients were grouped into the following four prototypic patterns: nodular/bronchiectatic disease (six patients), alveolar infiltrate (three patients), cavitary form (two patients), and pulmonary nodules (two patients). The most common CT pattern was nodular/bronchiectatic disease, in which diffuse centrilobular small nodules, tree-in-bud opacities, and multifocal bronchiolectasis/bronchiectasis were prominent (Figs. 3b, 6b, d). These abnormalities represent the presence of bronchiolitis. Focal consolidation and ground-glass opacities were occasionally seen in these patients. In patients with the alveolar infiltrate pattern, patchy or diffuse ground-glass infiltrates, ill-defined centrilobular small nodules, and areas of consolidation were characteristic CT findings (Figs. 4c, 5c, d). These abnormalities indicated an alveolar-interstitial process. Cavitary structures included thick walls and were often associated with focal consolidation, bronchiectasis/bronchiolectasis, and adjacent clusters of centrilobular small nodules and tree-in-bud opacities indicative of the endobronchial spread of infection (Fig. 2b, c). CT findings of patients with the pulmonary nodule pattern showed irregularly marginated nodules characterized by lobulation (Fig. 1b). Increased attenuation was seen in some nodules, which may suggest a granulomatous cause. These patterns were not mutually exclusive; several different types of abnormal findings could be seen in individual patients.
Treatments and outcomes of patients who developed pulmonary NTM disease during biological therapy for RA
All patients except one (case 11) stopped biological agents when pulmonary NTM disease was suspected (Table 3). Most patients received clarithromycin with or without rifampicin, ethambutol, and streptomycin. For one patient, azithromycin was introduced instead of clarithromycin (case 9). For some patients, a new quinolone (levofloxacin or moxifloxacin) was used concomitantly (cases 11–13). The clinical and radiological outcomes of these patients were satisfactory for the most part (Figs. 2d, 3c, 4d); sputum conversion was observed within a median duration of 6 months. Two patients were followed without any anti-NTM therapy (cases 2 and 4), and their general condition and radiological abnormalities were not exacerbated. In case 11, etanercept was continued for 13 months without anti-NTM therapy, leading to an exacerbation of pulmonary NTM disease. Etanercept was then discontinued and a combination anti-NTM therapy was started; 2 years later, the patient’s CT findings were improved (Fig. 5e, f). In case 3, tocilizumab therapy for RA was restarted after a 5-month discontinuation, because negative culture results of sputum specimens were repeatedly obtained following a combined anti-NTM therapy. No patients had experienced a relapse of pulmonary disease during their follow-up period.
Table 3.
Treatment regimens and outcomes of patients who developed pulmonary NTM disease during biological therapy for RA
| Case no. | Treatment of NTM disease (duration)a | RA therapy after NTM development | Cultures | Radiology | RA control |
|---|---|---|---|---|---|
| 1 | CAM, RFP, EB (16 m) | MTX, BUC | Negative | Improved | Well |
| 2 | None (19 m) | TAC, PSL | No change | No change | Well |
| 3 | CAM, RFP, EB (13 m) → none (15 m) | PSL (5 m) → TCZ, PSL | Negative | Improved | Exacerbated → well |
| 4 | None (3 years) | SASP, PSL | No change | No change | Exacerbated |
| 5 | CAM, RFP, EB, SM (20 m) | None | Negative | Improved | ND |
| 6 | CAM (12 m) → none (6 years) | MTX, BUC | Negative | Improved | Exacerbated |
| 7 | CAM, RFP, EB, SM (5 m) | MTX, PSL | Negative | Improved | Exacerbated |
| 8 | CAM, RFP, EB (18 m) | None | Negative | Improved | Well |
| 9 | CAM, RFP, EB (3 m) → AZM (3 m) | PSL | Negative | Improved | Well |
| 10 | CAM, RFP, EB (1 m) → CAM, EB (14 m) | MTX, SASP, PSL | Negative | Improved | Exacerbated |
| 11 | None (14 m) → CAM, RFP, EB, LVFX (2 years) | ETN, MTX, SASP, PSL (13 m) → MTX, SASP, PSL | Negative | Improved | Well → exacerbated |
| 12 | RFP, EB, MFLX (22 m) | BUC, PSL | Negative | Improved | Exacerbated |
| 13 | CAM, EB, LVFX (20 m) | MTX, TAC, PSL | Negative | Improved | Well |
NTM nontuberculous mycobacteria, RA rheumatoid arthritis, CAM clarithromycin, AZM azithromycin, RFP rifampicin, RBT rifabutin, EB ethambutol, SM streptomycin, MFLX moxifloxacin, LVFX levofloxacin, MTX methotrexate, BUC bucillamine, SASP salazosulfapyridine, TAC tacrolimus, TCZ tocilizumab, ETN etanercept, PSL prednisolone, ND no data, m months
aIn cases 3 and 6, anti-NTM therapy was completed; cases 2 and 4 had never received such therapy; and the other patients were still receiving anti-NTM agents at the time of enrollment in this study. Side effects of anti-NTM agents were observed in case 9 (hepatotoxicity) and case 10 (eruption)
Use of biological agents for RA patients who had a history of pulmonary NTM disease
Two patients had been given a diagnosis of pulmonary NTM disease before the introduction of tocilizumab therapy for RA (cases 12 and 13). In case 12, pulmonary NTM disease caused by M. avium had developed 4 years previously, when the patient had been receiving low-dose prednisolone and bucillamine for her RA. At that time, she had received rifampicin and levofloxacin as a combined anti-NTM therapy. Subsequent to this therapy, mycobacterial cultures of sputum specimens had tested negative, but abnormal CT findings remained. Two years after the first sputum conversion to negative culture results, the patient had started tocilizumab therapy due to exacerbation of her RA. During 3 months of tocilizumab therapy, the patient’s chest radiograph abnormalities and clinical symptoms were rapidly exacerbated. Tocilizumab was discontinued and anti-NTM therapy consisting of rifampicin, ethambutol, and moxifloxacin was introduced. Her chest radiographs were gradually improved.
The other patient (case 13) had developed pulmonary NTM disease caused by M. avium during anti-RA therapy with low-dose prednisolone, bucillamine, and salazosulfapyridine. Anti-NTM therapy with clarithromycin, ethambutol, and isoniazid had led to a favorable outcome (Fig. 6a). To control RA activity, the patient had received etanercept therapy for 6 months, followed by 9 months of infliximab therapy; at that point he developed pulmonary NTM disease caused by M. intracellulare (Fig. 6b). Combination therapy consisting of clarithromycin, ethambutol, and levofloxacin was started, and the patient’s CT findings improved (Fig. 6c). Two years after confirmation of negative culture results by repeated examinations, tocilizumab therapy was introduced. Three months later, M. intracellulare was again isolated from the patient’s sputum specimens, and pulmonary symptoms appeared (Fig. 6d). The previously used regimen for NTM disease was restarted. Clinical symptoms and radiological findings were improved and negative results of mycobacterial cultures were continuously obtained (Fig. 6e).
Discussion
In most cases in the present study, pulmonary NTM disease seems to have spread from a preexisting lesion, such as bronchial/bronchiolar lesions or nodular lesions. It is not clear whether these preexisting abnormalities may reflect the subclinical presence of pulmonary NTM infection (colonization). In the case of colonization, these pulmonary lesions may slowly progress to true NTM disease over time. The use of biological agents may have promoted this process into a more aggressive course. Another possible explanation is that the disruption of local host defense may play a central role in disease predisposition. Middleton et al. [12] have reported that, unlike M. tuberculosis,M. avium complex tends to adhere to damaged respiratory mucosa through a fibronectin-mediated process. Recently, we have shown that bronchiolar abnormalities are commonly seen in RA patients, especially those with long-standing RA [13, 14]. In addition, bronchiectasis was the most frequent finding in both patients with early RA and those with long-standing RA [14]. Such modifications of the structural and functional features of bronchi/bronchioles in RA may provide a favorable environment for infection and colonization of NTM organisms. In either case, rheumatologists should closely follow preexisting CT abnormalities in RA patients, even though their distribution and extent may be insufficient to warrant a diagnosis of pulmonary disease. Subtle changes in these lesions may be suggestive of the development of NTM disease.
In the present study, CT abnormalities of pulmonary NTM disease were protean and frequently overlapped. Jeong et al. [15] have shown that, regardless of the NTM species isolated, the most common CT findings of pulmonary NTM disease in immunocompetent patients are bilateral small nodules, cylindrical bronchiectasis, and branching centrilobular nodules; these CT findings reflected histological abnormalities such as bronchiolectasis and bronchiolar/peribronchiolar inflammation with or without granuloma formation. Further, Koh et al. [16] have reported that about one-third of patients with bilateral bronchiectasis and bronchiolitis on CT scans have pulmonary NTM infection; coexistence of these abnormalities involving more than five lobes, especially when associated with consolidation or cavitation, is highly suggestive of pulmonary NTM disease. Utilizing multiple logistic regression analysis, Chung et al. [17] have also shown that extensive bronchiectasis is one of the predictive factors associated with pulmonary NTM disease. Although their data were obtained from immunocompetent patients, they are compatible with the CT findings of our patients. The cavitary form (the so-called classic form) and the nodular/bronchiectatic form (the so-called non-classic form or Lady Windermere syndrome) are considered to be responsible for most NTM disease in immunocompetent individuals [1, 18], and this was certainly the case in the present study. In addition, two other forms (alveolar infiltrate and pulmonary nodule) were observed in 23 and 15% of our study patients, respectively. The true incidence of these forms of NTM disease is still unknown [18].
Takayanagi et al. [19] reported that pulmonary NTM disease was the most frequent pulmonary infection in RA patients. Among these patients, only 2% received anti-TNFα therapy, while most patients had received prednisolone (48%) or nonbiological disease-modifying antirheumatic drugs (DMARDs; 49%). Approximately 80% of patients had preexisting lung disease such as bronchiectasis, emphysema, and old tuberculosis. Most cases (85%) were caused by MAC. Through a literature search, we found five cases of pulmonary NTM occurring in Japanese RA patients who had never received biological agents: four were receiving prednisolone and/or nonbiological DMARDs and one was not receiving such medications [20–23]. Three patients were asymptomatic at the time of the development of NTM disease. Of these five cases, three were radiologically grouped into the cavitary form, one into the nodular/bronchiectatic form, and one was of the mixed type. One case caused by M. abscessus was fatal; this patient had pulmonary fibrosis, emphysema, and old tuberculosis. The others responded well to a combined anti-NTM therapy: sputum cultures repeatedly gave negative results and radiological improvement was observed. There seem to be no remarkable differences in the clinical features of NTM disease between patients receiving biological therapy and those without. Regardless of the use of biological agents, the NTM species involved and the patient’s general condition and pulmonary comorbidity may influence disease outcomes.
In Western countries, disseminated NTM diseases seem to be the common form in patients receiving biological agents for RA and other rheumatic diseases [24–28], but such cases were not observed in the present study. By reviewing the MedWatch database for NTM reports, Winthrop et al. [29] have shown that 9% of patients using anti-TNFα agents had died by the time their case was reported. In addition, we have found in the literature four cases of fatal pulmonary NTM infections that occurred during anti-TNFα therapy for rheumatic diseases [30–33]. Until now, however, no fatal case of NTM disease has been reported in the Japanese postmarketing surveillance of biological therapy for RA [34–37]. In the present study, therapeutic responses to anti-NTM therapy were also favorable. These satisfactory outcomes in Japan may be due to the strict obedience to doctors’ instructions exhibited by patients when taking anti-NTM agents.
Therapeutic decisions in cases of pulmonary NTM disease during biological therapy depend on individual physicians’ experience and data from case reports [2, 7]. In the present study, anti-NTM therapy was withheld for three patients because their symptoms were subclinical or mild and their disease was not the cavitary type. Two of these patients stopped anti-TNFα agents after the development of NTM disease, and since then, their CT abnormalities have not worsened. The other one continued etanercept therapy, and 1 year later, rapid radiological progression was observed. In one patient, tocilizumab therapy was restarted after a response to anti-NTM therapy became evident; and there were no relapses of pulmonary disease for 2 years. These data support the idea that patients with pulmonary NTM disease should discontinue biological therapy until they have completed anti-NTM therapy, or at least until a response to anti-NTM therapy is confirmed. If the decision is made to resume or continue biological therapy due to concerns about RA flares, anti-NTM therapy must be used concomitantly. Patients’ informed consent is mandatory. If the decision is made to observe patients without any anti-NTM therapy, biological agents should be discontinued.
There are no guidelines regarding the safety of introducing biological therapy in patients who have a history of pulmonary NTM disease. Two patients in the present study were treated with tocilizumab despite their history of pulmonary NTM disease. By the time they started tocilizumab therapy, both patients had achieved continued negative sputum culture results for 2 years. After a 3-month interval, however, their respiratory symptoms and radiological findings were rapidly exacerbated. The JST, the ATS/IDSA, and the British Thoracic Society have recommended that the optimal duration of anti-NTM treatment for pulmonary MAC diseases lasts until negative sputum cultures have been maintained for 12–24 months [6, 7, 38–40]. However, our data indicate that in the patients scheduled to receive biological agents for RA, the further continuation of anti-NTM therapy is required during biological therapy. In fact, case 3 successfully restarted tocilizumab therapy while also being treated for pulmonary NTM disease. Patients’ full compliance and physicians’ strict supervision are both essential.
In conclusion, pulmonary NTM disease seems to spread from preexisting structural abnormalities such as bronchial/bronchiolar lesions or nodular lesions. There were no particular radiological features that could characterize the biological agent-associated pulmonary NTM disease. Therapeutic responses to anti-NTM therapy were favorable in this clinical setting. Close follow-up of preexisting CT abnormalities can lead to early detection and the timely implementation of therapeutic strategies for pulmonary NTM disease in RA patients receiving biological therapy.
The NTM-BIORA Study Investigators
Naoki Ishiguro (Nagoya University Hospital, Nagoya, Japan), Mutuo Kuba (NHO Okinawa National Hospital, Okinawa, Japan), Nobuyuki Kobayashi (National Center for Global Health and Medicine, Tokyo, Japan), Hiro Kuroda (Jiseikai Memorial Hospital, Tokyo, Japan), Yumi Sakakibara (Tokyo Medical and Dental University, Tokyo, Japan), Shinichi Sasaki (Juntendo University Urayasu Hospital, Chiba, Japan), Yuko Takahashi (National Center for Global Health and Medicine, Tokyo, Japan), Hidehiro Yamada (St. Marianna University School of Medicine, Kawasaki, Japan), Hisashi Yamanaka (Tokyo Women’s Medical University, Tokyo, Japan), Kazuhiro Tateda (Toho University School of Medicine, Tokyo, Japan), and Hajime Goto (Kyorin University School of Medicine, Tokyo, Japan).
Acknowledgments
We thank all members of the relevant medical staff at the participating institutes for collecting data.
Conflict of interest
T. J. has received consultant fees and travel fees from Chugai Pharmaceutical Co., Ltd. N. N. has received consultant fees from Chugai Pharmaceutical Co., Ltd. and F. Hoffmann-La Roche, Ltd.; lecture fees from Chugai Pharmaceutical Co., Ltd.; and research grants from Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb Japan, and Pfizer Japan, Inc. S. M. has received licensing fees and subsidies from Chugai Pharmaceutical Co. and subsidies from Mitsubishi-Tanabe Pharmaceutical Co.
References
- 1.Taiwo B, Glassroth J. Nontuberculous mycobacterial lung diseases. Infect Dis Clin North Am. 2010;24:769–789. doi: 10.1016/j.idc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.McGrath EE, Blades Z, McCabe J, Jarry H, Anderson PB. Nontuberculous mycobacteria and the lung: from suspicion to treatment. Lung. 2010;188:269–282. doi: 10.1007/s00408-010-9240-9. [DOI] [PubMed] [Google Scholar]
- 3.Arend SM, Soolingen D, Ottenhoff TH. Diagnosis and treatment of lung infection with nontuberculous mycobacteria. Curr Opin Pulm Med. 2009;15:201–208. doi: 10.1097/MCP.0b013e3283292679. [DOI] [PubMed] [Google Scholar]
- 4.Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM. Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: case finding through the Emerging Infections Network. Clin Infect Dis. 2008;46:1738–1740. doi: 10.1086/587989. [DOI] [PubMed] [Google Scholar]
- 5.Ingen J, Boeree MJ, Dekhuijzen PN, Soolingen D. Mycobacterial disease in patients with rheumatic disease. Nat Clin Pract Rheumatol. 2008;4:649–656. doi: 10.1038/ncprheum0949. [DOI] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 7.Thomson RM, Yew WW. When and how to treat pulmonary non-tuberculous mycobacterial diseases. Respirology. 2009;14:12–26. doi: 10.1111/j.1440-1843.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for the diagnosis of pulmonary nontuberculous mycobacterial diseases—2008. Kekkaku. 2011;86:37–9. [PubMed]
- 9.Nakahara H, Kamide Y, Hamano Y, Hosokawa T, Nishide M, Lin Y et al. A case report of a patient with rheumatoid arthritis complicated with Mycobacterium avium during tocilizumab treatment. Mod Rheumatol. 2011;21:655–9. [DOI] [PubMed]
- 10.Okubo H, Iwamoto M, Yoshio T, Okazaki H, Kato T, Bandoh M, et al. Rapidly aggravated Mycobacterium avium infection in a patient with rheumatoid arthritis treated with infliximab. Mod Rheumatol. 2005;15:62–64. doi: 10.1007/s10165-004-0360-z. [DOI] [PubMed] [Google Scholar]
- 11.Esaki T, Sugimoto M, Mori S, Yamashita A, Matsumoto M, Kohrogi H. A case of pulmonary nontuberculous mycobacteriosis aggravated during treatment with etanercept for rheumatoid arthritis. Nihon Kokyuki Gakkai Zasshi. 2010;48:312–316. [PubMed] [Google Scholar]
- 12.Middleton AM, Chadwick MV, Nicholson AG, Dewar A, Groger RK, Brown EJ, et al. Inhibition of adherence of Mycobacterium avium complex and Mycobacterium tuberculosis to fibronectin on the respiratory mucosa. Respir Med. 2004;98:1203–1206. doi: 10.1016/j.rmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with rheumatoid arthritis. Mod Rheumatol. 2010;21:164–173. doi: 10.1007/s10165-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol. 2008;35:1513–1521. [PubMed] [Google Scholar]
- 15.Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004;231:880–886. doi: 10.1148/radiol.2313030833. [DOI] [PubMed] [Google Scholar]
- 16.Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282–288. doi: 10.1148/radiol.2351040371. [DOI] [PubMed] [Google Scholar]
- 17.Chung MJ, Lee KS, Koh WJ, Kim TS, Kang EY, Kim SM, et al. Drug-sensitive tuberculosis, multidrug-resistant tuberculosis, and nontuberculous mycobacterial pulmonary disease in nonAIDS adults: comparisons of thin-section CT findings. Eur Radiol. 2006;16:1934–1941. doi: 10.1007/s00330-006-0174-9. [DOI] [PubMed] [Google Scholar]
- 18.Martinez S, McAdams HP, Batchu CS. The many faces of pulmonary nontuberculous mycobacterial infection. AJR Am J Roentgenol. 2007;189:177–186. doi: 10.2214/AJR.07.2074. [DOI] [PubMed] [Google Scholar]
- 19.Takayanagi N, Tsuchiya Y, Tokunaga D, Miyahara Y, Yamaguchi S, Saito H, et al. Pulmonary infections in patients with rheumatoid arthritis. Nihon Kokyuki Gakkai Zasshi. 2007;45:465–473. [PubMed] [Google Scholar]
- 20.Kobashi Y, Miyashita N, Niki Y, Matsushima T. A case of pulmonary Mycobacterium avium complex disease complicated by interstitial pneumonia with collagen vascular disease. Kekkaku. 2003;78:487–490. [PubMed] [Google Scholar]
- 21.Fujita K, Tanaka E, Hatta K, Kori Y, Taguchi Y. An autopsy case of Mycobacterium abscessus pulmonary infection complicated with rheumatoid arthritis. Intern Med. 2008;47:1273–1276. doi: 10.2169/internalmedicine.47.1023. [DOI] [PubMed] [Google Scholar]
- 22.Uruga H, Izumi S, Hojo M, Sugiyama H, Toyota E, Kobayashi N, et al. A patient with Mycobacterium avium lung disease presenting with rapid, progressive and multiple cavity formation, who had been treated rheumatoid arthritis with disease modifying anti-rheumatic drugs (DMARDs) Nihon Kokyuki Gakkai Zasshi. 2008;46:195–201. [PubMed] [Google Scholar]
- 23.Origichi T, Migita K, Kawakami A, Yamanaka S, Hida A, Shibatomi K, et al. Atypical mycobacteriosis in two patients with rheumatoid arthritis. Mod Rheumatol. 2002;12:76–79. doi: 10.1007/s101650200013. [DOI] [PubMed] [Google Scholar]
- 24.Salvana EM, Cooper GS, Salata RA. Mycobacterium other than tuberculosis (MOTT) infection: an emerging disease in infliximab-treated patients. J Infect. 2007;55:484–487. doi: 10.1016/j.jinf.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Garzoni C, Adler S, Boller C, Furrer H, Villiger PM. Possible role of anti-TNF monoclonal antibodies in the treatment of Mycobacterium marinum infection. Rheumatology (Oxford) 2010;49:1991–1993. doi: 10.1093/rheumatology/keq146. [DOI] [PubMed] [Google Scholar]
- 26.Yim K, Nazeer SH, Kiska D, Rose FB, Brown D, Cynamon MH. Recurrent Mycobacterium xenopi infection in a patient with rheumatoid arthritis receiving etanercept. Scand J Infect Dis. 2004;36:150–154. doi: 10.1080/00365540310017474. [DOI] [PubMed] [Google Scholar]
- 27.Danko JR, Gilliland WR, Miller RS, Decker CF. Disseminated Mycobacterium marinum infection in a patient with rheumatoid arthritis receiving infliximab therapy. Scand J Infect Dis. 2009;41:252–255. doi: 10.1080/00365540902774599. [DOI] [PubMed] [Google Scholar]
- 28.Ramos JM, Garcia-Sepulcre MF, Rodriguez JC, Padilla S, Gutierrez F. Mycobacterium marinum infection complicated by anti-tumour necrosis factor therapy. J Med Microbiol. 2010;59:617–621. doi: 10.1099/jmm.0.017277-0. [DOI] [PubMed] [Google Scholar]
- 29.Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15:1556–1561. doi: 10.3201/eid1510.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besada E. Rapid growing mycobacteria and TNFalpha blockers: case report of a fatal lung infection with Mycobacterium abscessus in a patient treated with infliximab, and literature review. Clin Exp Rheumatol. 2011;29:705–707. [PubMed] [Google Scholar]
- 31.Marie I, Heliot P, Roussel F, Herve F, Muir JF, Levesque H. Fatal Mycobacterium peregrinum pneumonia in refractory polymyositis treated with infliximab. Rheumatology (Oxford) 2005;44:1201–1202. doi: 10.1093/rheumatology/keh700. [DOI] [PubMed] [Google Scholar]
- 32.Maimon N, Brunton J, Chan AK, Marras TK. Fatal pulmonary Mycobacterium xenopi in a patient with rheumatoid arthritis receiving etanercept. Thorax. 2007;62:739–740. doi: 10.1136/thx.2005.056952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas JE, Taoka CR, Gibbs BT, Fraser SL. Fatal pulmonary Mycobacterium abscessus infection in a patient using etanercept. Hawaii Med J. 2006;65:12–15. [PubMed] [Google Scholar]
- 34.Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70:2148–2151. doi: 10.1136/ard.2011.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koike T, Harigai M, Ishiguro N, Inokuma S, Takei S, Takeuchi T, et al. Safety and effectiveness of adalimumab in Japanese rheumatoid arthritis patients: postmarketing surveillance report of the first 3,000 patients. Mod Rheumatol. 2011. Epub ahead of print. [DOI] [PubMed]
- 36.Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189–194. doi: 10.1136/ard.2007.072967. [DOI] [PubMed] [Google Scholar]
- 37.Koike T, Harigai M, Inokuma S, Inoue K, Ishiguro N, Ryu J, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36:898–906. doi: 10.3899/jrheum.080791. [DOI] [PubMed] [Google Scholar]
- 38.Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999. Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 2000;55:210–8. [DOI] [PMC free article] [PubMed]
- 39.Research Committee of the British Thoracic Society First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax. 2001;56:167–172. doi: 10.1136/thorax.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opinions of Japanese Society for Tuberculosis committee on therapy of atypical mycobacterium infections. Kekkaku. 1998;73:599–605. [PubMed]


