Abstract
Objective
To describe the management of dyslipidaemia in patients with high risk of cardiovascular disease (CVD) and patients with a history of CVD identified by screening for diabetes in general practice in Denmark, concentrating on prescription of lipid-lowering drugs. Moreover, to analyse predicting factors for starting lipid-lowering drugs related to patient and general practice characteristics.
Design
Population-based cross-sectional study with follow-up.
Setting
A total of 139 general practices from three of five Danish regions, totalling 216 GPs.
Subjects
The study population comprised 4986 patients with a high risk of CVD and dyslipidaemia and 764 patients with a history of CVD and dyslipidaemia out of a population of 16 572 patients who completed screening for diabetes but were cleared for diabetes in the ADDITION study.
Results
Of patients with a high risk of CVD and dyslipidaemia not receiving lipid-lowering drugs at the time of screening (n = 4823), 20% started lipid-lowering therapy within the follow-up period (median 2.1 years). This percentage was 45% (n = 536) for patients with CVD and dyslipidaemia (median follow-up period 1.6 years). Age over 50, high cholesterol, impaired fasting glucose and/or impaired glucose tolerance, minor polypharmacy, use of heart/circulation drugs, and cholesterol measurements after screening predicted the prescription of lipid-lowering drugs for patients at high risk of CVD. For patients with CVD, male gender, high cholesterol and use of heart/circulation drugs predicted the prescription of lipid-lowering drugs. No general practice characteristics were associated with different prescription habits.
Conclusion
There is a gap between the recommended lipid-lowering drug therapy and current practice, with a substantial under-treatment and a considerable delay in the first prescription of lipid-lowering drugs.
Key Words: Cardiovascular risk factors, dyslipidaemia, family practice, prevention, screening
Management of dyslipidaemia in general practice is suspected to fall short of best practice.
Patients tients with CVD or high risk of CVD are exposed to a substantial under-treatment of dyslipidaemia in general practice
First prescription of lipid-lowering drugs is prescribed with a considerable delay.
Cardiovascular disease (CVD) is a major cause of mortality and morbidity worldwide and in Denmark [1,2]. Risk factor modification has been shown to reduce CVD mortality, particularly among patients with increased risk of CVD or a history of CVD [3,4]. In recent years, a steady decline in mortality from CVD has been seen in most European countries. Approximately two-thirds of the observed decrease can be ascribed to a decrease in three traditional risk factors: cholesterol level, blood pressure (BP), and smoking. The final third has been obtained by improved treatment of CVD [5–7].
The Danish College of General Practitioners (DSAM) has published guidelines for the prevention of CVD specifically for general practice [8–11]. The guidelines have focused on lipid-lowering drug therapy of patients with CVD or high risk of CVD.
Studies have shown an increase in patients receiving lipid-lowering drugs, but in the Euroaspire I and II studies, almost 50% of the patients with dyslipidaemia were not taking lipid-lowering drugs [12,13]. Taking the potential benefit into account, lipid-lowering drugs are widely underused.
Only few studies have focused on general practice's handling of patients with dyslipidaemia, from identifying the patients to starting lipid-lowering drug therapy [14]. General practitioners (GPs) might be reluctant to start lifelong drug therapy in asymptomatic patients. Conversely, polypharmacy could be a major concern with patients already receiving drug therapy [15]. Patient characteristics have also been suggested to influence drug therapy. Apart from known risk factors, sociodemographic characteristics and indicators of follow-up could be expected to predict drug therapy.
In order to improve our understanding of the management of lipid-lowering drug therapy in general practice, thorough analyses of current practice are necessary.
The aim of the study was to describe the management of dyslipidaemia in patients with a high risk of CVD and patients with CVD identified by screening for diabetes in general practice in Denmark concentrating on the prescription of lipid-lowering drugs. Moreover, to analyse predicting factors for starting lipid-lowering drugs related to patient and general practice characteristics.
Material and methods
Design
The study population was extracted from the ADDITION study, an ongoing international evaluation of screening procedures for type 2 diabetes in general practice [16]. The screening procedure is presented elsewhere [17].
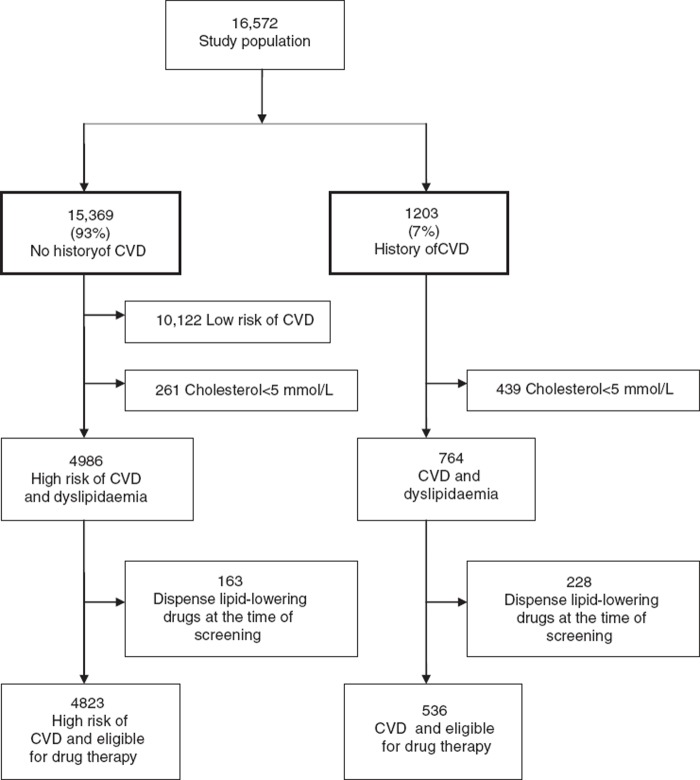
The study population comprised all 16 572 patients between 40 and 69 years of age who completed screening but were cleared for diabetes. Patients without available laboratory data or with a history of liver disease were excluded, since active liver disease is a contraindication of the most common lipid-lowering drugs, statins. Some 15 369 (93%) had no history of CVD and 1203 (7%) had a history of CVD (Figure 1). Patients were included from 2001 to 2006 and followed up until the end of 2006. Median time of follow-up was 4.5 years (5th to 95th interpercentile range: 0.7–5.6) for patients at high risk of CVD and 4.5 years (5th to 95th interpercentile range: 0.8–5.5) for patients with a history of CVD.
Figure 1.
Flow chart of inclusion and exclusion criteria applied to the study population.
Guidelines
The 1998 guideline from DSAM is used in this study and GPs were, when trained in the screening procedure, recommended to use this guideline to assess the CVD risk of their patients. The guidelines are in accordance with European guidelines for prevention of CVD [18]. They provide evidence-based recommendations on how to assess and manage individuals with asymptomatic atherosclerosis, based on their estimated total CVD risk. All necessary data to estimate the patient's CVD risk were obtained in the screening procedure.
The guideline recommends giving lipid-lowering drugs to patients with 20% risk of CVD within 10 years and total cholesterol >5mmol/L. Treatment goals for patients at high risk of CVD are total cholesterol <5mmol/L and LDL <3mmol/L. Lipid-lowering drug therapy is started if the treatment goal is not reached after six months of lifestyle changes.
The guidelines recommended similar treatment goals for patients with a history of CVD, but lipid-lowering drug therapy is started if treatment goals are not reached after three months of lifestyle changes [8]. Using these guidelines, we identified 4823 patients with dyslipidaemia at high risk of CVD who did not receive lipid-lowering drugs at screening, and 536 patients with dyslipidaemia and a history of CVD not taking lipid-lowering drugs.
Data sources
Data concerning patients’ demographic characteristics (gender, age, cohabitation, education, and ethnicity) and smoking status were obtained from questionnaires completed at screening by patients.
Systolic blood pressure (BP), total cholesterol, body mass index (BMI), and impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) were obtained from case record forms completed by GPs at screening.
Data on the GP's organizational data (number of GPs in the practice, GP's age and gender), were obtained from questionnaires completed by GPs. Number of inhabitants registered in the practice's postal code was obtained from the National Health Service. Clinics with postal codes with more than 10 000 inhabitants were classified as urban.
The Danish National Hospital Registry provided data on ICD-10 codes to identify CVD (ICD-10:I20–25, I60–64, I672, I69–70, I74) and liver diseases (ICD10: K70–77, C22). Prescription data were obtained from the Danish Prescription Database. Data on blood tests were obtained from the regional laboratory databases. Statistics Denmark provided demographic data on death and patients who had moved, that prevented their blood tests being registered in this study. Statistics Denmark connected all data using the unique civil registry number assigned to all Danish citizens.
Outcome measures
Outcomes assumed to influence the first prescription were polypharmacy, especially antihypertensive drug therapy and psychopharmaca [15], and number of blood tests. The extent of poly-pharmacy was assessed by prescription patterns three months prior to lipid-lowering drug therapy (mean time to lipid-lowering drug therapy in the medicated group was used in the non-medicated group).
Polypharmacy was assessed as the number of different prescriptions dispensed and categorized: 0–1, 2–4 (minor polypharmacy) and >5 (major polypharmacy) [19]. Antihypertensive drug therapy was assessed as drugs for heart/circulation disorders: atc classification: “CO”. Psychopharmaca was assessed as antipsychotic/antidepressive drugs: atc “NO5” or “NO6”. Furthermore, calculations were made for 11 of the other 14 medication groups with more than 100 prescriptions.
Repeating cholesterol measurements within six months after screening was used as a proxy for follow-up.
Statistics
Data are presented in percentages. Comparisons between the medicated and the non-medicated group were performed using the chi-squared test. Multiple logistic regression was used to determine factors associated with drug therapy. For variables concerning GPs, cluster analyses were done.
Ethics
The Scientific Ethics Committee and the Danish Data Protection Agency approved the ADDITION study.
Results
Of 4986 patients with high risk of CVD and dyslipidaemia (see Figure 1), 163 (3%) dispensed lipid-lowering drugs at the time of screening, and 1263 (25%) dispensed antihypertensive drugs. Of 4823 patients not receiving lipid-lowering drugs at screening, 942 (20%) started lipid-lowering drugs within follow-up. Median time to drug therapy was 2.1 years (5th to 95th interpercentile range: 0.1–4.6).
Of 764 patients with CVD and dyslipidaemia, 228 (30%) dispensed lipid-lowering drugs at the time of screening, and 507 (66%) dispensed antihypertensive drugs. Some 536 did not dispense lipid-lowering drugs despite CVD and dysplipidaemia; 242 (45%) started lipid-lowering drugs within follow-up. Median time to drug therapy was 1.6 years (5th to 95th interpercentile range: 0.1–4.3).
Medicated and non-medicated patients were compared with regard to factors possibly predicting treatment. Age >50 years, high cholesterol, IFG/ IGT, minor polypharmacy, prescriptions of heart/ circulation drugs, and cholesterol measurements after screening predicted drug therapy for patients at high risk of CVD (Table I). For patients with CVD, male gender, high cholesterol, and prescriptions of heart/circulation drugs predicted drug therapy (Table II). A total of 2130 (40%) of patients with high risk of CVD or CVD were not followed up, with neither lipid-profile measurements within six months after screening nor drug therapy in the follow-up-period.
Table I.
Factors predicting lipid-lowering drug therapy among patients with high risk of CVD.
| High risk of CVD |
||||||||
| Medicated n = 942 |
Non-medicated n = 3881 |
|||||||
| % | n | % | n | ORCrude | 95 % CI | ORAdjusted | 95 % CI | |
| Sex | ||||||||
| Male | 80 | 752 | 90 | 3491 | 1 | 1 | ||
| Female | 20 | 190 | 10 | 390 | 2.26* | 1.87–2.74 | 1.03 | 0.78–1.35 |
| Age | ||||||||
| < 50 | 10 | 98 | 14 | 542 | 1 | 1 | ||
| > 50 | 90 | 844 | 86 | 3339 | 1.40* | 1.11–1.76 | 1.48* | 1.12–1.97 |
| Systolic blood pressure | ||||||||
| < 140 | 32 | 290 | 41 | 1545 | 1 | 1 | ||
| 140–160 | 44 | 406 | 43 | 1641 | 1.27* | 1.08–1.49 | 1.07 | 0.89–1.30 |
| 160–180 | 20 | 181 | 14 | 540 | 1.72* | 1.40–2.11 | 1.05 | 0.81–1.36 |
| > 180 | 4 | 38 | 2 | 74 | 2.63* | 1.75–3.97 | 1.09 | 0.64–1.85 |
| Total cholesterol | ||||||||
| < 6 | 16 | 146 | 37 | 1422 | 1 | 1 | ||
| 6–7 | 37 | 346 | 45 | 1747 | 1.78* | 1.45–2.17 | 1.93* | 1.55–2.42 |
| 7–8 | 33 | 4309 | 16 | 609 | 4.55* | 3.68–5.63 | 5.55* | 4.31–7.13 |
| > 8 | 14 | 126 | 2 | 82 | 13.77* | 9.98–19.01 | 19.84* | 13.46–29.22 |
| Smoking status | ||||||||
| Non-smoker | 50 | 471 | 51 | 1971 | 1 | 1 | ||
| Smoker | 50 | 471 | 49 | 1910 | 1.03 | 0.89–1.19 | 0.98 | 0.83–1.20 |
| Body mass index | ||||||||
| < 30 | 71 | 670 | 75 | 2900 | 1 | 1 | ||
| > 30 | 29 | 272 | 25 | 981 | 0.83* | 0.71–0.98 | 0.96 | 0.79–1.16 |
| IFG and/or IGT | ||||||||
| No | 81 | 674 | 91 | 3118 | 1 | 1 | ||
| Yes | 19 | 160 | 9 | 321 | 2.31* | 1.87–2.84 | 2.68* | 2.11–3.39 |
| Higher education | ||||||||
| No | 19 | 175 | 17 | 625 | 1 | 1 | ||
| Short | 50 | 452 | 58 | 1866 | 0.90 | 0.75–1.07 | 1.09 | 0.88–1.37 |
| Long | 30 | 273 | 33 | 1213 | 0.83 | 0.68–1.02 | 1.03 | 0.81–1.31 |
| Ethnicity | ||||||||
| Danish | 90 | 845 | 88 | 3408 | 1 | 1 | ||
| Other | 10 | 97 | 12 | 473 | 1.21 | 0.96–1.52 | 1.15 | 0.88–1.52 |
| Cohabiting | ||||||||
| Single | 19 | 181 | 17 | 668 | 1 | 1 | ||
| Cohabiting | 81 | 758 | 83 | 3186 | 0.87 | 0.73–1.05 | 0.99 | 0.79–1.24 |
| Polypharmacy | ||||||||
| 0–1 | 48 | 452 | 70 | 2724 | 1 | 1 | ||
| 2–4 | 39 | 366 | 24 | 918 | 2.41* | 2.05–2.81 | 1.35* | 1.07–1.71 |
| > 5 | 13 | 124 | 6 | 239 | 3.11* | 2.45–3.95 | 1.27 | 0.88–1.84 |
| Heart/circulation drugs | ||||||||
| 0 | 52 | 489 | 79 | 3053 | 1 | 1 | ||
| 1 | 21 | 197 | 11 | 439 | 2.80* | 2.31-3.40 | 2.41* | 2.11-3.47 |
| > 1 | 27 | 256 | 10 | 389 | 4.11* | 3.42–4.94 | 4.07* | 3.07–5.42 |
| Psychopharmaca | ||||||||
| 0 | 85 | 804 | 90 | 3492 | 1 | 1 | ||
| 1 | 7 | 62 | 5 | 190 | 1.42* | 1.05–1.91 | 1.00 | 0.70–1.44 |
| > 1 | 8 | 76 | 5 | 199 | 1.66* | 1.26–2.18 | 1.06 | 0.72–1.54 |
| Number of cholesterol measurements | ||||||||
| 0 | 39 | 367 | 51 | 1978 | 1 | 1 | ||
| > 0 | 61 | 575 | 49 | 1903 | 1.63 | 1.41–1.88 | 1.65* | 1.39–1.96 |
Note: *Statistically significant difference between the medicated and the non-medicated group.
Table II.
Factors predicting lipid-lowering drug therapy among CVD patients.
| CVD |
||||||||
| Medicated n = 242 |
Non-medicated n = 294 |
|||||||
| % | n | % | n | ORCrude | 95 % CI | ORAdjusted | 95 % CI | |
| Sex | ||||||||
| Male | 63 | 153 | 56 | 164 | 1 | 1 | ||
| Female | 37 | 89 | 44 | 130 | 0.73 | 0.52–1.04 | 0.65* | 0.43–0.96 |
| Age | ||||||||
| < 50 | 6 | 15 | 6 | 19 | 1 | 1 | ||
| > 50 | 94 | 227 | 94 | 275 | 1.05 | 0.52–2.10 | 1.35 | 0.59–3.06 |
| Systolic blood pressure | ||||||||
| < 140 | 42 | 102 | 54 | 159 | 1 | 1 | ||
| 140–160 | 40 | 97 | 35 | 103 | 1.42 | 0.98–2.06 | 1.12 | 0.74–1.69 |
| 160–180 | 16 | 38 | 10 | 29 | 1.98* | 1.15–3.40 | 1.28 | 0.70–2.32 |
| > 180 | 0.2 | 5 | 0.7 | 2 | 0.75 | 0.07–8.43 | 0.60 | 0.05–7.19 |
| Total cholesterol | ||||||||
| < 6 | 40 | 95 | 57 | 166 | 1 | 1 | ||
| 6–7 | 44 | 105 | 33 | 95 | 1.93* | 1.33–2.80 | 1.86* | 1.24–2.78 |
| 7–8 | 13 | 31 | 10 | 29 | 1.86* | 1.06–3.28 | 2.23* | 1.20–4.14 |
| > 8 | 4 | 9 | 0.3 | 1 | 15.68* | 1.96–125.64 | 18.20* | 2.13–155.76 |
| Smoking status | ||||||||
| non smoker | 58 | 140 | 65 | 192 | 1 | 1 | ||
| smoker | 42 | 102 | 35 | 102 | 1.37 | 0.97–1.95 | 1.38 | 0.93–2.07 |
| Body mass index | ||||||||
| < 30 | 73 | 176 | 73 | 216 | 1 | 1 | ||
| > 30 | 27 | 66 | 27 | 78 | 0.96 | 0.66–1.41 | 0.89 | 0.58–1.37 |
| IFG and/or IGT | ||||||||
| No | 84 | 204 | 91 | 268 | 1 | 1 | ||
| Yes | 16 | 38 | 9 | 26 | 1.92* | 1.13–3.27 | 1.77 | 1.00–3.15 |
| Higher education | ||||||||
| No | 27 | 63 | 25 | 71 | 1 | 1 | ||
| Short | 50 | 117 | 50 | 140 | 0.97 | 0.65–1.45 | 0.94 | 0.60–1.48 |
| Long | 22 | 52 | 25 | 69 | 0.88 | 0.54–1.41 | 0.89 | 0.52–1.55 |
| Ethnicity | ||||||||
| Danish | 87 | 211 | 82 | 241 | 1 | 1 | ||
| Other | 13 | 31 | 18 | 53 | 1.50 | 0.93–2.42 | 1.24 | 0.73–2.11 |
| Cohabiting | ||||||||
| Single | 22 | 54 | 25 | 74 | 1 | 1 | ||
| Cohabiting | 78 | 187 | 75 | 218 | 1.18 | 0.79–1.76 | 1.22 | 0.78–1.90 |
| Polypharmacy | ||||||||
| 0–1 | 29 | 69 | 48 | 141 | 1 | 1 | ||
| 2–4 | 46 | 111 | 32 | 93 | 2.44* | 1.64–3.63 | 1.49 | 0.89–2.50 |
| > 5 | 26 | 62 | 21 | 60 | 2.11* | 1.34–3.34 | 1.18 | 0.58–2.40 |
| Heart/circulation drugs | ||||||||
| 0 | 40 | 96 | 64 | 187 | 1 | 1 | ||
| 1 | 24 | 59 | 17 | 49 | 2.45* | 1.49–3.68 | 1.92* | 1.11–3.33 |
| >1 | 36 | 87 | 20 | 58 | 2.92* | 1.93–4.42 | 2.80* | 1.57–5.01 |
| Psychopharmaca | ||||||||
| 0 | 79 | 190 | 82 | 240 | 1 | 1 | ||
| 1 | 10 | 24 | 7 | 21 | 1.44 | 0.78–2.67 | 1.02 | 0.51–2.05 |
| > 1 | 11 | 28 | 11 | 33 | 1.07 | 0.63–1.84 | 0.75 | 0.38–1.50 |
| Number of cholesterol measurements | ||||||||
| 0 | 50 | 120 | 52 | 152 | 1 | 1 | 1 | |
| > 0 | 50 | 122 | 48 | 142 | 1.09 | 0.77–1.53 | 1.19 | 0.82–1.74 |
Note: *Statistically significant difference between the medicated and the non-medicated group.
Some 2045 (38%) had their lipid profile measured, but did not start drug therapy. Follow-up with lipid-profile measurements was associated with slightly higher cholesterol level among patients at high risk of CVD. There was no difference in percentage close to treatment goal (<5.5mmol/L) between patients having no blood test and patients having blood tests taken (Table III). No general practice characteristics (gender, age, urban/rural area of clinic, number of GPs in the clinic) were associated with starting drug therapy.
Table III.
Cholesterol levels and percentage close to treatment goal (< 5.5 mmol/L) at screening in the medicated and the non-medicated groups related to follow-up of blood tests.
| Medicated |
Non-medicated |
|||
| Mean mmol/L (95% CI) | < 5.5mmol/L | Mean mmol/L (95% CI) | < 5.5mmol/L | |
| High risk of CVD | n = 942 | n = 3881 | ||
| No blood tests | 6.8* (6.7–6.9) n = 367 | 6% | 6.3* (6.2–6.3) n = 1978 | 13% |
| Blood tests | 7.0* (7.0–7.1) n = 575 | 4% | 6.4* (6.3–6.4) n = 1903 | 12% |
| CVD | n = 242 | n = 294 | ||
| No blood tests | 6.3 (6.1–6.5) n = 120 | 18% | 6.0 (5.9–6.2) n = 152 | 31% |
| Blood tests | 6.2 (6.1–6.4) n = 122 | 20% | 5.9 (5.8–6.0) n = 142 | 32% |
Notes: The population is divided into patients with high risk of CVD and patients with manifest CVD. *Significant statistical difference in mean cholesterol between no blood tests and blood tests.
Discussion
Main results
We identified 4986 patients with high risk of CVD and dyslipidaemia not prescribed lipid-lowering drugs at the time of screening. Some 20% started lipid-lowering drugs during the follow-up-period. Median time to drug therapy was 2.1 years.
Of the 764 patients identified with CVD and dyslipidemia not prescribed lipid-lowering drugs at the time of screening, 45% started drugs during follow-up. Median time to drug therapy was 1.6 years.
Of the investigated predictors, we found age over 50, high cholesterol level, diagnosis of IFG/IGT, minor polypharmacy, use of heart/circulation drugs, and cholesterol measurements after screening to predict drug therapy for patients at high CVD risk. For patients with CVD, male gender, high cholesterol, and two or more prescriptions of heart/circulation drugs, drug therapy was predicted.
Other important findings in this study include the fact that 40% were not followed for their high risk of CVD or CVD with either drug therapy or lipid-measurements and 38% had lipid measurements taken, but did not start drug therapy.
Prescribing pattern
The use of lipid-lowering drug therapy was surprisingly low, taking into account that GPs were explicitly recommended to use the guidelines on prevention of CVD. One explanation could be that patients were identified in connection with screening for diabetes. Elevated risk in complex screening is seen in the context of other results, which, if they prove normal, are often considered more important [20].
Only 25% of patients with CVD and dyslipidaemia were on lipid-lowering drugs at screening. Similarly, other studies have reported drug therapy rates of 27–71% in CVD patients.
Delay in treatment
Guidelines recommend lifestyle changes for six and three months before drug therapy for patients at high risk and with CVD, respectively. Studies report that 43–100% of GPs prescribe lifestyle changes as first-line therapy [21,22]. This indicates that starting lifestyle changes could account for some postponement of drug therapy, but not explain a median of 2.1/1.6 years. Time lags in the adoption of clinical guidelines could account for some delay and under-treatment [23,24].
Predictors for prescribing of lipid-lowering drugs
Backlund et al. showed that GPs use different judgement strategies for lipid-lowering drug prescriptions. CVD has the highest influence on GPs followed by cholesterol levels [25]. Our study supports this, as these factors strongly predict drug therapy. Backlund also showed that a large subgroup of GPs do not include CVD in their judgement [25], which could explain some of the drug therapy insufficiency found in the CVD group.
Our finding that antihypertensive drugs can predict drug therapy could indicate that (1) starting lipid-lowering drugs is easier in already medicated patients, (2) that increasing regimen complexity is not a significant barrier to drug therapy, or (3) that more diseases resulting in frequent visits to the GP provide more opportunities to start preventive drugs [26].
We found no general practice characteristics that significantly predicted drug therapy. This is in accordance with other studies in the field [22,27].
Blood tests
The large group not followed with lipid-profile measurements found in this study could again be due to the context of normal results of diabetes screening [20]. Finding 38% followed with lipid-profile measurements but not starting drugs was unexpected, indicating that some blood testing is done without clinical consequences.
Strengths of the study
Several strengths are present in this study. The follow-up period was long and data on prescriptions and blood tests are from complete databases [28,29]. Moreover, the study reflects real clinical practice not biased by trial set-up and a broad range of clinics participated. The participants were included in connection with screening for diabetes, avoiding selection bias by doctors including patients more likely to accept treatment.
Weaknesses of the study
The main weakness of the study is that the CVD risk was estimated on single measurements of blood pressure and cholesterol performed when screening for diabetes. Variation in blood pressure and cholesterol level by re-examination on another day was not included in the risk estimation, but could have eliminated the estimated high risk of CVD. The data reflect treatment in the period 2001–2006 and it is likely that treatment patterns have changed slightly since then. It has been shown that non-attendees to the screening were less likely to be cohabitant, skilled, or employed making this population slightly selected with regard to sociodemographic parameters [30]. A small group was followed for less than one year, which consequently slightly underestimated the percentage starting drugs. Since family history of CVD is a known risk factor, it would have been interesting to examine whether it is a predictor for drug therapy. Finally, no information on GP–patient communication was obtainable, leaving many unanswered questions about where and why treatment failed to start.
Conclusion
There is a gap between the recommended lipid-lowering drug therapy and current practice with substantial under-treatment with lipid-lowering drugs, and a considerable delay in first prescription of lipid-lowering drugs.
Acknowledgement
The study was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe and South Jutland, together with the Danish Council for Strategic Research, the Danish Research Foundation for General Practice, the Danish Centre for Evaluation and Health Technology Assessment, the diabetes fund of the National Board of Health, the Danish Medical Research Council, the Aarhus University Research Foundation, and the Novo Nordisk Foundation. The study received unrestricted grants from Novo Nordisk, Novo Nordisk Scandinavia, Astra Denmark, Pfizer Denmark, GlaxoSmithKline Pharma Denmark, Servier Denmark and HemoCue Denmark.
References
- 1.Konstantin Nissen N, Rasmussen S. Hjertestatistik 2008 – fokus på køn og sociale forhold [Heart statistics 2008 – focus on gender and socioeconomics] København: Hjerteforeningen; 2008. [Google Scholar]
- 2.Prevention of cardiovascular disease: Guideline for assessment and management of cardiovascular risk. Geneva: World Health Organization; 2007. [Google Scholar]
- 3.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: A network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–81. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): A major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41:105–14. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 6.Madsen M, Rasmussen S, Juel K. Acute myocardial infarction in Denmark. Incidence development and prognosis during a 20-year period. Ugeskr Laeger. 2000;162:5918–23. [PubMed] [Google Scholar]
- 7.Bjorkelund C, Andersson-Hange D, Andersson K, Bengtsson C, Blomstrand A, Bondyr-Carlsson D, et al. Secular trends in cardiovascular risk factors with a 36-year perspective: observations from 38- and 50-year-olds in the Population Study of Women in Gothenburg. Scand J Prim Health Care. 2008;26:140–6. doi: 10.1080/02813430802088403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dansk Selskab for Almen Medicin. Forebyggelse af hjertesygdom i almen praksis – med særligt henblik på dyslipidæmi [Prevention of heart disease in general practice – with particular focus on dyslipidaemia] Copenhagen: Dansk Selskab for Almen Medicin; 1998. [Google Scholar]
- 9.Christensen B. Forebyggelse af iskæmisk hjertekarsygdom i almen praksis [Prevention of ischaemic cardiovascular disease in general practice]. 2nd ed. Copenhagen: Dansk Selskab for Almen Medicin; 2002. [Google Scholar]
- 10.Christensen B. Forebyggelse af iskæmisk hjerte-kar-sygdom i almen praksis [Prevention of ischaemic cardiovascular disease in general practice]. 3rd ed. Copenhagen: Dansk Selskab for Almen Medicin; 2007. [Google Scholar]
- 11.Thomsen T, Christensen B, Hildebrandt P, Iversen HK, Larsen ML, Sillesen H, et al. Kliniske retningslinier for fore-byggelse af kardiovaskulær sygdom i Danmark [Clinical guidelines for prevention of cardiovascular disease in Denmark] Tillæg til Cardiologisk Forum August. 2004 [Google Scholar]
- 12.EUROASPIRE I and II Group, European Action on Secondary Prevention by Intervention to Reduce Events. Clinical reality of coronary prevention guidelines: A comparison of EUROASPIRE I and II in nine countries. EUROASPIRE I and II Group. European Action on Secondary Prevention by Intervention to Reduce Events. Lancet. 2001;357:995–1001. doi: 10.1016/s0140-6736(00)04235-5. [DOI] [PubMed] [Google Scholar]
- 13.Larsen J, Andersen M, Kragstrup J, Gram LF. Changes in the utilisation of lipid-lowering drugs over a 6-year period (1993–1998) in a Danish population. Eur J Clin. Pharmacol. 2001;57:343–8. doi: 10.1007/s002280100307. [DOI] [PubMed] [Google Scholar]
- 14.Carroll K, Majeed A, Firth C, Gray J. Prevalence and management of coronary heart disease in primary care: Population-based cross-sectional study using a disease register. J Public Health Med. 2003;25:29–35. doi: 10.1093/pubmed/fdg007. [DOI] [PubMed] [Google Scholar]
- 15.Shalansky SJ, Levy AR. Effect of number of medications on cardiovascular therapy adherence. Ann Pharmacother. 2002;36:1532–9. doi: 10.1345/aph.1C044. [DOI] [PubMed] [Google Scholar]
- 16.Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G, et al. The ADDITION study: Proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000:24S6–11. doi: 10.1038/sj.ijo.0801420. [DOI] [PubMed] [Google Scholar]
- 17.Christensen JO, Sandbaek A, Lauritzen T, Borch-Johnsen K. Population-based stepwise screening for unrecognised Type 2 diabetes is ineffective in general practice despite reliable algorithms. Diabetologia. 2004;47:1566–73. doi: 10.1007/s00125-004-1496-2. [DOI] [PubMed] [Google Scholar]
- 18.Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of coronary heart disease in clinical practice: Recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Atherosclerosis. 1998;140:199–270. doi: 10.1016/s0021-9150(98)90209-x. [DOI] [PubMed] [Google Scholar]
- 19.Bjerrum L. PhD thesis. Odense: Odense University; 1998. Pharmacoepidemiological studies of polypharmacy: Mmethodological issues, population estimates, and influence of practice patterns. [Google Scholar]
- 20.Bach Nielsen KD, Dyhr L, Lauritzen T, Malterud K. Long-term impact of elevated cardiovascular risk detected by screening: A qualitative interview study. Scand J Prim Health Care. 2005;23:233–8. doi: 10.1080/02813430500336245. [DOI] [PubMed] [Google Scholar]
- 21.Erhardt LR, Hobbs FD. A global survey of physicians’ perceptions on cholesterol management: The From The Heart study. Int J Clin Pract. 2007;61:1078–85. doi: 10.1111/j.1742-1241.2007.01420.x. [DOI] [PubMed] [Google Scholar]
- 22.Midlov P, Ekesbo R, Johansson L, Gerward S, Persson K, Nerbrand C, et al. Barriers to adherence to hypertension guidelines among GPs in southern Sweden: A survey. Scand J Prim Health Care. 2008;26:154–9. doi: 10.1080/02813430802202111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amsterdam EA, Laslett L, Diercks D, Kirk JD. Reducing the knowledge-practice gap in the management of patients with cardiovascular disease. Prev Cardiol. 2002;5:12–5. doi: 10.1111/j.1520-037x.2002.0548.x. [DOI] [PubMed] [Google Scholar]
- 24.Vægter K, Waldorff FB, Kirkegaard J, Kristensen K. Brug af DSAM's kliniske vejledning om forebyggelse af iskæmisk hjertesygdom i almen praksis. Resultater fra Storstrøms Amt [Use of DSAM's clinical guidelines on prevention of ischaemic heart disease in general practice]. Ugeskr Læger. 2000;162:5979–82. [Google Scholar]
- 25.Backlund L, Danielsson B, Bring J, Strender LE. Factors influencing GPs’ decisions on the treatment of hypercholesterolaemic patients. Scand J Prim Health Care. 2000;18:87–93. doi: 10.1080/028134300750018963. [DOI] [PubMed] [Google Scholar]
- 26.Tonstad S, Rosvold EO, Furu K, Skurtveit S. Undertreatment and overtreatment with statins: The Oslo Health Study 2000–2001. J Intern Med. 2004;255:494–502. doi: 10.1111/j.1365-2796.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 27.Heidrich J, Behrens T, Raspe F, Keil U. Knowledge and perception of guidelines and secondary prevention of coronary heart disease among general practitioners and internists: Results from a physician survey in Germany. Eur J Cardiovasc Prev Rehabil. 2005;12:521–9. doi: 10.1097/00149831-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–9. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 29.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register: A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–8. [PubMed] [Google Scholar]
- 30.Dalsgaard E, Lauritzen T, Christiansen T, Mai KS, Borch-Johnsen K, Sandbaek A. Socioeconomic factors related to attendance in a Type 2 diabetes screening programme. Diabet Med. 2009;26:518–25. doi: 10.1111/j.1464-5491.2009.02715.x. [DOI] [PubMed] [Google Scholar]