Abstract
Objective
Though the prevalence of COPD is related to the definition, even with this proviso COPD remains under-diagnosed. Screening can detect many new COPD cases, but its effects on smoking cessation remain unknown.
Design
To evaluate symptoms in “healthy” cigarette smokers, to screen new COPD cases using international and national guidelines, and to assess the success of a smoking cessation.
Subjects
Healthy asymptomatic smokers with a >20 pack-years smoking history were recruited. The first visit included a standardized personal interview, Fagerstom nicotine dependence test (FNDT) and individualized smoking counselling by Motivational Interviewing. At the follow-up visit two years later, the same analyses were repeated and smoking status assessed. To avoid bias in the counselling attributable to spirometry, the test was evaluated at the two-year follow-up assessment.
Results
Almost all, 93.2%, of 584 participants attended the second visit. Spirometry revealed COPD by GOLD criteria in 11.0% and by national guidelines in 15.3%, mid-expiratory flow (MEF50) had significantly declined in 19.5%, chronic cough or sputum production was detected in 62% of the subjects. After two years, 23.3% had succeeded in giving up smoking. There were four predictors of successful quitting, i.e. positive attitude to the intervention, pharmacotherapy, older age, and higher BMI, whereas other factors such as cough, obstruction, gender, pack-years, or nicotine dependence showed no association with ability to achieve successful cessation.
Conclusion
Significant numbers of “healthy” smokers experience symptoms, according to detailed questionnaires, and have COPD. Motivation is the most significant factor in determining the chance of stopping smoking.
Key Words: COPD, GOLD, Motivational Interviewing, screening, smoking counselling, spirometry
Screening can detect many new COPD cases, but its effects on smoking cessation and disease progression are unknown.
Significant numbers of “asymptomatic” smokers (n = 544) with a >20-year smoking history actually experienced symptoms, >10% had COPD.
One individualized counselling using the technique of Motivational Interviewing combined with pharmaceutical therapies achieved 23% success in smoking cessation.
The rate of success displayed a clear association with motivation and with pharmacotherapy but not with the extent of airway obstruction.
It is well known that the prevalence of chronic obstructive pulmonary disease (COPD) is related to which definition/classification is in use [1], but nonetheless COPD is under-diagnosed. There is wide debate not only about the best COPD definition, but especially on the role of screening of COPD [2–4]. Globally the prevalence and mortality of COPD is predicted to increase [5], and thus there is an urgent need to identify efficient ways for smoking cessation.
COPD is internationally classified by GOLD criteria (Global Initiative for Chronic Obstructive Lung Disease) available from http://www.goldcopd.com, (see also Rabe et al. [6]). The fixed FEV1/FVC limit in the GOLD criteria can cause COPD over-diagnosis in older individuals [7,8], though there are also results indicating that GOLD criteria can also identify at-risk patients in this population [6,9]. It is virtually impossible to adapt international post-bronchodilator reference values, since earlier databases do not contain post-bronchodilator investigations [10]. In addition, GOLD Stage 0, i.e. those subjects with chronic cough and sputum production but a normal FEV1/FVC ratio, have been omitted from the newest GOLD guidelines [11–13]. On the other hand, recent results suggest that symptoms such as chronic cough and sputum production may precede COPD development and/or be associated with increased mortality [14–17]. The evaluation of mid-expiratory flow (MEF 50) may reflect early COPD but it is subject to extensive variability, it is effort dependent, and its association with other spirometric values or symptoms characteristic for COPD is unknown. Despite the problems in MEF assessment [18], it has been found to have significance at least in the differentiation between asthma and COPD [19].
COPD screening is not recommended in the US Preventive Services Task Force (USPSTF) Guidelines [2], largely because of concerns about the cost/benefits/harms in screening for COPD (time and effort required by both patients and the healthcare system, false positive screening tests, and adverse effects of subsequent unnecessary therapy). However, this has to be balanced against the huge costs of treating individuals with severe COPD and hospitalizations [20]. The recommendation by USPSTF is in agreement with those of the American College of Physicians [21], but ATS/ERS or GOLD recommendations do not strictly forbid COPD screening ([3], http://www.GOLD.com). Some studies do indicate that success in smoking cessation is higher in those subjects with airway obstruction but these studies have investigated the lung function values before the smoking cessation counselling [22–24]. It has been claimed that those studies are biased because the type of counselling provided may have been dependent on the lung function values themselves [25].
This study was undertaken to focus on the diagnosis of early COPD in a group of smokers who considered themselves as symptom-free and healthy with no diagnosed diseases or medications, and to assess what factors influenced their success in quitting smoking in a two-year prospective follow-up. Spirometry with bronchodilatation was only conducted as part of the second visit, in order to avoid any bias attributable to knowledge of the extent of obstruction on the success of smoking cessation.
Material and methods
The study was conducted in Northern Finland district, where the prevalence of smokers is high, 35.6% [26]. Voluntary smokers who agreed to be followed up for their health state were recruited through a newspaper announcement. The inclusion criteria consisted of smokers who considered themselves as healthy but who had smoked for at least 20 pack-years; none of the subjects had been diagnosed with lung or other chronic diseases, allergies, allergic diseases, or were taking any prescribed medications. Their clinical history was gone through in detail to exclude a previous asthma diagnosis. Originally 584 smokers were recruited, but 5.5% did not attend the follow-up visit, i.e. 15 subjects had moved away from the region, 13 declined to participate in the follow-up visit for miscellaneous reasons, and four had died (one pneumonia, two lung cancers, and one heart disease); eight subjects were not able to perform acceptable spirometry because they were unable to follow the instructions strictly. Thus 544 subjects participated in the study, 331 men and 213 women.
The first visit consisted of the recording of the standardized personal history, assessment of the symptoms (chronic cough, sputum production), test for nicotine dependence (FNDT), and individualized smoking cessation counselling (30 min) by the technique of Motivational Interviewing [27] conducted by the same experienced nurse. This was a non-randomized longitudinal study, i.e. counselling was forwarded to each participant for ethical reasons since the systematic Cochrane review has highlighted the benefits associated with individualized counselling in efforts to stop smoking [28]. Nicotine replacement therapy and bupropion were offered to each participant if not contraindicated. An alternative drug, varenicline, was not on the market at the start of this study. The participants were asked to attend the control visit, which would take place two years later. During the second visit, the same questionnaires were repeated accompanied by more detailed assessment of the symptoms (St George). Each subject underwent flow-volume spirometry. COPD diagnosis was based both on GOLD criteria (FEV/FVC <70% after bronchodilatation) and on Finnish national criteria for obstruction using reference values where FEV/FVC <88% predicted and FEV1 <80% predicted are indicative for obstruction [29]. Mid-expiratory flow (MEF50) was assessed from the flow-volume spirometry. Spirometry was conducted both before and after bronchodilatation (0.4 mg salbutamol); if a greater than 12% increase was observed after salbutamol, detailed history and peak flow follow-up was conducted (2 weeks) with sympathomimetic therapy to exclude asthma. In all, 25 smokers had 12% or ≤200 ml increase by bronchodilatation, 17 of them were confirmed to have COPD and five were healthy, one subject with COPD fulfilled asthma criteria as estimated from the PEF recordings. Abstinence from smoking was verified by urine cotinine analyses.
Statistical analyses
The prevalence of COPD classified by lung function values is reported in percentage distributions. The association of lung functioning categories and cough or sputum production with pack-years and nicotine dependence was analysed using the Spearman correlation coefficient. We have used box plots to illustrate the association between age and BMI with smoking cessation status. Analysis of variance was used to evaluate the statistical significance of differences in age and BMI between smoking cessation groups. Since pack-years and the nicotine dependence variable, FNDT, were skewed to the right, differences were summarized using medians and quartiles. Mann–Whitney tests were used to evaluate differences in these variables. We also compared smoking cessation distributions by gender, cough, sputum, lung function categories, and attitude to cessation intervention. A chi-squared test was used to evaluate the statistical significance. McNemar’s test was used to evaluate the significance of the change in the cough and sputum production between the baseline and the two-year control visit. SPSS for Windows version 16.0 (SPSS Inc.) was used for the analysis.
This study was approved by the Ethical Committee of Lapland Central Hospital with written consent being obtained from every subject.
Results
A total of 544 smokers formed the study population; 60.8% were men and 39.2% women. The mean age of the subjects was 53.7 (SD 9.1) years, mean BMI was 27.3 (4.1), and median smoking history was 29.0 (lower quartile 21.1 and upper quartile 38) pack-years.
Sixty (11.0%) subjects had FEV1/FVC <0.7 and 83 (15.3%) subjects had FEV1 <80% predicted. MEF50 was decreased in 19.5% of the subjects. FEV1/FVC (rs = –0.28), FEV1% predicted (Spearman rs = –0.31), and MEF50 (rs = –0.23) displayed a significant correlation with pack-years. These variables were not significantly associated with nicotine dependence (FNDT). Altogether 339 (62%) subjects reported chronic cough or sputum production; 186 had both sputum production and cough. The presence of cough and/or sputum production did not correlate with pack-years or nicotine dependence.
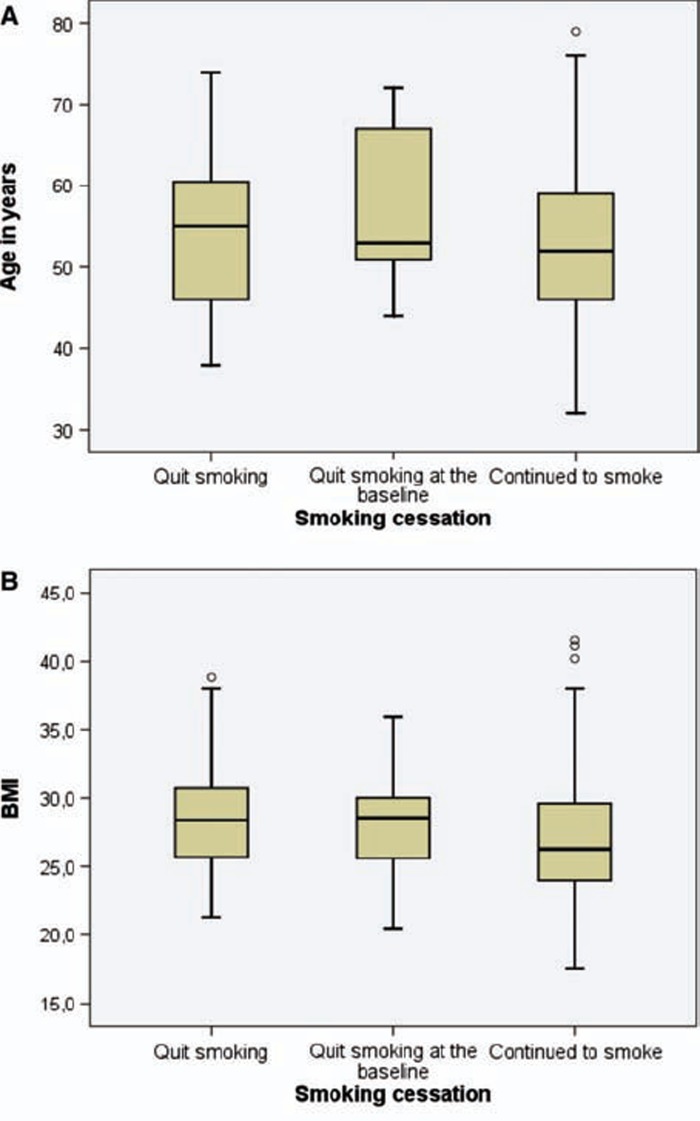
A total of 127 (23.3%) subjects stopped smoking, 93 of them during the two years’ follow-up and 34 after reading the newspaper announcement before attending the first visit or at the time of the baseline investigations. Smoking cessation was statistically significantly associated with age and BMI (Figure 1). Smokers with higher BMI (p < 0.001, analysis of variance) and older age (p < 0.001, analysis of variance) were more likely to quit smoking. A positive attitude towards the intervention strongly predicted smoking cessation (p < 0.001), the effect with pharmacotherapy also being very significant (p < 0.001). The presence of cough did not have any significant effect on the success of smoking cessation (p = 0.073) (Table I).
Figure 1.
Distributions of age and BMI measured during the first visit are shown with box plots according to the smoking status at the two-year control visit. Smokers with higher BMI (p < 0.001, analysis of variance) and older age (p < 0.001, analysis of variance) were more likely to have stopped smoking.
Table I.
Distribution of smoking cessation status by gender, symptoms, attitude to intervention, and use of pharmacological aids to quitting.
| Continued to smoke n (%) | Quit smoking n (%) | Quit smoking at the baseline n (%) | All n (%) | P-value of chi-square test | |
| Gender | |||||
| Men | 246 (74.3) | 60 (18.1) | 25 (7.6) | 331 (100) | 0.181 |
| Women | 171 (80.3) | 33 (15.5) | 9 (4.2) | 213 (100) | |
| Cough | |||||
| Yes | 182 (79.1) | 40 (17.4) | 8 (3.5) | 230 (100) | 0.0731 |
| No | 233 (74.8) | 53 (16.9) | 26 (8.3) | 312 (100) | |
| Sputum | |||||
| Yes | 230 (78.0) | 51 (17.3) | 14 (4.7) | 295 (100) | 0.2881 |
| No | 187 (75.1) | 41 (16.9) | 20 (8.0) | 248 (100) | |
| Pharmacological therapy | |||||
| Yes | 5 (6.9) | 57 (79.2) | 10 (13.9) | 72 (100) | <0.0012 |
| No | 412 (87.6) | 36 (7.6) | 22 (4.8) | 470 (100) | |
| NRT | 3 (5.6) | 42 (77.8) | 9 (16.7) | 54 (100) | 0.3963 |
| Bupropion | 2 (11.1) | 15 (83.3) | 1 (5.6) | 18 (100) | |
| Attitude to cessation intervention | |||||
| Positive | 271 (73.6) | 88 (23.9) | 9 (2.4) | 368 (100) | <0.0014 |
| Negative | 146 (97.3) | 3 (2.0) | 1 (0.7) | 150 (100) | |
| All n (%) | 417 (76.7) | 93 (17.1) | 34 (6.2) | 544 (100) |
Notes: 1Total number of subjects varies due to missing data for some variables;
2two quitters at the baseline missing;
3NRT = nicotine replacement therapy;
4Intervention was not given to 26 cases (24 of these had stopped smoking before this study).
There was no difference in mean (SD) age of starting smoking, i.e. 16.9 (3.6) years for those who continued smoking and 16.9 (2.9) for those who succeeded in quitting (p-value of t-test was 0.973). Pack-years and FNDT values did not significantly differ between subjects who continued smoking compared with those who stopped: median values (lower and upper quartiles) were 29 (20, 38) vs. 30.0 (23,40) (p-value of Mann–Whitney test = 0.077) for pack-years and 3.0 (2, 4) vs 3.0 (2, 4) (p = 0.280) for FNDT. Interestingly, those who managed to stop smoking had similar cough and sputum production (p-value of McNemar’s test = 0.658) at the two-year visit compared with the baseline. Moreover, success in quitting was not associated with the extent of airway obstruction (Table II).
Table II.
Smoking cessation according to COPD GOLD stages and the national Finnish criteria of obstruction and classification of airway limitation by national MEF50 criteria.
| Continued to smoke n (%) | Quit smoking n (%) | Quit smoking at the baseline n (%) | All n (%) | P-value of chi-square test | |
| COPD GOLD criteria | 0.134 | ||||
| Smokers with NLF1 | 241 (73.9) | 58 (17.8) | 27 (8.3) | 328 (100) | |
| Stage 0 (cough and sputum) | 129 (81.6) | 24 (15.2) | 5 (3.2) | 158 (100) | |
| Stage I | 24 (88.9) | 3 (11.1) | 0 (0) | 27 (100) | |
| Stage II -IV | 23 (69.7) | 8 (24.2) | 2 (6.1) | 33 (100) | |
| COPD national criteria | 0.125 | ||||
| No | 359 (77.9) | 77 (16.7) | 25 (5.4) | 461 (100) | |
| Yes | 58 (69.9) | 16 (19.3) | 9 (10.6) | 83 (100) | |
| MEF50 (% of predicted) | 0.758 | ||||
| > / = 62 | 338 (77.2) | 74 (16.) | 26 (5.9) | 438 (100) | |
| 35–61 | 61 (75.3) | 13 (16.0) | 7 (8.6) | 81 (100) | |
| < / = 34 | 18 (72.0) | 6 (24.0) | 1 (40) | 25 (100) | |
| All n (%) | 417 (76.7) | 93 (17.1) | 34 (6.2) | 544 (100) |
Notes: 1NLF = normal hing function; MEF = mean expiratory flow.
Discussion
This study was not powered to assess the prevalence of COPD in healthy smokers since it was directed towards a selected group of subjects. This certainly explains why the success in this relatively long follow-up was high: over 90% attended the second visit and 23% succeeded in quitting smoking. The link between smoking cessation and its association with obstruction examined in this study is not subject to counselling bias since the spirometric evaluation was only conducted on the second visit. It was observed that motivation, pharmacotherapy, higher BMI, and older age were the most significant predictors for quitting smoking.
This study confirms earlier studies that the diagnosis of COPD is dependent on the COPD definition in use and that COPD is under-diagnosed, especially among those who consider themselves as healthy and asymptomatic. However, the higher number of reduced MEF50 values and the number of symptoms mentioned in the detailed questionnaire are evidence that this group of “healthy smokers” contains a significant number of subjects who are at risk of COPD development. This study (two-year follow-up) is too short to be able to determine whether MEF50 or chronic cough leads to COPD. It has to be noted also that middle-aged smokers with normal lung function can have emphysema in high resolution computed tomography (HRCT) in as many as 43% of cases, indicating one additional reason for the under-diagnosis of COPD [30].
Several studies have revealed that many (31–45%) cigarette smokers without a previous COPD diagnosis do in fact suffer from this disease [31–34], indicating that screening should perhaps be directed only at symptomatic smokers, since COPD is so common in this group of smokers. On the other hand, persistent respiratory symptoms can predict worse clinical outcomes even in the group with mild COPD [35]. Asymptomatic smokers may have COPD. For example, two-thirds of the COPD cases did not report chronic productive cough in a recent study conducted in the same district in Finland [26]. At the onset of this study all smokers considered themselves as healthy and asymptomatic, but when they were filling in the detailed questionnaire as many as 62% of the listed symptoms and over 10% of these subjects had COPD (Stage I or more) based on GOLD criteria. The fact that we could identify these cases is crucial in providing counselling at the individual level even though cost/benefit advantages at the population level remain controversial.
The present study, which involved a two-year follow-up, succeeded well in the smoking cessation of chronic smokers with one individualized counselling session combined with anti-smoking drug therapies. This study involved individuals who were concerned about their health; moreover, those who succeeded in smoking cessation were more receptive to the anti-smoking information (96.7%) than those who continued smoking (65.2%). These results are also in agreement with studies where quitting rates can improve by up to 22% if spirometry is combined with education and nicotine replacement therapy [36]. A recent meta-analysis emphasized the importance of smoking cessation counselling in addition to pharmacotherapy in achieving prolonged abstinence [37] while recent Cochrane analysis [38] failed to detect significant effects of behavioural interventions in unselected cigarette smokers. In our study, the smoking cessation counselling followed the technique of Motivational Interviewing [27]. This was combined with pharmacotherapy and was found to be a very effective way to achieve success in smoking cessation in this selected population.
As far as we are aware, our study is the first to include one individualized counselling for smoking cessation, recommending pharmaceutical aids for quitting, and correlating the results from blinded examination of spirometry with the success in quitting in subjects with obstruction. The results of this study differ from those of Bednarek and co-workers [22], who noted that smoking cessation did correlate with the extent of obstruction. In that particular study spirometry was conducted at the beginning of the study and no pharmaceutical aid for smoking cessation was provided.
COPD develops slowly and thus the cost/benefit advantages of screening are difficult to justify. COPD typically includes a number of co-morbidities, which are further worsened by cigarette smoking. However, medical treatment of both COPD and its co-morbidities is improving. Finding motivated smokers for counselling and screening of COPD by microspirometry [31] or spirometry [34] can be judged by the facts that these methods are relatively easy, non-invasive, non-dangerous, and informative. This study reveals the importance of motivation and one efficient individualized counselling combined with pharmacotherapy in smoking cessation, while cough, obstruction, pack-years, and nicotine dependence appear to have no/minor significant roles in determining the success in smoking cessation.
Acknowledgements
The authors would like to thank Helsinki University Hospital and Lapland Central Hospital Funds, Finnish Antituberculosis Association Foundation, Yrjö Jahnsson Foundation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Shirtcliffe P, Weatherall M, Marsh S, Travers J, Hansell A, McNaughton A, et al. COPD prevalence in a random population survey: A matter of definition. Eur Respir J. 2007;30:232–9. doi: 10.1183/09031936.00157906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Screening for chronic obstructive pulmonary disease using spirometry: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;148:529–34. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 4.Enright P. Does Screening for COPD by primary care physicians have the potential to cause more harm than good? Chest. 2006;129:833–5. doi: 10.1378/chest.129.4.833. [DOI] [PubMed] [Google Scholar]
- 5.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabe KF, Beghe B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175:1222–32. doi: 10.1164/rccm.200704-586UP. [DOI] [PubMed] [Google Scholar]
- 7.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20:1117–22. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 8.Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006;173:1316–25. doi: 10.1164/rccm.200601-023OC. [DOI] [PubMed] [Google Scholar]
- 9.Mannino DM, Sonia BA, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: What defines abnormal lung function? Thorax. 2007;62:237–41. doi: 10.1136/thx.2006.068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. COPD. 2006;3:95–100. doi: 10.1080/15412550600651552. [DOI] [PubMed] [Google Scholar]
- 11.Mannino DM. GOLD Stage 0 COPD: Is it real? Does it matter? Chest. 2006;130:309–10. doi: 10.1378/chest.130.2.309. [DOI] [PubMed] [Google Scholar]
- 12.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 13.Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329–32. doi: 10.1164/rccm.2112048. [DOI] [PubMed] [Google Scholar]
- 14.Medbo A, Melbye H. What role may symptoms play in the diagnosis of airflow limitation? A study in an elderly population. Scand J Prim Health Care. 2008;26:92–8. doi: 10.1080/02813430802028938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Marco R, Accordini S, Cerveri I, Corsico A, Anto JM, Kunzli N, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32–9. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 16.Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Nilsson PM, Lofdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res. 2005;6:98. doi: 10.1186/1465-9921-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, Lundback B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127:1544–52. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 18.Van den Bemt L, Schermer T, van Weel C. Rational monitoring of COPD: Where do current clinical guidelines stand? Eur Respir J. 2007;29:1078–81. doi: 10.1183/09031936.00043107. [DOI] [PubMed] [Google Scholar]
- 19.Goedhart DM, Zanen P, Lammers JW. Relevant and redundant lung function parameters in discriminating asthma from COPD. COPD. 2006;3:33–9. doi: 10.1080/15412550500493261. [DOI] [PubMed] [Google Scholar]
- 20.Mannino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 21.Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: A randomised clinical trial. Respir Med. 2006;100:2012–17. doi: 10.1016/j.rmed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Bednarek M, Gorecka D, Wielgomas J, Czajkowska-Malinowska M, Regula J, Mieszko-Filipczyk G, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869–73. doi: 10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorecka D, Bednarek M, Nowinski A, Puscinska E, Goljan-Geremek A, Zielinski J. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123:1916–23. doi: 10.1378/chest.123.6.1916. [DOI] [PubMed] [Google Scholar]
- 24.Stratelis G, Molstad S, Jakobsson P, Zetterstrom O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care. 2006;24:133–9. doi: 10.1080/02813430600819751. [DOI] [PubMed] [Google Scholar]
- 25.Kotz D, van Schayck CP, Huibers MJ, Wesseling GJ. Assessing the efficacy of spirometry for smoking cessation. Thorax. 2007;62:74. [PMC free article] [PubMed] [Google Scholar]
- 26.Kotaniemi J-T, Sovijarvi A, Lundback B. Chronic obstructive pulmonary disease in Finland: Prevalence and risk factors. COPD. 2005;2:331–9. doi: 10.1080/15412550500218122. [DOI] [PubMed] [Google Scholar]
- 27.Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from Motivational Interviewing across behavioral domains: A systematic review. Addiction. 2001;96:1725–42. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2000:CD001292. doi: 10.1002/14651858.CD001292. [DOI] [PubMed] [Google Scholar]
- 29.Viljanen AA, Halttunen PK, Kreus KE, Viljanen BC. Spirometric studies in non-smoking, healthy adults. Scand J Clin Lab Invest Suppl. 1982;159:5–20. [PubMed] [Google Scholar]
- 30.Stratelis G, Fransson SG, Schmekel B, Jakobsson P, Molstad S. High prevalence of emphysema and its association with BMI: A study of smokers with normal spirometry. Scand J Prim Health Care. 2008;26:241–7. doi: 10.1080/02813430802452732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rytila P, Helin T, Kinnula V. The use of microspirometry in detecting lowered FEV1 values in current or former cigarette smokers. Prim Care Respir J. 2008;17:232–7. doi: 10.3132/pcrj.2008.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stratelis G, Jakobsson P, Molstad S, Zetterstrom O. Early detection of COPD in primary care: Screening by invitation of smokers aged 40 to 55 years. Br J Gen Pract. 2004;54:201–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Van Schayck CP, Chavannes NH. Detection of asthma and chronic obstructive pulmonary disease in primary care. Eur Respir J Suppl. 2003;39:16s–22s. doi: 10.1183/09031936.03.00040403. [DOI] [PubMed] [Google Scholar]
- 34.Zielinski J, Bednarek M. Early detection of COPD in a high-risk population using spirometric screening. Chest. 2001;119:731–6. doi: 10.1378/chest.119.3.731. [DOI] [PubMed] [Google Scholar]
- 35.Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD Stage 1 COPD. Thorax. 2008;63:768–74. doi: 10.1136/thx.2007.093724. [DOI] [PubMed] [Google Scholar]
- 36.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 37.Strassmann R, Bausch B, Spaar A, Kleijnen J, Braendli O, Puhan MA. Smoking cessation interventions in COPD: A network meta-analysis of randomised trials. Eur Respir J. 2009;34:634–40. doi: 10.1183/09031936.00167708. [DOI] [PubMed] [Google Scholar]
- 38.Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2009:CD003999. doi: 10.1002/14651858.CD003999.pub3. [DOI] [PubMed] [Google Scholar]