Abstract
Leishmania ISPs are ecotin-like natural peptide inhibitors of trypsin-family serine peptidases, enzymes that are absent from the Leishmania genome. This led to the proposal that ISPs inhibit host serine peptidases and we have recently shown that ISP2 inhibits neutrophil elastase, thereby enhancing parasite survival in murine macrophages. In this study we show that ISP1 has less serine peptidase inhibitory activity than ISP2, and in promastigotes both are generally located in the cytosol and along the flagellum. However, in haptomonad promastigotes there is a prominent accumulation of ISP1 and ISP2 in the hemidesmosome and for ISP2 on the cell surface. An L. major mutant deficient in all three ISP genes (Δisp1/2/3) was generated and compared with Δisp2/3 mutants to elucidate the physiological role of ISP1. In in vitro cultures, the Δisp1/2/3 mutant contained more haptomonad, nectomonad and leptomonad promastigotes with elongated flagella and reduced motility compared with Δisp2/3 populations, moreover it was characterized by very high levels of release of exosome-like vesicles from the flagellar pocket. These data suggest that ISP1 has a primary role in flagellar homeostasis, disruption of which affects differentiation and flagellar pocket dynamics.
Introduction
The leishmaniases are an array of diseases ranging symptomatically from relatively mild, local, cutaneous ulceration to fatal, visceral dissemination with accompanying fever and anaemia (Murray et al., 2005). They are caused by the protozoan parasites of the Leishmania genus and are transmitted by phlebotomine sand flies. Leishmania are digenetic parasites that alternate between disease-causing, non-motile, intracellular amastigotes and flagellated, extracellular promastigotes in their mammalian hosts and insect vectors respectively. Leishmania amastigotes are ingested by sand flies in a blood meal and undergo serial differentiation before they are injected, as metacyclic promastigotes, into their subsequent mammalian host. The intermediate stages in differentiation from procyclic to metacyclic promastigote are often overlooked as they are seldom seen in vitro (Rogers et al., 2002; Gossage et al., 2003). Nevertheless the distinct phenotypes afforded during these intermediate life cycle stages are critical for the survival of the parasite, establishment of infection and transmission to the next host. Procyclic promastigotes differentiate from amastigotes in the blood meal surrounded by the peritrophic matrix (PM), where they rapidly multiply. Simultaneously with the end of blood meal digestion and disintegration of the PM, procyclic promastigotes transform into nectomonads (Sadlova and Volf, 2009), long, non-replicative forms that escape from the remains of the PM-encased blood meal and specifically attach to the midgut epithelium to prevent expulsion from thegut during defecation (Wilson et al., 2010). Here they develop into leptomonad promastigotes (a synonym for short nectomonads) (Cihakova and Volf, 1997) which enter another proliferative cycle and finally transform into the mammalian infective stage, metacyclic promastigotes (Rogers et al., 2002; Gossage et al., 2003). Leptomonads and metacyclics colonize the stomodeal valve (junction between midgut and foregut) while tear-shaped haptomonads adhere to the stomodeal valve (Molyneux and Killick-Kendrick, 1987). Ultimately, the valve is blocked by accumulated parasites and promastigote secretory gel (Rogers et al., 2002), and its chitin lining is destroyed by Leishmania chitinase (Volf et al., 2004; Rogers et al., 2008). These changes facilitate transmission of the infective metacyclic promastigotes to the mammalian host (Rogers et al., 2004).
Leishmania amastigotes are often erroneously referred to as aflagellate however they have a short internal flagellum that extends upon differentiation into procyclic promastigotes (Gluenz et al., 2010a). The promastigote flagellum consists of the canonical 9+2 microtubules, motile axoneme and an associated paraflagellar rod (PFR), which is also critical for motility (Santrich et al., 1997). Flagellum length is dynamic during the various promastigote stages of the parasite's life cycle, where it is crucial for directed motility and attachment within the sand fly. It varies from a relatively short, < 7 µm, in procyclic promastigotes, to in excess of 20 µm in the colonizing nectomonad and leptomonad promastigotes, while adherent haptomonads have reduced flagella with an extended tip (Killick-Kendrick et al., 1974b; Rogers et al., 2002). Eukaryotic flagella are assembled/disassembled using an intraflagellar transport (IFT) system. IFT in Leishmania has not been widely studied but an in silico genome screen has shown that the IFT pathway is present (Gouveia et al., 2007) and it has been investigated in the closely related Trypanosoma brucei, where silencing of either anterograde or retrograde transport results in shortening of the flagellum (Absalon et al., 2008). Variations in Leishmania flagellum length may also be dictated by IFT-independent mechanisms affecting axonemal microtubule dynamics and protein trafficking (Gluenz et al., 2010b). While motility and attachment are clearly of vital importance in promastigotes, accumulating evidence suggests that the flagellum also functions as a sensory organelle promoting environmentally induced morphological changes during parasite differentiation (Zilberstein and Shapira, 1994; Rotureau et al., 2009) and amastigote–host interactions (Gluenz et al., 2010a).
The Leishmania flagellum exits the cell body from an invagination in the cell membrane that forms the flagellar pocket, the parasite's sole site for endo/exocytosis (Field and Carrington, 2009). However, while the parasite assembles and routes most surface molecules, such as lipophosphoglycan and GP63, via the classical ER-Golgi-plasma membrane pathway (McConville et al., 2002), it is recognized that many Leishmania-secreted proteins have no N-terminal secretion signal indicating that the parasite employs non-classical methods of secretion (Stegmayer et al., 2005). Recent evidence suggests that more than 50% of Leishmania-secreted proteins are trafficked in vesicles morphologically similar to mammalian exosomes (Silverman et al., 2010); but while there is accumulating evidence for the stimulated secretion of exosomes and shedding vesicles in macrophages and neutrophils (Cocucci et al., 2009) the mechanism for vesicle-mediated non-classical secretion by Leishmania is unknown.
Leishmania has three genes encoding ecotin-like inhibitors of serine peptidases (ISPs) (Eschenlauer et al., 2009). Ecotin from Escherichia coli is a strong competitive inhibitor of trypsin-fold serine peptidases (Chung et al., 1983) and its postulated in vivo targets include mammalian serine peptidases, such as neutrophil elastase (NE), tryptase and cathepsin G, expressed by cells of the innate immune system (Eggers et al., 2004). Trypsin-fold serine peptidases of clan PA, family S1A are not apparently encoded in the Leishmania genome (Ivens et al., 2005), so we hypothesized that host-derived serine peptidases are the major target for parasite ISPs. ISP2 is expressed in all life cycle stages of Leishmania major with particularly high levels in infective metacyclic promastigotes and amastigotes, where it influences the early stages of macrophage infection and parasite intracellular survival through inhibition of NE present at the surface of macrophages (Eschenlauer et al., 2009; Faria et al., 2011). L. major lines lacking ISP2 and ISP3 (Δisp2/3) are internalized more efficiently than wild-type (WT) parasites by BALB/c or C57B6 macrophages, due to upregulation of phagocytosis and selective engagement of CR3 (Eschenlauer et al., 2009; Faria et al., 2011). However, deficiency in ISP2 and ISP3 results in the partial elimination of intracellular parasites 24 h post-infection, suggesting that these ISPs are important for the initiation and persistence of infection in the mammalian host. NE is the main target of ISP2 in macrophages and NE activity, subject to control by ISP2, promotes the phagocytosis of L. major through Toll-like receptor 4 (TLR4) and provokes the killing of a proportion of the internalized parasites within 24 h (Faria et al., 2011). These studies indicate that ISP2 regulates host serine peptidase activity, which influences the outcome of anti-parasite defences.
In this study we created an L. major mutant deficient in all three ISP proteins (Δisp1/2/3). By comparing the phenotype of Δisp2/3 andΔisp1/2/3 mutants we have been able to define distinct roles for ISP1 and ISP2 in Leishmania.
Results
Generation of L. major ISP1, ISP2 and ISP3 triple null mutants
Leishmania major ISP1–ISP2–ISP3 triple null mutants (Δisp1/2/3) were created by sequential removal of two ISP1 alleles from the previously described Δisp2/3 parasites (Eschenlauer et al., 2009) (Fig. 1A and B). Southern blotting confirmed the removal of both ISP1 alleles, as the 3.6 kb WT alleles detected with a 5′ flank probe on SalI digested genomic DNA (Fig. 1C, lane 1) were present in Δisp2/3 (lane 2), while one allele was replaced with a drug resistance marker in heterozygous parasites (lane 3) and both replaced in Δisp1/2/3 parasites (lanes 4 and 5). ISP1 was re-introduced into the ribosomal locus of Δisp1/2/3 to generate Δisp1/2/3:ISP1 and ISP2 and ISP3 were likewise re-introduced to generate Δisp1/2/3:ISP2-3. A third re-expressing cell line Δisp1/2/3:ISP2-3[pXG-ISP1] was generated by transfecting an episomal copy of ISP1 into Δisp1/2/3:ISP2-3 parasites. Additionally, episomal plasmids for ISP1 and ISP2 were transfected into WT L. major to generate overexpression lines WT [pXG-ISP1] and WT [pXG-ISP2] respectively. Western blotting confirmed that ISP1 (Fig. 1D, lane 2) and ISP2 (Fig. 1E, lane 2) were absent from Δisp1/2/3 parasites and that ISP1 and ISP2 were expressed in all the add-back and overexpression cell lines (Fig. 1D–G).
Fig. 1.
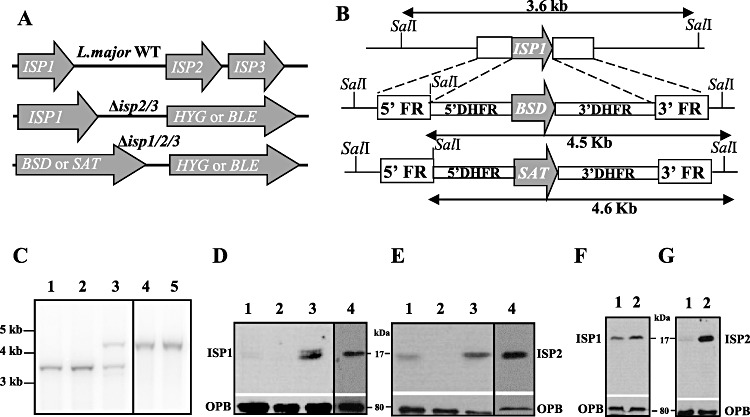
Generation of L. major ISP1, ISP2 and ISP3 triple null mutants.
A. Schematic representation of the ISP loci of L. major in wild-type (WT, upper), Δisp2/3 (middle) and Δisp1/2/3 (lower) parasites.
B. Schematic representation of the ISP1 locus in WT L. major (upper) and the constructs for gene deletion. ORFs are shown as grey arrows and 5′ and 3′ flanking regions (FR) as boxes representing the DNA sequences used for targeting. The predicted fragment sizes after restriction digest are shown. BSD, blasticidin resistance gene; SAT, nourseothricin resistance gene.
C. Southern blot of WT L. major (lane 1), Δisp2/3 (lane 2), first allele ISP1 knockout heterozygous Δisp1/2/3 (lane 3) and two Δisp1/2/3 clones (lanes 4 and 5) digested with SalI and probed with radiolabelled 5′ FR.
D. Western blots of cell extracts from 1 × 107 stationary-phase promastigotes of WT L. major (lane 1), Δisp1/2/3 (lane 2), Δisp1/2/3:ISP1 (lane 3), Δisp1/2/3:ISP2-3[pXG-ISP1] (lane 4), using purified αISP1 primary antibody raised in sheep.
E. Western blot of cell extracts from 1 × 107 stationary-phase promastigotes of WT L. major (lane 1), Δisp1/2/3 (lane 2), Δisp1/2/3:ISP2-3 (lane 3), Δisp1/2/3:ISP2-3[pXG-ISP1] (lane 4) using purified α-ISP2 primary antibody raised in sheep.
F. Western blot of cell extracts from 1 × 107 stationary-phase promastigotes of WT L. major (lane 1) and WT [pXG-ISP1] (lane 2).
G. Western blot of cell extracts from 1 × 107 stationary-phase promastigotes of WT L. major (lane 1) and WT [pXG-ISP2] (lane 2). Antibodies to Oligopeptidase B (OPB) were used as loading control.
ISP1 has low peptidase inhibitory activity and is not involved in the uptake and initial survival of L. major in macrophages
To determine whether ISP1 has a role in modulating mammalian host serine peptidase activity and thereby influencing uptake and survival within murine macrophages, as has been shown for ISP2 (Eschenlauer et al., 2009), activity and infection assays were performed. Recombinant ISP1 was produced in E. coli, purified using affinity and ion-exchange chromatography and tested for inhibitory activity against mammalian clan PA, family S1A serine peptidases. ISP1 inhibited approximately 50% of human NE activity when pre-incubated with the enzyme at 80-fold excess, while ISP2 showed approximately the same degree of inhibition at 20-fold excess (Fig. 2A). The determination of the inactivation constant revealed a Ki of 107 ± 22 nM using MeOSuc-AAPV-AMC as a substrate. This was 10-fold higher than ISP2 assayed under the same conditions(Ki 8.8 ± 2.0 nM). ISP1 did not inhibit bovine trypsin, even when tested at 10 µM, in contrast to ISP2, which inhibited the enzyme completely at 2 µM and gave 50% inhibition at 0.2 µM (Fig. 2A). These results suggest that ISP1 is more selective than ISP2 and is a less potent inhibitor of these representative peptidases. Next, the inhibitory activity against serine peptidases present in the invertebrate host Phlebotomus papatasi was evaluated using extracts of the sand fly midgut as a source of peptidases (Fig. 2B). We observed full inhibition of the measured peptidase activity by ISP2 at 0.2 µM while ISP1 at 6.7 µM gave approximately 40% inhibition, showing that while both ISPs are capable of modulating the activity of sand fly midgut peptidases, ISP2 is the more efficacious.
Fig. 2.
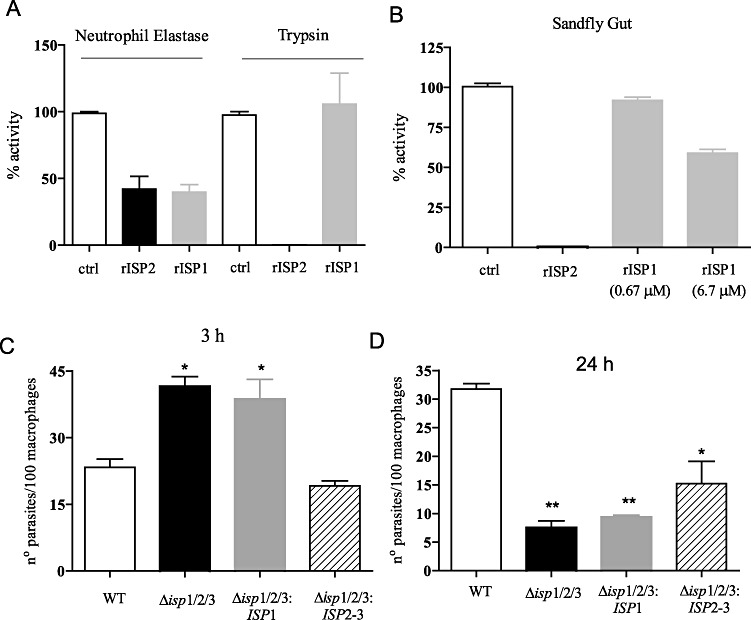
No role for ISP1 in uptake and survival of L. major in macrophages
A. Inhibition of serine peptidases by recombinant ISPs. Human neutrophil elastase or bovine trypsin were incubated with rISP2 (200 nM) or rISP1 (670 nM) in 100 mM Tris-HCl pH 8, for 20 min on ice, and the residual peptidase activity was determined using MeOSuc-AAPV-AMC or Z-R-AMC as substrates respectively.
B. Extracts from the midgut of Phlebotomus papatasi were incubated with rISP2 (200 nM) or rISP1 in 100 mM Tris-HCl pH 8, 100 mM NaCl, for 5 min on ice and the residual peptidase activity was determined using Boc-LGR-AMC as substrate. The graphs show average ± SEM of duplicates and are representative of two independent experiments.
C. Number of parasites/100 macrophages was determined following incubation of stationary-phase promastigotes with elicited peritoneal macrophages from BALB/c mice at a 5:1 parasite : macrophage ratio for 3 h.
D. Extracellular parasites were removed after 3 h interaction and the cells were cultured for 24 h to evaluate intracellular survival of amastigotes. The experiments were performed in triplicate, two independent times. The asterisk indicates statistical significance at P < 0.05 in relation to wild type.
Δisp1/2/3 parasites were used to infect elicited BALB/c macrophages in parallel with the cell lines re-expressing ISP1 or ISP2 (Δisp1/2/3:ISP1 and Δisp1/2/3:ISP2-3). Δisp1/2/3 promastigotes were internalized twofold more efficiently than WT and the phenotype was compensated in the Δisp1/2/3:ISP2-3 but not the Δisp1/2/3:ISP1 parasites (Fig. 2C). The survival of Δisp1/2/3 parasites was severely reduced at 24 h and the phenotype was partly reversed in the Δisp1/2/3:ISP2-3, but not Δisp1/2/3:ISP1 parasites (Fig. 2D). These experiments show that whereas ISP2 influences the uptake and initial survival of L. major in macrophages, ISP1 does not.
Δisp1/2/3 parasite populations have a growth defect and contain aggregates of cells with often tortuous morphology
Given the lack of trypsin-fold serine peptidases in the L. major genome, we had anticipated that the ISPs would likely have an extracellular role that involved inhibition of host enzymes. The triple null mutant promastigotes, however, displayed a distinct growth phenotype. Cultured Δisp1/2/3 parasites grew normally during early log phase (up to 2 days) but, by mid-log phase, had a decreased rate of growth and reached stationary phase slightly earlier and at half the culture density of WT parasites (Fig. 3A). This was partially alleviated in ISP1, ISP2-3 and the ISP1-2-3 add-back cell lines. In conjunction with a reduced growth rate, about 30% of the parasites in the two clonal Δisp1/2/3 populations had unusual morphology, characterized by an enlargement of the flagellar pocket region (Fig. 3C, white asterisk and Fig. 3D, at higher magnitude). Concomitantly with the decrease in the growth rate (at approximately 48 h), the Δisp1/2/3 cultures contained unusually large parasite aggregates (Fig. 3B), formed from high numbers of often misshapen cells aggregating with their anterior pole at the centre of the mass (Fig. 3G). This was over and above the rosetting phenomenon commonly observed in late stage L. major cultures in vitro. As the growth cycle progressed, we observed increasing numbers of parasite aggregates in the Δisp1/2/3 cultures (Fig. 3B), but not in cultures of Δisp1/2/3:ISP1. All the Δisp1/2/3:ISP1 parasites were of WT appearance (Fig. 3E) and formed fewer clumps, implicating the lack of ISP1 in the abnormal parasite morphology and tendency to form aggregates. Aggregate formation was also abrogated in the Δisp1/2/3:ISP2-3 parasites, but abnormal cells with distended, twisted flagellar pockets were still apparent in the population at low numbers (Fig. 3F). These data suggest that ISP1 has a role in L. major promastigotes which is not complemented by ISP2-3.
Fig. 3.
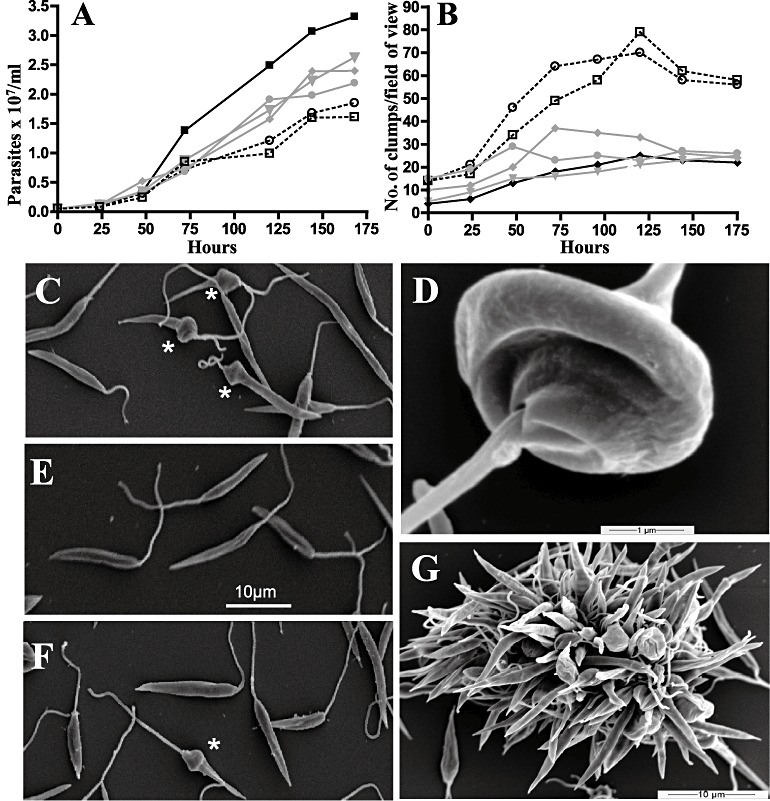
Growth and appearance of Δisp1/2/3 parasite populations
A. Growth curve showing WT L. major (black squares), Δisp1/2/3, two independent clones (black dashed, squares and circles) and Δisp1/2/3:ISP1,Δisp1/2/3:ISP2-3,Δisp1/2/3:ISP2-3[pXG-ISP1] parasites (grey triangle, diamond and circle respectively).
B. Formation of clumps as determined by light microscopy in 10 fields of view (×40 objective) in six-well plates. (A) and (B) were co-determined and the graphs are representative of four independent experiments.
C, E and F. SEM of Δisp1/2/3, Δisp1/2/3:ISP1 and Δisp1/2/3:ISP2-3, the scale bar in (E) is applicable to all three images. Misshapen cells in (C) and (F) are denoted by a white asterisk.
D. Anterior view of Δisp1/2/3 distended flagellar pocket.
G. SEM of typical Δisp1/2/3 aggregate.
Δisp1/2/3 parasite populations have longer flagella and are less motile than WT populations
Given the identified distortion in the flagellar pocket region and that proteins orthologous to both ISP1 and ISP2 had been identified in the T. brucei flagellar proteome (Broadhead et al., 2006), we used scanning electron microscopy and iTEM software to measure flagella lengths in WT and mutant parasites in late log cultures. Only cells not in aggregates were considered in the measurements. The mean length of a flagellum in an L. major WT population was 11.8 µm (Fig. 4A), while that of Δisp1/2/3 parasite populations was significantly higher at 13.7 µm (Fig. 4B, Table S1). Reintroduction of either ISP1 or ISP2-ISP3 alone did not revert the phenotype as Δisp1/2/3:ISP1 and Δisp1/2/3:ISP2-3 parasites had mean flagella lengths of 13.8 µm and 14.9 µm respectively (Fig. 4C and D). The triple add-back parasites Δisp1/2/3:ISP2-3[pXG-ISP1], however, had a mean flagellum length of 10.3 µm (Fig. 4E), which was significantly lower than that of WT cells (Table S1), suggesting that the presence of all three ISP genes is required for flagellar homeostasis and so correct flagellar length. A more detailed analysis of the flagella lengths, whereby they were classified into length groupings of 0–4.9 µm, 5–9.9 µm, 10–14.9 µm, 15–19.9 µm or 20–24.9 µm revealed that the increased flagella length of the Δisp1/2/3,Δisp1/2/3:ISP1 and Δisp1/2/3:ISP2-3 populations was due to a shift from the submedian 5–9.9 µm to the post-median 15–19.9 µm grouping in all these genotypes. The reverse was true in Δisp1/2/3:ISP2-3[pXG-ISP1] parasites accounting for their shorter mean flagella length. We postulated that this may be due to excess ISP1. To address this, we determined the mean flagellum lengths of WT [pXG-ISP1] and WT [pXG-ISP2] to be 8.7 µm and 9.7 µm respectively (Fig. 4F and G). The data show that overexpression of either ISP1 or ISP2, in the presence of endogenous levels of ISP1 and ISP2, results in a reduction in mean flagellum length. As ISP1 was expressed from a multicopy plasmid in Δisp1/2/3:ISP2-3[pXG-ISP1] and ISP2 was previously identified as overexpressed in the ribosomal locus of Δisp2/3:ISP2-3 (Eschenlauer et al., 2009), the results generally show that overexpression of either ISP in the triple add-back parasites could account for their short flagellum.
Fig. 4.
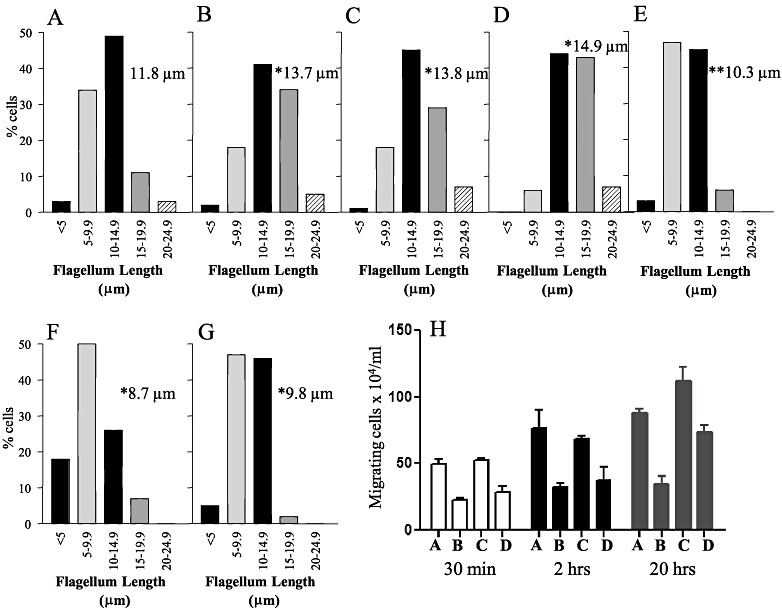
Δisp1/2/3 parasite populations have longer flagella and are less motile than WT populations.
A–G. (A) WT L. major; (B) Δisp1/2/3; (C) Δisp1/2/3:ISP1; (D) Δisp1/2/3:ISP2-3; (E) Δisp1/2/3:ISP2-3[pXG-ISP1]; (F) WT [pXG-ISP1]; (G) WT [pXG-ISP2]. Grouped flagella length measurements expressed as a % of 200 flagella measured using iTEM software and ×550 SEM microscopy. Figures in grey shaded boxes are the mean flagella lengths for each graph and (*) or (**) denotes statistically difference at P < 0.001 or P < 0.01 of the entire data set from the WT (A), as measured by one-way anova with a Tukey post test. A full statistical analysis is shown in Table S1.
H. Crossing assay showing the number of parasites (A, B, C and D as described above) crossing a 3.0 µM Transwell® membrane in 30 min (white bars), 2 h (black bars) and 20 h (grey bars). Error bars are SD of the mean for four replicates.
We observed that a great number of the Δisp1/2/3 parasites in the cultures with no apparent morphological abnormalities displayed altered movement. We investigated this by testing the ability of un-aggregated promastigotes to cross pored membranes in Transwell chambers. Reduced motility was observed, Δisp1/2/3 parasites were found to have crossed a membrane with only half the efficiency of WT parasites at each of the three time points studied (Fig. 4H). This was fully compensated in the Δisp1/2/3:ISP1 parasites but not the Δisp1/2/3:ISP2-3 parasites, which only showed a motility equivalent to that of WT at the 20 h time point. Taken together, these data suggest that the balance between the levels of the different ISPs is important for the maintenance of flagellum length and function.
Increased abundance of haptomonad promastigotes in Δisp1/2/3 parasite populations
Flagellum length, particularly in relation to cell body size and morphology, is a distinguishing feature in different promastigote life cycle stages, so we quantified, based on both morphology (Fig. 5) and flagellum length, the numbers of procyclic, nectomonad, leptomonad, haptomonad and metacyclic promastigotes present in late log phase cultures. WT populations contained more than twice as many procyclic promastigotes than Δisp1/2/3 populations. This difference was also apparent in Δisp1/2/3:ISP2-3 populations, but Δisp1/2/3:ISP1 populations contained as many procyclic promastigotes as WT populations; thus ISP1 complemented the phenotype but ISP2 did not. While slightly increased numbers of nectomonad and leptomonad promastigotes in the Δisp1/2/3 population partially accounted for the decrease in procyclic promastigotes, the most marked increase was in the number of haptomonad parasites. In addition, the WT populations had nearly twice the number of metacyclic promastigotes than the Δisp1/2/3 populations, a change that was also compensated by ISP1 but not ISP2 (Fig. 5). While unclassified cells also increased in the Δisp1/2/3 populations, the major differentiation shift in Δisp1/2/3 populations was due to the decrease in procyclic and metacyclic promastigotes and an increase in the intermediate stages with predominance towards haptomonad promastigotes.
Fig. 5.
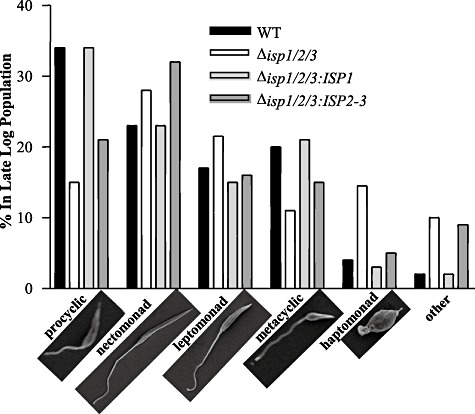
Differentiation of Δisp1/2/3. Parasites in late log phase cultures were examined at ×550 magnification using SEM and classified into the morphological categories for promastigotes as previously described (Rogers et al., 2002). The experiment was performed with duplicate SEM samples independently processed and the graph is typical of 200 parasites in each population.
ISP1 and ISP2 localize to the flagellar collar in haptomonad promastigotes
We purified polyclonal anti-ISP1 and anti-ISP2 antibodies to a high specificity, and neither was found to have cross-reactivity with Δisp1/2/3 parasites in fluorescence microscopy (Fig. 6A and B). Both ISP1 and ISP2 showed a prominent labelling of the hemidesmosome in haptomonad promastigotes, where a very strong signal was detected (Fig. 6C and D), in addition to a cell surface localization for ISP2. In other promastigote life cycle stages, ISP1 and ISP2 were found in the cytosol as well as in distinct foci along the flagellum, with a noticeable accumulation at the tip of the flagellum in approximately 70% of cells analysed (Fig. 6E–G).
Fig. 6.
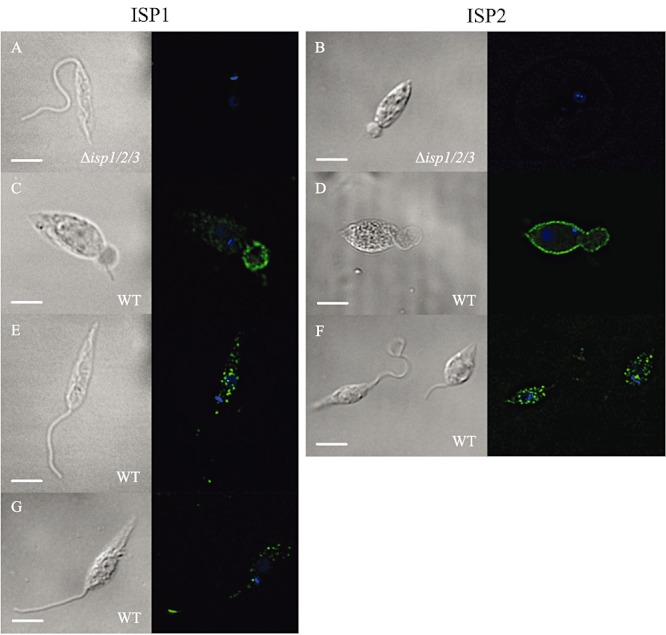
ISP1 and ISP2 localization. Fixed L. major were labelled with anti-ISP1 (A, C, E and G) or anti-ISP2 (B, D and F) antibody and donkey anti-sheep Alexa 488-secondary antibody. Nuclear and kinetoplast DNA were stained with DAPI. Left panel, DIC image. Right panel, merged image of ISP (green) and DAPI (blue). Scale bar = 5 µm.
ISP1 and ISP2 orthologues were absent from the T. brucei flagellar proteome performed on snl-1 RNAi mutants (Hart et al., 2009), although they had previously been identified in this organelle in WT T. brucei (Broadhead et al., 2006) (Table S2). The snl-1 mutants are severely deficient in the PFR protein, PFR-A, and lack the intermediate and distal regions of the PFR which results in parasite paralysis (Bastin et al., 1999). An equivalent snl-1 mutant has been generated in Leishmania mexicana by deletion of the PFR2 gene (Santrich et al., 1997). We therefore aimed to determine the localization of ISP1 and ISP2 in WT L. mexicana and in Δpfr1 and Δpfr2 mutants. Western blot analysis showed that ISP1 could be detected in WT L. mexicana parasites and that expression was significantly reduced in both Δpfr1 and Δpfr2 mutants (Fig. S1A), a finding that correlates with the proteomics analysis of the T. brucei snl-1 mutants. ISP1 had the same subcellular localization in both L. major and L. mexicana, including the hemidesmosome of haptomonads but was absent from the flagellum of Δpfr1 and Δpfr2 L. mexicana (Fig. S1B). Similar analyses for ISP2 were not possible as antibody raised against L. major ISP2 did not detect L. mexicana ISP2 in either Western blots or immunofluorescence (Fig. S1C and D), despite the presence of an intact gene in L. mexicana (Rogers et al., 2011).
Excessive vesicles and membraneous whorls in the flagellar pockets of Δisp1/2/3 parasites
To identify the cause of the distortion in the flagellar pocket of Δisp1/2/3, we examined longitudinal and cross-sections of the flagellar pocket region by transmission electron microscopy (TEM). WT parasites (Fig. 7A and B) have largely clear unobstructed flagellar pockets. The Δisp1/2/3 parasites, however, accumulated dense granular vesicles and membranous whorls (Fig. 7C and D). Release of this material into the flagellar pocket was not observed in the Δisp1/2/3:ISP1 (Fig. 7E and F) or Δisp1/2/3:ISP2-3[pXG-ISP1] (Fig. 7I and J), whereas vesicles and whorls were detected in the Δisp1/2/3:ISP2-3 parasites (Fig. 7G and H). The Δisp1/2/3 parasites have a normal 9+2 axoneme and PFR (Fig. 7D). This demonstrates that loss of ISP1, but not ISP2, causes a disruption in membrane dynamics within the flagellar pocket but does not affect the structure of the flagellum.
Fig. 7.
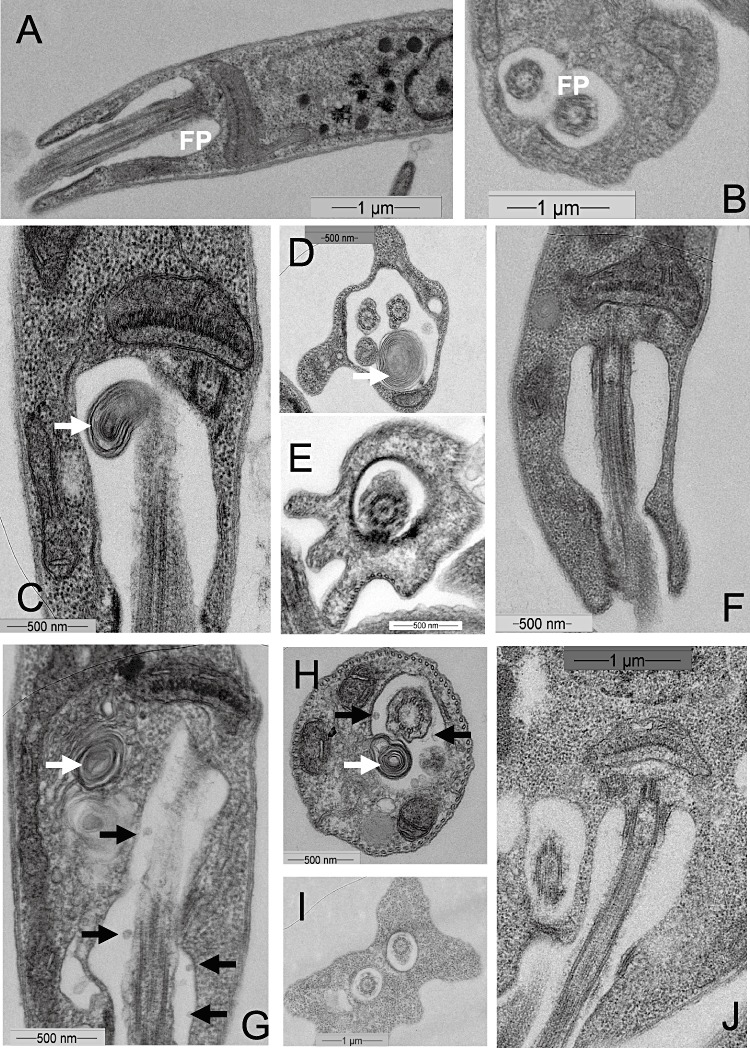
Flagellar pocket structure in Δisp1/2/3 parasites. Transmission EM images of WT (A, B), Δisp1/2/3 (C, D), Δisp1/2/3:ISP1 (E, F), Δisp1/2/3:ISP2:3 (G, H) and Δisp1/2/3:ISP2-3[pXG-ISP1] (I, J) promastigote flagellar pockets (FP). Membranous whorls in Δisp1/2/3 (C, D) and Δisp1/2/3:ISP2-3 (G, H) are indicated with a white arrow and small vesicles in Δisp1/2/3:ISP2-3 (G, H) with a black arrow. FP flagellar pocket, DG dense granule.
Excessive secretion from Δisp1/2/3 parasites
Scanning electron microscopy (SEM) and TEM also revealed that Δisp1/2/3 promastigotes secreted/excreted excess material from their anterior poles. SEM showed that the material was emerging from the flagellar pocket (Fig. 8A–C) and in some cases appeared to slough down the flagellum and shed from the tip (Fig. 8C). TEM gave a more detailed picture of the exudation, and material was visualized emitting from the flagellar pocket, at the hemidesmosome (Fig. 8D and E) and apparently budding from the flagellum itself (Fig. 8F–H). While some dense granules could be seen, the majority of the material released from the Δisp1/2/3 parasites comprised relatively transparent, apparently membrane-bound vesicles of ∼ 100 nm diameter. These vesicles were found in regions of TEM sections with a concentration of flagellum (Fig. 8E), but this was not exclusive (Fig. 8D and F–H). It is also apparent that, while the exuded vesicles are clearly membrane-bound as they leave the parasite, their membrane integrity diminishes as they dissipate from the point of release (Fig. 8D–H). A similar release of membrane material was observed in Δisp1/2/3 (Fig. 8J, arrowed and in higher magnification in 8M–O) and Δisp1/2/3:ISP1 (Fig. 8L, arrowed and in higher magnification in 8P) intracellular amastigotes within macrophages but not in WT (Fig. 8I) or Δisp1/2/3:ISP2-3 (Fig. 8K).
Fig. 8.
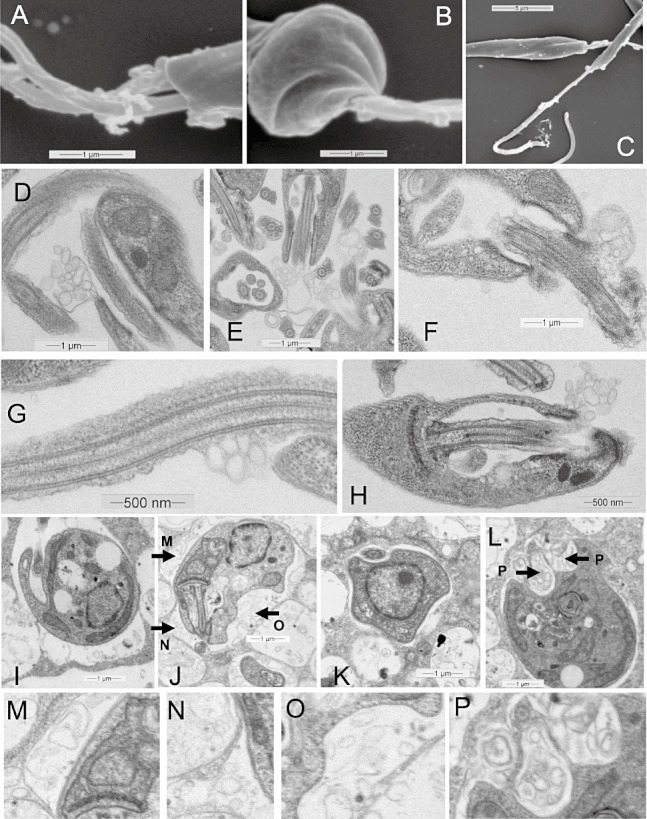
Release of membrane vesicles in Δisp1/2/3.
A–C. SEM of Δisp1/2/3 parasites.
D–H. TEM of Δisp1/2/3.
I–L. RAW macrophage infected with (I) WT L. major, (J) Δisp1/2/3, (K) Δisp1/2/3:ISP1, (L) Δisp1/2/3:ISP2-3. Membrane vesicles released in (J) and (L) are indicated with black arrows and magnified in (M)–(P).
Discussion
Leishmania major ISP1 and ISP2 share only 36% sequence identify with each other and have very distinct inhibitory properties. While rISP2 is a relatively potent inhibitor of all the clan PA, family S1A serine peptidases tested so far (Eschenlauer et al., 2009), the inhibitory activity of rISP1 is more restricted and is at least 10-fold lower than that of rISP2. The interaction of bacterial ecotins with the active site of serine peptidases occurs via the primary binding site composed of the 50s and 80s loops, the latter bears the reactive Met84 residue (Yang et al., 1998). While the residues composing the 80s loop of ISP1 have similarity to those of ecotins (more so than ISP2), ISP1 lacks the reactive Met which would be predicted to influence its inhibitory capacity. Furthermore, the tripeptide that comprises the putative 50s loop of ISP1 (HQT) has no conservation with ecotin (LHR), whereas ISP2 (VYR) bears conserved substitutions in those positions. Thus ISP1 and ISP2 appear to present significant differences in the binding site to target enzymes, which are consistent with, and suggestive of, important functional diversity. This diversity must reside within the ecotin-binding domain as the predicted molecular weight of ISP1 is 16.4 kDa and the ecotin domain (Pfam domain: PF03974) covers 95% of the protein. Our results indeed show that the two natural inhibitors have different inhibitory specificities, with rISP2 inhibiting NE and trypsin with Kis in the low nM range, whereas rISP1 is less active against NE and has no inhibitory activity towards trypsin. ISP2, but not ISP1, has been demonstrated to prevent TLR4 activation through inhibition of mammalian NE, thus promoting survival in macrophages (Eschenlauer et al., 2009; Faria et al., 2011). The demonstration that rISP1 is 10-fold less potent than ISP2 as an inhibitor of NE, possibly accounts for the observed lack of effect of ISP1 on macrophage invasion.
ISP1 is predominantly expressed in promastigotes, so a role in the inhibition of sand fly gut serine peptidases could be predicted. Sand flies express an abundance of trypsin-like and chymotrypsin-like serine peptidases and Leishmania must withstand the hydrolytic environment of the sand fly gut. Moreover, various Leishmania species have demonstrable trypsin-modulating activity in vivo (Dillon and Lane, 1993; Sant'Anna et al., 2009; Telleria et al., 2010; Dostalova et al., 2011). In this study, however, we found that whereas rISP2 was able to inhibit the serine peptidase activity of a sand fly midgut extract (showing 60% inhibition at 2 nM), under the same conditions rISP1 inhibited with less potency (with 40% inhibition at 6.7 µM). Thus these results reinforce the concept that ISP1 is less active against trypsin-like peptidases than ISP2, although it cannot yet be ruled out that ISP2 and ISP1 have functions that target different peptidases in the sand fly.
The current study has revealed, however, that ISP1 has an intracellular role within the parasite itself, which we did not anticipate as L. major apparently lacks endogenous S1A enzymes. Nevertheless, the distinctive in vitro phenotype discovered for Δisp1/2/3 is strongly suggestive of an intracellular role for ISP1, either targeting an as yet unidentified non-family S1 peptidase or a role that is independent of the inhibition of serine peptidase activity. The increased flagella length in Δisp1/2/3 parasite cultures may be explained in part by a change in the balance between the procyclic, nectomonad and leptomonad promastigote forms that occur during differentiation. Our detailed analysis of late log phase promastigote populations showed that Δisp1/2/3 cultures did vary from WT populations in containing more forms intermediate in the procyclic–metacyclic transformation. Potentially relevant to this, the intracellular distribution of both ISP1 and ISP2 also varied with promastigote differentiation. ISP1 and ISP2 in haptomonad promastigotes were found to be prominently located in the hemidesmosome (Fig. 6), a structure that is involved in attachment to the chitinous parts of the stomodeal valve (Killick-Kendrick et al., 1974b; Molyneux and Killick-Kendrick, 1987), although ISP2 also had a surface location. Parasite attachment results in destruction of the chitin lining and unique filaments on the apical end of cylindrical cells of the valve (Volf et al., 2004). These changes, together with the plug formed from the promastigote secretory gel, facilitate the regurgitation of Leishmania into the vertebrate host (Rogers et al., 2004; Volf et al., 2004). With this location, both ISP1 and ISP2 are well situated to interact with sand fly peptidases. In other promastigote forms the ISPs occur in the cytosol and in discreet foci at the tip of the flagellum. Localization to the flagellum is clearly dependent on a functional PFR, as ISP1 in Δpfr1 and Δpfr2 parasites was absent from the flagellum, which is consistent with proteomics analysis in trypanosomes (Broadhead et al., 2006; Hart et al., 2009).
The changes in the proportion of the different promastigotes forms in Δisp1/2/3 cultures cannot, however, explain fully the elongated flagellum phenotype in these parasites, because while re-expression of ISP1 alone restored the differentiation balance of the triple null mutant culture, the re-introduction of ISP2 was also required to complement fully the flagellum length phenotype. This suggests that whereas ISP1 is involved in promastigote differentiation, both ISP1 and ISP2 are required for flagellum homeostasis. The Δisp1/2/3 parasite flagella are not structurally disrupted, having a normal 9+2 microtubule arrangement and PFR, indicating that IFT is operating, but it is possible that the elongated flagella present in the Δisp1/2/3 parasites arise from disruption of the regulation of trafficking along the IFT rather than any loss of structural integrity itself. Alternatively, excess components not properly turned over during re-modelling and homeostasis that become accumulated in the flagellum itself could account for the alterations in flagellar length. It appears that ultimately some of the excess material is shed, based on the materials observed in the flagellar pocket region of the mutant promastigotes. It is not known how flagellar assembly is regulated in Leishmania, but mitogen kinases involved in the control of flagellar length by putative phosphorylation of flagellar components have been described (Wiese et al., 2003; Erdmann et al., 2006) and similar mechanisms have been proposed to modulate cargo recognition in a myosin-dependent flagellar protein trafficking pathway operating beside the IFT system (Katta et al., 2010). IFT, powered by kinesin and dynein motors, has an essential, if not solo, role in Leishmania flagella biosynthesis and silencing of either anterograde or retrograde transport results in shortening of the flagellum in trypanosomes (Absalon et al., 2008). This is similar to the phenotype seen in this study with the overexpression of ISP1 or ISP2 in Leishmania (Fig. 4). The ISP mutants characterized in the current study also have similar phenotypes to the L. major kinesin mutants, which have shortened flagella upon overexpression of kinesin, while comparable siRNA knock-down mutants of the equivalent T. brucei gene have elongated flagella due to the micro-tubule binding and depolymerizing activity of kinesin (Blaineau et al., 2007). Intriguingly, Leishmania donovani parasites lacking a component of the outer dynein arm docking complex (LdDG2 null mutants) also demonstrated a link between flagellum length regulation and differentiation, and it was proposed that unincorporated flagellar proteins trigger stress responses which promote differentiation between parasite forms (Harder et al., 2010).
Another interesting feature of the Δisp1/2/3 parasites is the accumulation of vesicles and membranous whorls in their flagellar pocket. The accumulation of whorls was previously attributed to the breakdown of membranes during the shortening of the flagellum as parasites differentiated from the nectomonad to haptomonad life cycle stages (Killick-Kendrick et al., 1974a). This may account for this phenotype in the Δisp1/2/3 parasites cultures, as they contain more parasites undergoing differentiation; however, it does not explain the accumulation of large and small dense vesicles in the flagellar pockets, as previously reported in the T. brucei IFT mutants (Absalon et al., 2008). It therefore seems possible that the excessive accumulation of vesicles in the flagellar pockets of Δisp1/2/3 parasites is the result of a combination of altered differentiation and disruption of IFT, both of which are reversed by ISP1 but not ISP2.
The vesicles observed emerging from Δisp1/2/3 parasites were not confined to the flagellar pockets, but also occurred at the hemidesmosome where they surrounded the collars. The exudate does not appear to be the filamentous acid phosphatase known to be released from Leishmania promastigotes, as it is less fibrous in appearance (Stierhof et al., 1994). Rather, many of the vesicles secreted resemble in size and morphology those recently described as exosomes secreted from L. donovani (Silverman et al., 2010). Exosome secretion is a recent discovery in Leishmania, where the presence of an alternative secretory pathway has long since been accepted although elucidation of the mechanism and occurrence has been elusive (Stegmayer et al., 2005). However, unlike this recent L. donovani study, and a previous proteomic analysis of the L. donovani secretome (Silverman et al., 2008), we can see no evidence of exosome budding from the body of L. major WT or Δisp1/2/3 cells; however, we did detect significant release from the flagellum, flagellar pocket and hemidesmosome collar of the Δisp1/2/3 parasites which appear identical in appearance to the L. donovani exosomes and is not present in WT L. major. While it is unlikely that altered differentiation in the Δisp1/2/3 parasites results in upregulated release of vesicles via the exosome pathway, excessive secretion may arise as the parasites try to dispose of surplus or mis-trafficked intracellular material accumulating in their flagellar pockets due to erroneous flagellum homeostasis.
The findings of this study clearly show that ISP1 has an endogenous role in Leishmania promastigotes, although as yet its exact function in the parasite's homeostasis is not clear. We propose that ISP1 is involved in flagellum homeostasis and that its loss results in flagellar pocket distension. Together these culminate in excessive secretion and stress response-induced differentiation. This supports recent reports that the flagellum is a sensory organelle promoting morphological changes during trypanosomatid differentiation (Rotureau et al., 2009) and host–parasite interactions in amastigotes (Gluenz et al., 2010b). Further investigation of the Δisp1/2/3 parasites will aid understanding of flagellum homeostasis as well as the Leishmania exosomal secretory pathways.
Experimental procedures
Parasites
Leishmania major Friedlin (MHOM/JL/80/Friedlin) were grown as promastigotes in modified Eagle's medium (designated HOMEM medium) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS) at 25°C as described previously (Besteiro et al., 2006). Gene deletion mutants were selected with 50 µg ml−1 hygromycin B (Roche), 20 µg ml−1 phleomycin (Invivogen), 10 µg ml−1 blasticidin (Merck), 100 µg ml−1 nourseothricin (Sigma) and 50 µg ml−1 puromycin (Calbiochem). 25 µg ml−1 G418 (Invitrogen) was used for re-expressing lines. Metacyclic promastigotes were isolated from stationary-phase culture by agglutination of promastigotes with peanut lectin as previously described (Sacks et al., 1985).
Generation of L. major ISP mutants
Leishmania major triple ISP null mutants (designated Δisp1/2/3) were generated from Δisp2/3 parasites (Eschenlauer et al., 2009). The two plasmids containing the antibiotic resistance cassettes used for the double allele deletion of ISP1 were produced as follows. The 480 bp 5′ flanking region (FR) was generated by PCR with primers OL1434 (CGAAGCTTTAAGCACTTAAAGCCGTGGACG) and OL1435 (TAGTCGACGATGGGAATGTGGGTTCGGTTA). The 546 bp 3′ FR was generated with PCR primers OL1436 (TACCCGGGACATCTGCTTTCTAGCTCGCTC) and OL1437 (GCAGATCTGGTCAGTGTGGAGGTGAAGAGG). The PCR fragments were subcloned into pGEM-T and then released by restriction digest with HindIII/SalI for the 5′ FR and XmaI/BglII for the 3′ FR. The fragments were sequentially cloned into a similarly digested hygromycin-resistant plasmid pGL792 (Besteiro et al., 2004). To produce blasticidin- and nourseothricin-resistant plasmids, the hygromycin cassette was replaced with the SpeI/BamHI resistance cassettes from pGL 434 and pGL227 to give plasmids pGL1027 and pGL1028. The integration cassettes were digested from the plasmids with BglII/HindIII prior to transfection. For the re-expression of ISP1, a 440 bp PCR fragment containing ISP1 ORF was amplified from L. major genomic DNA with the primers OL1474 (CGAGATCTTCATACTGCAAGATCGAGGCC) and OL1475 (ATGCGGCCGCCTCACTCCGTGGCTGCCTGCATC). The PCR fragment was subcloned into pGEM-T and the insert then cloned into the pRIB expression vector using BglII and NotI to give pGL1229. The re-expression construct for ISP2-ISP3 is pGL1005 as previously described (Eschenlauer et al., 2009). The integration cassettes from pGL1229 and pGL1005 were excised by digestion with PacI and PmeI before transfection. L. major promastigotes were electroporated and selected as previously described (Eschenlauer et al., 2009). To generate triple add-back parasites, Δisp1/2/3:ISP2-3 parasites were transfected with an episomal copy of ISP1 (pGL1003) which was generated by amplifying ISP1 from genomic DNA with the primers OL1457 (TACCCGGGATGTCATACTGCAAGATCGAG) and OL1316 (GCGGATCCTCATCCGTGGCTGCCTGCATC), digesting with XmaI/BamHI and ligating into the similarly digested pXG vector. The same construct was used to generate pXG-ISP1-overexpressing parasites by transfection into WT parasites. ISP2-overexpressing parasites (pXG-ISP2) were generated by amplifying ISP2 from genomic DNA with the primers OL1458 (TACCCGGGATGTCCGACGCCGCTGGAAAG) and OL1318 (GCGGATCCTCACATCTCCCTTGCCTTGGTG) using the same methodology. Southern blots were performed as previously described (Eschenlauer et al., 2009).
Macrophage infections
Macrophage infections were performed as previously described (Eschenlauer et al., 2009). Briefly, elicited peritoneal macrophages from BALB/c mice were infected at a 5:1 ratio with stationary-phase promastigotes, for 3 h in RPMI supplemented with 0.1% bovine serum albumin (BSA). For the survival assays, after 3 h the extracellular parasites were removed by extensive washing and the culture was placed at 37°C in RPMI-FCS medium overnight, before fixation and staining. RAW macrophages were infected at a 1:10 ratio with stationary-phase promastigotes and incubated at 37°C for 5 days prior to preparation for TEM microscopy. For each sample, 107 macrophages were infected and the cells were gently lifted from flasks by the addition of cold PBS and gentle agitation prior to fixing as detailed below.
Immunofluorescence analyses
For immunolocalization of ISP1 and ISP2, parasites and slides were prepared as previously described (Ambit et al., 2011). Freshly affinity-purified primary antibodies were used at a 1:50 (v/v) dilution in 0.1% (v/v) Triton X-100, 0.1% (w/v) BSA, in PBS (designated TB) and slides were incubated for 2 h at room temperature. The slides were washed and incubated with donkey anti-sheep Alexa Fluor 488-conjugated antibody (Invitrogen) at 1:2000 (v/v) in TB for 1 h at room temperature. VectaShield Mounting Medium with DAPI (Vector Laboratories) was then applied to the slide. Imaging was performed using a DeltaVision RT epifluorescent microscope under a ×100 objective and images were captured with a CoolSNAP HQ camera.
Electron microscopy
For TEM, promastigotes were washed in PBS and resuspended in 250 mM trehalose. They were applied to a Formvar/carbon-coated grid and imaging was done at 120 kV in a Zeiss LEO 912 energy filtering transmission electron microscope at 30 eV. For sectioned parasites, promastigotes were fixed by adding a mixture of 2.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer pH 7.4 for 40 min. Subsequent processing followed standard methods. Resin sections (100 nm thick) were zero-loss imaged with the Zeiss LEO 912 EFTEM. For SEM parasites were allowed to settle out from culture medium onto poly-l-lysine-coated glass coverslips then briefly rinsed in PBS to remove unattached cells, then fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer. The attached parasites were then osmicated, acetone dehydrated and critical point dried from liquid CO2, sputter-coated with Au/Pd and imaged at 6 kV in a JSEM 6400.
Crossing assay
Assays were performed in Transwell® Permeable Supports (Corning). The assays were performed in complete Leishmania growth-conditioned medium obtained from stationary-phase cultures of wild-type L. major (centrifuged at 1500 g to remove parasites and filtered using 0.2 µm syringe filters). Prior to the assay, 600 µl of filtered conditioned medium was added to each well and plates were allowed to equilibrate at 25°C for 1 h prior to addition of 100 µl of parasites at a density of 5 × 106 ml−1 to the upper chamber. Following incubations of 30 min, 2 h and 20 h, the transwells were removed and the solution in the lower chamber was gently resuspended. The parasites were counted by placing aliquots of the homogenized solution in a haemocytometer.
Ki determination for recombinant ISP1
Expression and purification of recombinant L. major ISP1 (rISP1) has been described previously (Eschenlauer et al., 2009). Ki determination of rISP1 against human trypsin and NE (Calbiochem) was carried out as described previously for rISP2 (Eschenlauer et al., 2009). Briefly, 20 nM NE was pre-incubated with increasing concentrations of rISP1 (10 nM–2 µM) in 100 mM Tris-HCl pH 8.0, 2.5% (v/v) dimethyl sulfoxide (DMSO), for 20 min at 4°C. The reaction was initiated by addition of substrate (300 µM MeOSuc-AAPV-AMC) and enzyme substrate hydrolysis was monitored in a spectrofluorimeter in a continuous assay, by measuring the release of fluorescence (excitation 380 nm, emission at 460 nm). Kiapp values were determined by non-linear regression with GraphPad Prism 5 using the Morrison equation for tight binding inhibitors to fit the curves and the Ki was calculated using the equation: Ki = (Kiapp[S])/([S] + Km), where the Km of NE for MeOSuc-AAPV-AMC, determined experimentally, was 600 µM. Data are the mean ± standard deviation from three independent experiments. The effect of rISP1 on bovine trypsin (Sigma) was determined in a similar manner using 100 nM trypsin, 10 µM rISP1, 50 µM Bz-R-AMC, 2.5% (v/v) DMSO. The enzyme and rISP1 were pre-incubated for 40 min before starting the reaction. rISP2 was used as a positive control and gave 50% inhibition at 0.2 µM, as described previously (Eschenlauer et al., 2009).
Inhibition of sand fly midgut peptidase activity by recombinant ISP1 and ISP2
Recombinant ISP1 and ISP2 (rISP1 and rISP2) were tested for inhibitory effects on the peptidase activity of extracts from the midgut of P. papatasi, a sand fly that is the natural vector for L. major. Sand flies were kept at 25°C in standard conditions as described by Volf and Volfova (2011) and dissected 48 h after bloodfeeding on a BALB/c mouse, when the protease activity in the midgut is at its maximal level. Pools of 10 guts in 100 µl of Tris buffer (20 mM Tris, 150 mM NaCl, pH 7.8) were homogenized, cleared by centrifugation (5000 g, 5 min) and stored at −70°C. The protein concentration of the soluble fraction was determined using the Bradford assay (Bio-Rad). The fly gut extract (0.75 µg−1) was pre-incubated with increasing concentrations of rISP1 (0.7 µM–7 µM) in 100 mM Tris-HCL pH 8.0, 100 mM NaCl, 2.5% (v/v) DMSO, for 5 min at 4°C. For ISP2, the fly gut extract (0.5 µg−1) was pre-incubated with increasing concentrations of rISP2 (2 nM–2 µM) in 100 mM Tris-HCl pH 8, 100 mM NaCl, for 5 min at 4°C. The reactions were initiated by addition of substrate (20 µM Boc-LGR-AMC) and the amount of AMC released was determined by monitoring fluorescence (excitation 380 nm, emission at 460 nm).
Western blot analyses
Promastigote cultures (108 cells) were centrifuged at 1000 g for 10 min, washed with PBS pH 7.2 and resuspended in 100 µl of SDS-PAGE sample buffer [45 mM Tris pH 6.8, 10% (v/v) glycerol, 1% (w/v) SDS, 0.01% bromophenol blue, 50 mM DTT]. The samples were vortexed then boiled for 5 min and 10 µl per sample was loaded on a 15% SDS-PAGE. Proteins were transferred onto Hybond C-Super nitrocellulose membranes (Amersham Pharmacia) and blocked in Tris-buffered saline (TBS) supplemented with 0.05% Tween-20 (TBST) and 5% (w/v) Marvel at 4°C overnight. Affinity-purified primary antibodies to either rISP1 or rISP2 were used at 1:50 (v/v) in TBST with 5% (w/v) Marvel, and purified anti-OPB antibodies were used at 1:20 000 (v/v) (Munday et al., 2011) for 2 h at room temperature. Secondary antibodies, donkey anti-sheep IgG-HRP (Santa Cruz Biotechnology) were used at a 1:10 000 dilution. The reactive bands were detected using ECL Western Blotting Detection Reagent (Amersham).
Acknowledgments
We are grateful to Keith Gull for providing the L. mexicana PFR mutants and Alan Scott for support in protein purification. This work was supported by the Wellcome Trust (Grant number 081877), the Medical Research Council (Grant No. 0700127) and in part by FAPERJ and CNPq to A.P.C.A.L. and M.S.F. A.P.C.A.L. is a CNPq fellow. The Wellcome Trust Centre for Molecular Parasitology is supported by core funding from the Wellcome Trust [085349].
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Analysis of ISP1 and ISP2 expressionin L. mexicana.
Table S1. Statistical analysis of mean flagellar length measurements.
Table S2. Flagellar proteins identified in WT but not snl2 RNAi mutant trypanosomes.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Absalon S, Blisnick T, Bonhivers M, Kohl L, Cayet N, Toutirais G, et al. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J Cell Sci. 2008;121:3704–3716. doi: 10.1242/jcs.035626. [DOI] [PubMed] [Google Scholar]
- Ambit A, Woods KL, Coombs GH, Mottram JC. Morphological events during the cell cycle of Leishmania major. Eukaryot Cell. 2011;10:1429–1438. doi: 10.1128/EC.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin P, Pullen TJ, Sherwin T, Gull K. Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J Cell Sci. 1999;112:3769–3777. doi: 10.1242/jcs.112.21.3769. [DOI] [PubMed] [Google Scholar]
- Besteiro S, Coombs GH, Mottram JC. A potential role for ICP, a leishmanial inhibitor of cysteine peptidases, in the interaction between host and parasite. Mol Microbiol. 2004;54:1224–1236. doi: 10.1111/j.1365-2958.2004.04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteiro S, Williams RAM, Morrison LS, Coombs GH, Mottram JC. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J Biol Chem. 2006;281:11384–11396. doi: 10.1074/jbc.M512307200. [DOI] [PubMed] [Google Scholar]
- Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, et al. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol. 2007;17:778–782. doi: 10.1016/j.cub.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- Chung CH, Ives HE, Almeda S, Goldberg AL. Purification from Escherichia coli of a periplasmic protein that is a potent inhibitor of pancreatic proteases. J Biol Chem. 1983;258:11032–11038. [PubMed] [Google Scholar]
- Cihakova J, Volf P. Development of different Leishmania major strains in the vector sandflies Phlebotomus papatasi and P. duboscqi. Ann Trop Med Parasitol. 1997;91:267–279. doi: 10.1080/00034989761120. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Lane RP. Bloodmeal digestion in the midgut of Phlebotomus papatasi and Phlebotomus langeroni. Med Vet Entomol. 1993;7:225–232. doi: 10.1111/j.1365-2915.1993.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Dostalova A, Votypka J, Favreau A, Barbian K, Volf P, Valenzuela J, et al. The midgut transcriptome of Phlebotomus (Larroussius) perniciosus, a vector of Leishmania infantum: comparison of sugar fed and blood fed sand flies. BMC Genomics. 2011;12:223. doi: 10.1186/1471-2164-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers CT, Murray IA, Delmar VA, Day AG, Craik CS. The periplasmic serine protease inhibitor ecotin protects bacteria against neutrophil elastase. Biochem J. 2004;379:107–118. doi: 10.1042/BJ20031790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann M, Scholz A, Melzer IM, Schmetz C, Wiese M. Interacting protein kinases involved in the regulation of flagellar length. Mol Biol Cell. 2006;17:2035–2045. doi: 10.1091/mbc.E05-10-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenlauer SC, Faria MS, Morrison LS, Bland N, Ribeiro-Gomes FL, DosReis GA, et al. Influence of parasite encoded inhibitors of serine peptidases in early infection of macrophages with Leishmania major. Cell Microbiol. 2009;11:106–120. doi: 10.1111/j.1462-5822.2008.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria M, Reis FCG, Azevedo-Pereira RL, Morrison LS, Mottram JC, Lima APCA. Leishmania inhibitor of serine peptidase inhibitor 2 prevents TLR4 activation by neutrophil elastase promoting parasite survival in murine macrophages. J Immunol. 2011;186:411–422. doi: 10.4049/jimmunol.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- Gluenz E, Hoog JL, Smith AE, Dawe HR, Shaw MK, Gull K. Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 2010a;24:3117–3121. doi: 10.1096/fj.09-151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluenz E, Ginger ML, McKean PG. Flagellum assembly and function during the Leishmania life cycle. Curr Opin Microbiol. 2010b;13:473–479. doi: 10.1016/j.mib.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Gossage SM, Rogers ME, Bates PA. Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. Int J Parasitol. 2003;33:1027–1034. doi: 10.1016/s0020-7519(03)00142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia JJ, Vasconcelos EJ, Pacheco AC, Araujo-Filho R, Maia AR, Kamimura MT, et al. Intraflagellar transport complex in Leishmania spp. In silico genome-wide screening and annotation of gene function. Genet Mol Res. 2007;6:766–798. [PubMed] [Google Scholar]
- Harder S, Thiel M, Clos J, Bruchhaus I. Characterization of a subunit of the outer dynein arm docking complex necessary for correct flagellar assembly in Leishmania donovani. PLoS Negl Trop Dis. 2010;4:e586. doi: 10.1371/journal.pntd.0000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SR, Lau KW, Hao Z, Broadhead R, Portman N, Huhmer A, et al. Analysis of the trypanosome flagellar proteome using a combined electron transfer/collisionally activated dissociation strategy. J Am Soc Mass Spectrom. 2009;20:167–175. doi: 10.1016/j.jasms.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katta SS, Tammana TVS, Sahasrabuddhe AA, Bajpai VK, Gupta CM. Trafficking activity of myosin XXI is required in assembly of Leishmania flagellum. J Cell Sci. 2010;123:2035–2044. doi: 10.1242/jcs.064725. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R, Molyneux DH, Ashford RW. Leishmania in phlebotomid sandflies. I. Modifications of the flagellum associated with attachment to the mid-gut and oesophageal valve of the sandfly. Proc R Soc Lond B Biol Sci. 1974a;187:409–419. doi: 10.1098/rspb.1974.0085. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R, Molyneux DH, Ashford RW. Ultrastructural observations on the attachment of Leishmania in the sandfly. Trans R Soc Trop Med Hyg. 1974b;68:269. [PubMed] [Google Scholar]
- McConville MJ, Mullin KA, Ilgoutz SC, Teasdale RD. Secretory pathway of trypanosomatid parasites. Microbiol Mol Biol Rev. 2002;66:122–154. doi: 10.1128/MMBR.66.1.122-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux DH, Killick-Kendrick R. Morphology, ultrastructure and life cycles. In: Peters W, Killick-Kendrick R, editors. The Leishmaniases in Biology and Medicine. San Diego, CA: Academic Press; 1987. pp. 121–176. [Google Scholar]
- Munday JC, McLuskey K, Brown E, Coombs GH, Mottram JC. Oligopeptidase B-deficient mutants of Leishmania major. Mol Biochem Parasitol. 2011;175:49–57. doi: 10.1016/j.molbiopara.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Rogers MB, Hilley JD, Dickens NJ, Wilkes JM, Bates PA, Depledge DP, et al. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 2011;21:2129–2142. doi: 10.1101/gr.122945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology. 2002;124:495–507. doi: 10.1017/s0031182002001439. [DOI] [PubMed] [Google Scholar]
- Rogers ME, Ilg T, Nikolaev AV, Ferguson MA, Bates PA. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430:463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Hajmová M, Joshi MB, Sadlova J, Dwyer DM, Volf P, Bates PA. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008;10:1363–1373. doi: 10.1111/j.1462-5822.2008.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotureau B, Morales MA, Bastin P, Spath GF. The flagellum-mitogen-activated protein kinase connection in Trypanosomatids: a key sensory role in parasite signalling and development? Cell Microbiol. 2009;11:710–718. doi: 10.1111/j.1462-5822.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigen changes between non-infective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- Sadlova J, Volf P. Peritrophic matrix of Phlebotomus duboscqi and its kinetics during Leishmania major development. Cell Tissue Res. 2009;337:313–325. doi: 10.1007/s00441-009-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Anna MR, Diaz-Albiter H, Mubaraki M, Dillon RJ, Bates PA. Inhibition of trypsin expression in Lutzomyia longipalpis using RNAi enhances the survival of Leishmania. Parasit Vectors. 2009;2:62. doi: 10.1186/1756-3305-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santrich C, Moore L, Sherwin T, Bastin P, Brokaw C, Gull K, et al. A motility function for the paraflagellar rod of Leishmania parasites revealed by PFR-2 gene knockouts. Mol Biochem Parasitol. 1997;90:95–109. doi: 10.1016/s0166-6851(97)00149-7. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, et al. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008;9:R35. doi: 10.1186/gb-2008-9-2-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang C, et al. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- Stegmayer C, Kehlenbach A, Tournaviti S, Wegehingel S, Zehe C, Denny P, et al. Direct transport across the plasma membrane of mammalian cells of Leishmania HASPB as revealed by a CHO export mutant. J Cell Sci. 2005;118:517–527. doi: 10.1242/jcs.01645. [DOI] [PubMed] [Google Scholar]
- Stierhof Y-D, Ilg T, Russell DG, Hohenberg H, Overath P. Characterization of polymer release from the flagellar pocket of Leishmania mexicana promastigotes. J Cell Biol. 1994;125:321–331. doi: 10.1083/jcb.125.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria EL, de Araujo AP, Secundino NF, d'Avila-Levy CM, Traub-Cseko YM. Trypsin-like serine proteases in Lutzomyia longipalpis – expression, activity and possible modulation by Leishmania infantum chagasi. PLoS ONE. 2010;5:e10697. doi: 10.1371/journal.pone.0010697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011;36(Suppl. 1):S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- Volf P, Hajmova M, Sadlova J, Votypka J. Blocked stomodeal valve of the insect vector: similar mechanism of transmission in two trypanosomatid models. Int J Parasitol. 2004;34:1221–1227. doi: 10.1016/j.ijpara.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wiese M, Kuhn D, Grunfelder CG. Protein kinase involved in flagellar-length control. Eukaryot Cell. 2003;2:769–777. doi: 10.1128/EC.2.4.769-777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Bates MD, Dostalova A, Jecna L, Dillon RJ, Volf P, et al. Stage-specific adhesion of Leishmania promastigotes to sand fly midguts assessed using an improved comparative binding assay. PLoS Negl Trop Dis. 2010;4:e816. doi: 10.1371/journal.pntd.0000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SQ, Wang CI, Gillmor SA, Fletterick RJ, Craik CS. Ecotin: a serine protease inhibitor with two distinct and interacting binding sites. J Mol Biol. 1998;279:945–957. doi: 10.1006/jmbi.1998.1748. [DOI] [PubMed] [Google Scholar]
- Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


