Abstract
Background
Testosterone supplementation has been shown to increase muscle mass and strength in healthy older men. The safety and efficacy of testosterone treatment in older men who have limitations in mobility have not been studied.
Methods
Community-dwelling men, 65 years of age or older, with limitations in mobility and a total serum testosterone level of 100 to 350 ng per deciliter (3.5 to 12.1 nmol per liter) or a free serum testosterone level of less than 50 pg per milliliter (173 pmol per liter) were randomly assigned to receive placebo gel or testosterone gel, to be applied daily for 6 months. Adverse events were categorized with the use of the Medical Dictionary for Regulatory Activities classification. The data and safety monitoring board recommended that the trial be discontinued early because there was a significantly higher rate of adverse cardiovascular events in the testosterone group than in the placebo group.
Results
A total of 209 men (mean age, 74 years) were enrolled at the time the trial was terminated. At baseline, there was a high prevalence of hypertension, diabetes, hyperlipidemia, and obesity among the participants. During the course of the study, the testosterone group had higher rates of cardiac, respiratory, and dermatologic events than did the placebo group. A total of 23 subjects in the testosterone group, as compared with 5 in the placebo group, had cardiovascular-related adverse events. The relative risk of a cardiovascular-related adverse event remained constant throughout the 6-month treatment period. As compared with the placebo group, the testosterone group had significantly greater improvements in leg-press and chest-press strength and in stair climbing while carrying a load.
Conclusions
In this population of older men with limitations in mobility and a high prevalence of chronic disease, the application of a testosterone gel was associated with an increased risk of cardiovascular adverse events. The small size of the trial and the unique population prevent broader inferences from being made about the safety of testosterone therapy.
Limited mobility is a common geriatric condition that is a predictor of disability, poor quality of life, and death.1–7 In men, an age-related decline in the serum testosterone concentration is associated with reduced muscle mass and lower-extremity strength, limitations in physical function, and poor mobility.8–13 Testosterone supplementation increases muscle mass and strength and leg power, all of which are important determinants of mobility.14–21 Previous trials of testosterone supplementation have been conducted primarily among healthy older men. The safety and efficacy of testosterone treatment in improving muscle performance and physical function in older men with limitations in mobility have not been studied.
The Testosterone in Older Men with Mobility Limitations (TOM) trial was a placebo-controlled, randomized trial that was designed to determine the effects of testosterone administration on lower-extremity strength and physical function in older men with limitations in mobility and low serum levels of total or free testosterone.22 In December 2009, a data and safety monitoring board, established by the National Institute on Aging, determined that the incidence of adverse cardiovascular events in the TOM trial was significantly higher in the testosterone group than in the placebo group. The members of the data and safety monitoring board therefore recommended that enrollment and administration of the study medications be discontinued. We report here the adverse events associated with testosterone therapy in the TOM trial. The effects of testosterone therapy on efficacy outcomes are reported briefly.
Methods
Study Design
The TOM trial was a parallel-group, randomized, placebo-controlled, double-blind trial involving community-dwelling men. The trial was conducted at three recruitment sites: Boston University Medical Center, New England Research Institutes, and the Veterans Affairs Boston Healthcare System. The trial protocol and amendments, as well as the statistical analysis plan, are available with the full text of this article at NEJM.org.
The trial was funded primarily by the National Institute on Aging of the National Institutes of Health under a cooperative agreement. Additional support was provided by the resources of the Boston Claude D. Pepper Older Americans Independence Center and the Boston University Clinical and Translational Science Institute. Testosterone and placebo gels for the study were provided by Auxilium Pharmaceuticals, which had no role in the design or implementation of the study, the analysis or interpretation of the data, or the preparation of the manuscript.
The trial was designed by the authors, and the trial protocol was approved by the institutional review board at each participating center. The design and maintenance of the database and the data collection were supervised by the Boston University School of Public Health Data Coordinating Center. The manuscript was written by the authors, and the decision to submit the manuscript for publication was made jointly by the authors and the trial’s data and safety monitoring board (see the Appendix). The authors vouch for the accuracy and completeness of the data and all analyses.
Study Participants
The study participants were men, 65 years of age or older, who had a total serum testosterone level between 100 and 350 ng per deciliter (3.5 to 12.1 nmol per liter) or a free serum testosterone level of less than 50 pg per milliliter (173 pmol per liter), as measured in a blood sample obtained in the morning. Participants were also required to have evidence of limitations in mobility, defined as having difficulty walking two blocks on a level surface or climbing 10 steps and having a score between 4 and 9 on the Short Physical Performance Battery (which measures performance on a scale of 0 to 12, with higher scores indicating better performance).22 Exclusion criteria were uncontrolled hypertension, unstable angina, myocardial infarction within 3 months before enrollment, New York Heart Association class III or class IV congestive heart failure, prostate or other active cancer, severe lower urinary tract symptoms, untreated severe obstructive sleep apnea, glucocorticoid or anabolic steroid therapy, a glycated hemoglobin level higher than 8.5%, a hematocrit higher than 48%, a prostate-specific antigen level higher than 4 ng per milliliter, and a bodymass index (the weight in kilograms divided by the square of the height in meters) of more than 40. Further details of the trial inclusion and exclusion criteria are provided in Section A in the Supplementary Appendix, available at NEJM.org. All participants provided written informed consent.
Procedures
Participants were stratified according to age (65 to 75 years or older than 75 years) and were randomly assigned to receive 10 g of a transdermal gel containing either placebo or 100 mg of testosterone (Testim 1%,23 Auxilium Pharmaceuticals), to be applied once daily for 6 months. Two weeks after randomization was performed, the dose was adjusted if the average of two testosterone measurements was less than 500 ng per deciliter (17.4 nmol per liter), in which case the dose was increased to 15 g daily, or more than 1000 ng per deciliter (34.7 nmol per liter), in which case the dose was decreased to 5 g daily.
The primary efficacy outcome was the change from baseline in maximal voluntary muscle strength in a leg-press exercise.24 Secondary efficacy outcomes included changes from baseline in chest-press strength, 50-m walking speed, stair-climbing speed and power, and a lift-and-lower test.24 Measurements were performed at baseline and at week 24, or at the last visit in the case of participants who discontinued the study medication before 6 months. Testosterone was measured with the use of an immunoassay25 (Quest) with a sensitivity of 10 ng per deciliter (0.35 nmol per liter), and sex hormone–binding globulin was measured with the use of an immunofluorometric assay15 (Delfia, Wallac [now PerkinElmer]) with a sensitivity of 2.5 nmol per liter; free testosterone was calculated.26 Further details of the trial procedures are provided in Section A in the Supplementary Appendix.
Safety Monitoring
Safety monitoring included measurements of hemoglobin, hematocrit, prostate-specific antigen, and serum chemical levels; a prostate examination; an assessment of urinary tract symptoms; and an assessment of adverse events. Adverse events were categorized by personnel at KAI Research (Rockville, MD), a contract research organization, according to the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class categorization. The data and safety monitoring board reviewed serious adverse events as they occurred and cumulative adverse events every 6 months.
An imbalance in a broad array of adverse cardiac events between the two study groups prompted the data and safety monitoring board to review unblinded data in December 2009 and to request analyses of two categories of adverse events in addition to the MedDRA-classified cardiac events. The first, “cardiovascular-related events,” included MedDRA-classified cardiac events as well as events that the data and safety monitoring board considered to be cardiovascular in nature but that were included in other MedDRA System Organ Class categories: stenting and bypass procedures (included in the MedDRA category of surgical and medical procedures), peripheral edema (included in the category of general disorders), elevated blood pressure, arrhythmias, and electrocardiographic changes (included in the investigations category), and stroke and syncope (included in the category of nervous system disorders). The second analysis included “atherosclerosis-related events,” such as myocardial infarction, sudden death, angioplasty, coronary-artery bypass surgery, and stroke. Further details of the safety monitoring procedures are provided in Section A in the Supplementary Appendix.
Statistical Analysis
We had planned to enroll 252 men in the trial, since we had estimated that with this sample size, the study would have 90% power to show an increase of 245 newtons (N) (25 kg) in bilateral legpress strength with testosterone therapy, with a standard deviation for the treatment effect of 540 N (55 kg), on the basis of previous studies,27 assuming a 20% loss to follow-up and a probability of type I error of 0.05. At the time the analyses of adverse events were performed (December 15, 2009), a total of 209 men had been randomly assigned to a trial group.
The primary analysis reported here was a between-group comparison of the incidence of adverse events among the 209 participants who had been randomly assigned before the termination of the trial. The proportion of subjects in each group with one or more adverse events was calculated for each MedDRA System Organ Class, and these proportions were compared with the use of chi-square tests and Fisher’s exact tests. Estimated odds ratios for events, both unadjusted and adjusted for baseline risk factors, and associated 95% confidence intervals were calculated with the use of logistic regression. The time to the report of the first adverse event or to data censoring was determined for cardiovascular-related events, dermatologic events, and events that necessitated referral for medical evaluation and was compared between the two trial groups with the use of the Kaplan–Meier method and Cox proportional-hazards models. Post hoc sensitivity analyses were performed in which subjects who had specific risk factors at baseline that might have affected the outcomes were excluded. We also performed a limited efficacy analysis for this report, comparing mean changes in efficacy outcomes with the use of two-sample Student’s t-tests. Further details of the statistical methods are provided in Section A in the Supplementary Appendix.
Results
Enrollment of Subjects and Discontinuation of the Study
Enrollment in the trial took place between September 2005 and December 2009. On December 31, 2009, the data and safety monitoring board recommended that the study intervention and enrollment be discontinued, owing to a higher proportion of adverse events in the testosterone group than in the placebo group. Of the target sample of 252 men, 209 had been randomly assigned at the time the analyses of adverse events were performed (December 15, 2009), and were included in the safety analyses (Fig. 1 in the Supplementary Appendix). Of the 209 randomly assigned men, 129 had completed the 6-month intervention period, and an additional 47 had received the study medication for 12 or more weeks and had undergone at least one outcome assessment after randomization. The 176 men with a baseline assessment and at least one outcome assessment were included in the efficacy analyses.
Baseline Characteristics of the Men
The mean age of the men was 74 years. The mean level of total testosterone was 243 ng per deciliter (8.4 nmol per liter), and the mean level of free testosterone was 46 pg per milliliter (160 pmol per liter). The men had substantial limitations in mobility, as indicated by a mean score on the Short Physical Performance Battery of 7.6 and a mean 4-m walking speed of 0.94 m per second (Table 1). Both study groups had a high prevalence of hypertension, obesity, diabetes, hyperlipidemia, and known cardiovascular disease (Table 2). A greater proportion of men in the testosterone group than in the placebo group reported that they had received a diagnosis of hyperlipidemia or were taking a statin.
Table 1.
Baseline Characteristics of the Study Participants.*
| Characteristic | Testosterone Gel (N = 106) |
Placebo Gel (N = 103) |
P Value† |
|---|---|---|---|
| Enrollment site — no. (%) | 0.72 | ||
| Boston University Medical Center | 26 (25) | 30 (29) | |
| New England Research Institutes | 70 (66) | 65 (63) | |
| Veterans Affairs Boston Healthcare System | 10 (9) | 8 (8) | |
| Age — yr | 74±6 | 74±5 | 0.84 |
| Body-mass index‡ | 29.7±4.1 | 30.0±4.2 | 0.58 |
| Race — no./total no. (%)§ | 0.04 | ||
| Black | 15/105 (14) | 4/102 (4) | |
| White | 87/105 (83) | 93/102 (91) | |
| Asian or Pacific Islander | 1/105 (1) | 1/102 (1) | |
| Other¶ | 2/105 (2) | 4/102 (4) | |
| Hispanic or Latino ethnic group — no./total no. (%)§ | 2/97 (2) | 2/88 (2) | 0.99 |
| Testosterone level‖ | |||
| Total — ng/dl | 250±57 | 236±66 | 0.11 |
| Free — pg/ml | 48±12 | 43±14 | 0.003 |
| Score on the Short Physical Performance Battery** | 7.6±1.5 | 7.7±1.4 | 0.74 |
| Measure of strength | |||
| Leg press | 0.51 | ||
| No. who performed the test | 75 | 69 | |
| Force — newtons | 1947±430 | 1991±365 | |
| Chest press | 0.74 | ||
| No. who performed the test | 66 | 62 | |
| Force — newtons | 424±112 | 418±102 | |
| Dominant-hand grip | 0.46 | ||
| No. who performed the test | 97 | 92 | |
| Force — kg | 27.3±6.7 | 26.6±7.4 | |
| 50-m walking test | |||
| Without a load | 0.64 | ||
| No. who performed the test | 84 | 79 | |
| Speed — m/sec | 1.62±0.40 | 1.60±0.37 | |
| With a load | 0.99 | ||
| No. who performed the test | 54 | 49 | |
| Speed — m/sec | 1.63±0.34 | 1.63±0.36 | |
| Stair-climbing test | |||
| Without a load | 0.59 | ||
| No. who performed the test | 72 | 68 | |
| Power — W | 329±113 | 320±90 | |
| With a load | 0.44 | ||
| No. who performed the test | 69 | 66 | |
| Power — W | 363±135 | 346±115 | |
| Lift-and-lower test†† | 0.77 | ||
| No. who performed the test | 64 | 58 | |
| Score | 24.4±9.2 | 23.9±9.8 |
Plus–minus values are means ±SD.
The P values were calculated with the use of Fisher’s exact test and Student’s t-test.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Race or ethnic group was determined by self-report.
Included in this category were subjects who described themselves as being of mixed race, who did not report their race, or who described themselves in categories that were not black, white, or Asian–Pacific Islander.
To convert the values for total testosterone to nanomoles per liter, multiply by 0.0347. To convert the values for free testosterone to picomoles per liter, multiply by 3.467. Total and free testosterone levels were measured in a single blood sample that was obtained between 7 a.m. and 11 a.m. The subjects were eligible if they had a total testosterone level between 100 and 350 ng per deciliter or a free testosterone level of less than 50 pg per milliliter.
Scores on the Short Physical Performance Battery range from 0 to 12, with higher scores indicating better performance.
The scores on the lift-and-lower test refer to the number of shelves a subject was able to touch with a weighted basket in 1 minute. Higher scores indicate better performance.
Table 2.
Baseline Characteristics Related to Cardiovascular Risk.*
| Characteristic | Testosterone (N = 106) |
Placebo (N = 103) |
P Value† |
|---|---|---|---|
| Preexisting cardiovascular disease — no. (%)‡ | 56 (53) | 48 (47) | 0.41 |
| Obesity — no. (%)§ | 48 (45) | 50 (49) | 0.68 |
| Blood pressure — mm Hg | |||
| Systolic | 137±15 | 137±14 | 0.98 |
| Diastolic | 77±10 | 75±10 | 0.21 |
| Hypertension — no. (%)¶ | 90 (85) | 80 (78) | 0.21 |
| Antihypertensive therapy — no. (%) | 90 (85) | 75 (73) | 0.04 |
| Diabetes mellitus — no. (%)¶ | 25 (24) | 28 (27) | 0.63 |
| Glycated hemoglobin — % | 6.2±0.7 | 6.1±0.7 | 0.32 |
| Lipids — mg/dl‖ | |||
| Cholesterol | |||
| Total | 165±35 | 171±39 | 0.24 |
| LDL | 89±30 | 92±33 | 0.51 |
| HDL | 46±13 | 48±18 | 0.20 |
| Triglycerides | 159±111 | 143±69 | 0.20 |
| Hyperlipidemia — no. (%)¶ | 67 (63) | 51 (50) | 0.05 |
| Statin therapy — no. (%) | 66 (62) | 48 (47) | 0.03 |
| Framingham Risk Score — %** | 22±6 | 21±6 | 0.31 |
| Smoking status — no./total no. (%) | 0.66 | ||
| Never smoked | 27/104 (26) | 21/103 (20) | |
| Former smoker | 68/104 (65) | 73/103 (71) | |
| Current smoker | 9/104 (9) | 9/103 (9) |
Plus–minus values are means ±SD. HDL denotes high-density lipoprotein, and LDL low-density lipoprotein.
The P values were calculated with the use of Fisher’s exact test and Student’s t-test.
Preexisting cardiovascular disease was defined as self-reported coronary artery disease, cerebrovascular disease, peripheral vascular disease, aortic aneurysm, congestive heart failure, or arrhythmia.
Obesity is defined as a body-mass index (the weight in kilograms divided by the square of the height in meters) of 30 or more.
Hypertension, diabetes, and hyperlipidemia were considered to be present if the participant reported having received the diagnosis or if he was receiving medication for the condition.
In the testosterone group, data on total and HDL cholesterol and triglyceride levels were available for 105 men, and data on LDL cholesterol level for 103 men; in the placebo group, data on HDL and LDL cholesterol and triglyceride levels were available for 101 men, and data on total cholesterol level for 102 men.
Data on the Framingham Risk Score were available for 103 men in the testosterone group and 101 men in the placebo group.
Testosterone Dose and Adherence
After adjustment of the testosterone dose to achieve the target range, 29 men in the testosterone group received 5 g of the testosterone gel daily, 61 received 10 g, and 16 received 15 g. Adherence, as assessed by a count of the unused gel tubes, was greater than 90% in both groups.
Laboratory and Physiological Data
The mean (±SD) testosterone levels were 574±403 ng per deciliter (19.9±14.0 nmol per liter) in the testosterone group (after adjustment of the dose to achieve the target range) and 292±160 ng per deciliter (10.1±5.6 nmol per liter) in the placebo group. In the testosterone group, as compared with the placebo group, there was a significant increase in hemoglobin and hematocrit levels and a significant decrease in high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels (Table 1 in the Supplementary Appendix). Changes in blood pressure did not differ significantly between the testosterone and placebo groups (change in systolic pressure, −2.9±12.9 mm Hg vs. −4.6±14.8 mm Hg; change in diastolic pressure, −1.3±7.1 mm Hg vs. −1.3±7.8 mm Hg).
Adverse Events
In the testosterone group, as compared with the placebo group, there were significantly more adverse events and significantly more subjects who reported one or more adverse events (Table 2 in the Supplementary Appendix). Twice as many men in the testosterone group as in the placebo group were referred for medical evaluation owing to an adverse event. Men who were assigned to testosterone also reported a greater number of serious adverse events and a greater number of adverse events that were considered to be life-threatening, although the differences between the groups were not significant (Table 3 in the Supplementary Appendix).
Significantly more men in the testosterone group than in the placebo group had adverse events in three MedDRA categories: cardiac disorders; respiratory, thoracic, and mediastinal disorders; and skin and subcutaneous tissue disorders (Table 2 in the Supplementary Appendix). Of particular concern to the data and safety monitoring board was the greater number of subjects with adverse cardiac events in the testosterone group than in the placebo group (10 vs. 1) (Table 3). In accordance with the recommendation of the data and safety monitoring board, two additional analyses of cardiovascular events were performed (Table 3). A total of 23 men in the testosterone group and 5 in the placebo group had cardiovascular-related events; 7 men in the testosterone group and 1 in the placebo group had atherosclerosis-related events.
Table 3.
Subjects with One or More Cardiovascular-Related Adverse Events.*
| Subject No. | Adverse Event | MedDRA- Classified Cardiac Event |
Cardiovascular- Related Event |
Atherosclerosis- Related Event |
Method of Confirmation† |
|---|---|---|---|---|---|
| no. of events | |||||
| Testosterone group | |||||
| 1 | Acute coronary syndrome and chest pain | 1 | 2 | 1 | Review of medical records |
| 2 | Chest pain | 1 | 1 | Examination by study physician | |
| 3 | Syncope | 1 | Self-report | ||
| 4 | Syncope | 1 | Self-report | ||
| 5 | Myocardial infarction treated with angioplasty and placement of pacemaker | 1 | 1 | 1 | Review of medical records |
| 6 | Myocardial infarction | 1 | 1 | 1 | Review of medical records |
| 7 | Angioplasty and coronary-artery bypass grafting | 1 | 1 | Review of medical records | |
| 8 | Peripheral edema | 1 | Examination by study physician | ||
| 9 | Peripheral edema | 1 | Examination by study physician | ||
| 10 | Ectopy on ECG (premature ventricular contractions, couplets) | 1 | 1 | ECG evaluation by study physician | |
| 11 | Left ventricular strain pattern during exercise testing | 1 | 1 | ECG evaluation by study physician | |
| 12 | ST-segment depression during exercise testing | 1 | 1 | ECG evaluation by study physician | |
| 13 | Elevated blood pressure | 1 | Examination by study physician | ||
| 14 | Atrial fibrillation with rapid ventricular rate and shortness of breath and exacerbation of congestive heart failure, which necessitated hospitalization | 2 | 2 | Examination by study physician (atrial fibrillation) and report of primary care physician (exacerbation of congestive heart failure) | |
| 15 | Stroke | 1 | Review of medical records | ||
| 16 | Elevated blood pressure and atrial fibrillation | 1 | 1 | Review of medical records | |
| 17 | Peripheral edema | 1 | Examination by study physician | ||
| 18 | Peripheral edema | 1 | Examination by study physician | ||
| 19 | Elevated blood pressure | 1 | Examination by study physician | ||
| 20 | Tachycardia with fatigue | 1 | 1 | Self-report | |
| 21 | Death, suspected myocardial infarction | 1 | 1 | 1 | Notification by physician |
| 22‡ | Peripheral edema | 1 | Examination by study physician | ||
| 23 | Congestive heart failure exacerbation | 1 | 1 | Review of medical records | |
| Placebo group | |||||
| 1 | Syncope resulting in hospitalization | 1 | Self-report | ||
| 2 | Tachycardia | 1 | Examination by study physician | ||
| 3 | Elevated blood pressure | 1 | Examination by study physician | ||
| 4 | Arrhythmia–ectopy noted on ECG before exercise testing | 1 | 1 | Examination by study physician | |
| 5 | Carotid bruit and carotid-artery plaque identified on ultrasonography | 1 | 1 | Examination by study physician and review of medical records | |
In addition to an assessment of cardiac events as categorized with the use of the Medical Dictionary for Regulatory Activities (MedDRA) classification, the data and safety monitoring board requested two analyses. The first included MedDRA-classified cardiac events plus events that the data and safety monitoring board considered to be cardiovascular in nature but that were included in other MedDRA System Organ Class categories: stenting and bypass procedures (included in the MedDRA category of surgical and medical procedures); peripheral edema (included in the category of general disorders); elevated blood pressure, arrhythmias, and electrocardiographic changes (included in the investigations category); and stroke and syncope (included in the category of nervous system disorders). For clarity, these adverse events are listed as cardiovascular-related events. The second analysis included atherosclerosis-related events, which were events that were directly related to atherosclerotic vascular disease, such as myocardial infarction, sudden death, angioplasty, coronary-artery bypass grafting, and stroke. ECG denotes electrocardiogram.
Medical records were reviewed if an adverse event was reported and records were available to document that event.
Two men, both in the testosterone group, underwent elective vascular procedures during treatment that were not included in these analyses since they were deemed to have been related to preexisting conditions: Subject 22 underwent elective surgery to repair an aortic aneurysm, and another participant, not listed in this table, underwent prescheduled elective angioplasty and placement of a stent in his leg owing to peripheral vascular disease. The results of the analyses did not change when these two events were included.
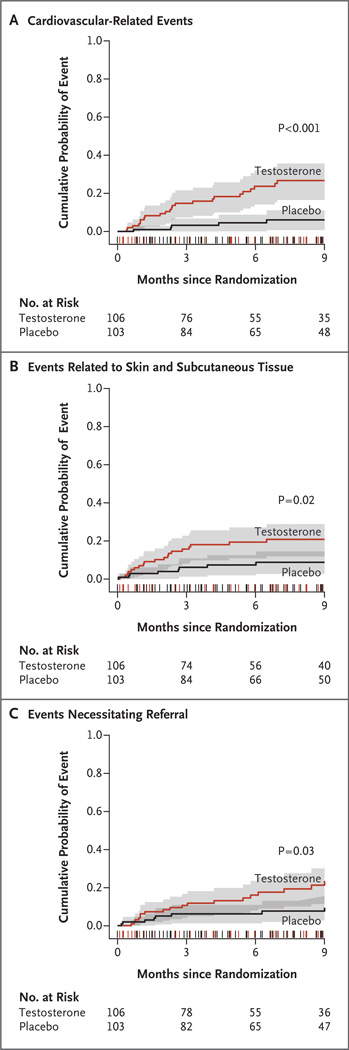
The risk of a cardiovascular-related adverse event remained significantly greater among men in the testosterone group than among men in the placebo group after adjustment for age group, body-mass index, smoking status, high-density lipoprotein cholesterol level, and presence or absence of diabetes, hyperlipidemia, and hypertension (Table 4). In time-to-event analyses, the relative risk of a cardiovascular-related event remained constant throughout the 24-week intervention period (Fig. 1). There were few adverse events during the 3-month observation phase after the end of the intervention period.
Table 4.
Risk of Adverse Events with Testosterone Therapy, According to Category.*
| Event Category | Total Risk | Instantaneous Risk | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | Hazard Ratio (95% CI) | |||
| unadjusted | adjusted | unadjusted | adjusted | |
| MedDRA cardiac† | 10.6 (1.3–84.5) | NA‡ | 10.5 (1.3–82.4) | NA‡ |
| Atherosclerosis-related§ | 7.2 (0.9–59.7) | NA‡ | 7.1 (0.9–57.8) | NA‡ |
| Cardiovascular-related¶ | 5.4 (2.0–14.9) | 5.8 (2.0–16.8) | 5.0 (1.9–13.2) | 5.5 (2.0–14.8) |
| Dermatologic‖ | 2.6 (1.1–6.2) | 4.9 (1.7–14.6) | 2.6 (1.1–5.9) | 4.8 (1.7–13.0) |
| Necessitating referral for medical evaluation | 2.3 (0.98–5.3) | 5.2 (1.8–14.6) | 2.3 (1.1–5.2) | 5.2 (2.0–13.5) |
Total risk refers to the risk of an adverse event occurring over the entire study period after randomization, which included the 24-week treatment period and the subsequent 12-week observation phase. Instantaneous risk refers to the risk of an adverse event occurring at any specific time. Unadjusted odds ratios and hazard ratios were estimated with the use of simple logistic regression. Adjusted odds ratios and hazard ratios were estimated with the use of multiple logistic regression, with adjustment for age group; body-mass index; presence or absence of diabetes, hypertension, and hyperlipidemia; and high-density lipoprotein cholesterol level. Given the small number of subjects in each cell, the ability to adjust for baseline risk factors is limited, and results should be interpreted conservatively. When 104 men with preexisting self-reported cardiovascular, cerebrovascular, or peripheral vascular disease, with or without congestive heart failure and arrhythmias, were excluded, 9 men treated with testosterone had cardiovascular-related events, as compared with 2 men receiving placebo gel (odds ratio, 5.8; 95% confidence level [CI] 1.2 to 28.4; P = 0.03). Three men in the testosterone group had MedDRA cardiac events and two had atherosclerosis-related events, as compared with no men with these events in the placebo group.
Included are adverse events that were classified as cardiac according to the Medical Dictionary for Regulatory Activities (MedDRA) classification system.
Adjusted estimates are not applicable (NA) because there was only one event in the placebo group.
Included are adverse events that were classified, according to the recommendation of the data and safety monitoring board, as having been directly related to atherosclerotic vascular disease (e.g., myocardial infarction, sudden death, angioplasty, coronary-artery bypass grafting, and stroke).
At the recommendation of the data and safety monitoring board, we included in this analysis, in addition to cardiac events in the MedDRA “Cardiac” category, those that were considered to be cardiovascular in nature but which were included in other MedDRA System Organ Class categories: stenting and bypass procedures (included in the MedDRA “Surgical and Medical Procedures” category); peripheral edema (included in the “General Disorders” category); elevated blood pressure, arrhythmias, and electrocardiographic changes (included in the “Investigations” category); and stroke and syncope (included in the “Nervous System Disorders” category).
For dermatologic events, unadjusted odds ratios and hazard ratios were estimated with the use of Cox proportionalhazards models. Adjusted odds ratios and hazard ratios were estimated with the use of multivariate proportional-hazards models, with adjustment for age group, body-mass index, presence or absence of diabetes, hypertension, and hyperlipidemia, and high-density lipoprotein cholesterol level.
Figure 1. Time-to-Event Analysis of Adverse Events, According to Body System.
Kaplan–Meier estimates of the cumulative probability of incident cardiovascular-related adverse events (Panel A), events related to skin and subcutaneous tissue (Panel B), and events necessitating referral for medical evaluation (Panel C), from randomization to the end of the planned observation phase (9 months after randomization) are shown for the testosterone and placebo groups. The 95% confidence intervals are indicated by the shaded areas. The notches on the x axis show the distribution of censoring times before 9 months among participants in both groups. The P values were calculated from an unadjusted comparison of curves with the use of the log-rank test.
There was no evidence of a significant relationship between potential risk factors and cardiovascular-related events in time-to-event analyses (Fig. 2 in the Supplementary Appendix). Men with testosterone levels in the highest quartile during the intervention period, as compared with all other subjects, were at elevated risk for cardiovascular-related events (hazard ratio, 2.4; P = 0.05). Among subjects who were randomly assigned to the testosterone group, testosterone levels during the intervention period were available for 81 subjects. Cardiovascular-related events were reported in 4 of 14 subjects with testosterone levels higher than 1000 ng per deciliter during the treatment period, by 5 of 21 with levels of 500 to 1000 ng per deciliter, and by 7 of 46 subjects with levels of less than 500 ng per deciliter.
One man in the testosterone group had a hematocrit that was higher than 54%, and one reported having received a diagnosis of prostate cancer. One man in the placebo group underwent transurethral resection of the prostate.
Sensitivity Analyses
Sensitivity analyses were performed to determine the effect on the results when subjects who had baseline characteristics that might have affected the outcomes were excluded. The results of these sensitivity analyses with respect to the risk of adverse events associated with assignment to the testosterone group were consistent with the results of the analyses of data from the overall population (Section B in the Supplementary Appendix). Adjustment for baseline self-reported mobility status and Short Physical Performance Battery score had no effect on the results.
Efficacy Outcomes
In an analysis involving all the men who underwent at least one outcome assessment after randomization, men who were assigned to testosterone, as compared with those assigned to placebo, had significantly greater increases in leg-press strength, chest-press strength, and stair-climbing power while carrying a load. Changes in gait speed without a load and stair-climbing power without a load did not differ significantly between the groups (Table 5). Adjustment for baseline self-reported mobility status and Short Physical Performance Battery score had no effect on the results.
Table 5.
Changes in Measures of Muscle Performance and Physical Function from Baseline to the End-of-Study Assessment, among Men Receiving Testosterone Therapy.*
| Strength and Performance Measures |
Testosterone | Placebo | Difference between Treatments | |||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Baseline Mobility | |||||||
| no. of men | mean (95% CI) | no. of men | mean (95% CI) | mean (95% CI) | P value† | mean (95% CI) | P value‡ | |
| Leg-press strength (newtons) | 49 | 156.9 (89.2 to 224.6) | 54 | 27.1 (−28.3 to 82.6) | 129.8 (43.9 to 215.6) | 0.003 | 129.4 (43.5 to 215.4) | 0.004 |
| Chest-press strength (newtons) | 38 | 34.7 (18.7 to 51.4) | 44 | 0.28 (−13.8 to 14.3) | 34.5 (13.2 to 55.8) | 0.002 | 34.5 (13.1 to 56.2) | 0.002 |
| Grip strength in dominant hand (kg) | 63 | 1.0 (−0.1 to 2.0) | 70 | 0.7 (−0.3 to 1.7) | 0.27 (−1.1 to 1.7) | 0.69 | 0.26 (−1.1 to 1.7) | 0.71 |
| 50-m walking speed (m/sec) | ||||||||
| Without a load | 39 | 0.074 (−0.004 to 0.153) | 43 | 0.024 (−0.018 to 0.066) | 0.050 (−0.035 to 0.135) | 0.26 | 0.048 (−0.037 to 0.133) | 0.27 |
| With a load | 26 | 0.139 (0.023 to 0.256) | 29 | 0.065 (0.013 to 0.116) | 0.074 (−0.05 to 0.19) | 0.24 | 0.090 (−0.030 to 0.209) | 0.14 |
| Stair-climbing power (W) | ||||||||
| Without a load | 49 | 19.5 (7.4 to 31.5) | 52 | 10.7 (−2.9 to 24.3) | 8.7 (−9.2 to 26.7) | 0.34 | 8.1 (−9.9 to 26.2) | 0.37 |
| With a load | 46 | 39.2 (14.4 to 64.0) | 50 | 9.0 (−8.4 to 26.4) | 30.2 (0.3 to 60.1) | 0.05 | 29.7 (0.2 to 59.3) | 0.05 |
| Lift-and-lower test§ | 36 | 4.4 (2.5 to 6.4) | 45 | 2.6 (0.8 to 4.3) | 1.9 (−0.8 to 4.5) | 0.16 | 1.9 (−0.7 to 4.4) | 0.15 |
The end-of-study assessment was performed either at the end of the 6-month intervention period or at the last visit, if possible, in cases in which the study medication was discontinued before 6 months. Results are reported only for men who were able to perform the relevant tests at baseline. CI denotes confidence interval.
We calculated the P values in the unadjusted analysis using two-sample Student’s t-tests of equal change in the trial groups, allowing unequal variance and using Satterthwaite’s approximation to degrees of freedom.
We calculated the P values in the adjusted analysis using multiple linear regression, with adjustment for baseline total score on the Short Physical Performance Battery and self-report of limitations in mobility.
The scores on the lift-and-lower test refer to the number of shelves a subject was able to touch with a weighted basket in 1 minute. Higher scores indicate better performance.
Discussion
In this study of older men with low testosterone levels and limitations in mobility, random assignment to daily application of a testosterone gel, as compared with a placebo gel, was associated with a greater frequency of adverse events, particularly cardiovascular, respiratory, and dermatologic events. The divergence between the groups in the incidence of cardiovascular adverse events was maintained over the 6-month intervention period and did not diminish during the 3-month observation phase that followed the intervention period. The increased cardiovascular risk in the testosterone group was seen with all three definitions of cardiovascular events, and the increase persisted after adjustment for baseline risk factors. The increased risk was also evident in sensitivity analyses adjusted for baseline mobility status and Short Physical Performance Battery score and in sensitivity analyses performed after the exclusion of subjects whose eligibility deviated from the planned criteria. The pattern of adverse cardiovascular events associated with testosterone therapy was considered by the data and safety monitoring board to be of sufficient concern to warrant termination of the trial.
The generalizability of our data about the safety of testosterone therapy is limited by several factors. First, cardiovascular events were not a planned primary or secondary outcome, and therefore, a structured evaluation of cardiovascular events was not performed, a factor that may have influenced the ascertainment of events. Most of the cardiovascular-related events were verified from medical records or by direct examination. Second, the sample, although larger than those in most previous trials, was small, and the number of adverse events was small. The results of individual small trials may not be confirmed in large trials,28 and trials that have been stopped early tend to overestimate treatment differences. Third, the clinical characteristics of our study population differ from those of most other populations in which testosterone therapy has been administered in a clinical setting or as part of a clinical trial. Men who were younger than 65 years of age and men with severe hypogonadism were excluded from the trial. Participants had substantial limitations in mobility and a high prevalence of chronic conditions, including preexisting heart disease, obesity, diabetes, and hypertension. Frail elderly men with limitations in mobility are more likely to have clinical and subclinical cardiovascular disease than are those who do not have limitations in mobility.29,30
Previous studies provide very limited data to either reinforce or contradict the findings in this study with respect to the effects of testosterone therapy in older men with limited mobility. Meta-analyses of previous trials of testosterone therapy have not shown significant increases in cardiovascular risk with testosterone therapy, although nonsignificant increases have been noted among participants of all ages,31–33 as well as among older men.31,33 The trials in these meta-analyses were limited by inadequate methods of ascertaining adverse events or the poor quality of data on adverse events, by the small numbers of events or the small numbers of older participants, or by intervention periods that were shorter than the 6-month intervention in this trial. Some epidemiologic studies have shown that low testosterone levels are an independent risk factor for death from cardiovascular causes and from all causes.34–37 However, differences between the effects of endogenous hormones and those of pharmacologic hormonal therapy, as well as differences in the duration of exposure to testosterone, could contribute to the apparent discrepancies between these epidemiologic data and the results of our trial.
It is not likely that the adverse cardiovascular events seen in the TOM trial are a consequence of an unusual protocol for testosterone administration (Table 4 in the Supplementary Appendix). The upper limit of the testosterone threshold used for inclusion in the trial is not dissimilar to that used in most other trials.16–19,38–47 The testosterone doses in this trial may have been higher than those that are typically used in clinical practice48 and were higher than the doses used in some previous trials17,18,39–42 but were similar to those in other trials.16,21,43–46 The average testosterone concentrations during the intervention period among men in our testosterone group were in the middle of the normal range for young men; these levels were higher than those in some testosterone trials17,18,39–42 but did not differ from levels reported in other trials.16,19,21,43–46
The cardiovascular adverse events reported in the TOM trial were diverse and may have variable clinical importance. The lack of a consistent pattern in these events and the small number of overall events suggest the possibility that the differences detected between the two trial groups may have been due to chance alone. The results of several separate analyses were consistent with the initial observation of a significant difference, but these analyses were not entirely independent of one another. In interpreting these findings, it is essential to recognize the role that chance may have played in the outcomes we observed.
The diversity of cardiac adverse events also renders the events less susceptible to a single mechanistic explanation. Testosterone causes salt and water retention,49–51 particularly in older men,14 and this could contribute to edema, hypertension, and congestive heart failure, although there are some trials in which testosterone has been administered in men with congestive heart failure.39,40 Testosterone and associated increases in estradiol may promote inflammation, coagulation, and platelet aggregation.52 The use of anabolic steroids has been associated with left ventricular hypertrophy and systolic and diastolic dysfunction.53,54 Changes in plasma lipid levels would not account for the rapid divergence in rates of cardiovascular adverse events.
Testosterone therapy was associated with significant improvements in leg-press and chest-press strength and in stair-climbing power with a load. Inferences regarding efficacy are limited because of the attenuation of statistical power owing to the early termination of the trial.
In conclusion, we evaluated the effect of testosterone supplementation in men 65 years of age or older who had limitations in mobility and low serum levels of total or free testosterone. The trial was stopped before enrollment had been completed because of an incidence of adverse cardiovascular events that was higher in the testosterone group than in the placebo group. However, caution is warranted in interpreting this finding, because of the small numbers of events and because of limitations with respect to the ascertainment of adverse events. Caution is also warranted in extrapolating these findings to other doses and formulations of testosterone or to other populations, particularly young men who have hypogonadism without cardiovascular disease or limitations in mobility.
Supplementary Material
Acknowledgments
Supported primarily by the National Institutes on Aging under a cooperative agreement (1UO1AG14369). Additional support was provided by grants from the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679) and the Boston University Clinical and Translational Science Institute (1UL1RR025771). A part of the work was supported by the resources and facilities of the Veterans Affairs Boston Healthcare System. Testosterone and placebo gel for the study were provided by Auxilium Pharmaceuticals, Norristown, PA.
Dr. Bhasin reports receiving consulting fees and payments for travel or accommodation expenses from Novartis and GlaxoSmithKline and grant support from Solvay Pharmaceuticals, Merck, and Ligand Pharmaceuticals; Dr. Coviello, receiving compensation from Endo Pharmaceuticals for participation in a 1-day discussion group; Dr. Mazer, receiving consulting fees from Lipocine and Solvay Pharmaceuticals and a fee for expert testimony from Watson Laboratories and being a full-time employee of Hoffmann–La Roche Pharmaceuticals; and Dr. Tennstedt, receiving grant support from Auxilium for data analysis.
We thank Drs. Sergei Romashkan and Evan Hadley of the National Institute on Aging for their oversight and guidance throughout the trial, the staff of the General Clinical Research Unit of Boston University’s Clinical and Translational Science Institute for their help with the study, the staff of the Veterans Affairs Boston Healthcare System, and the study participants for their commitment and generosity.
Appendix
The members of the data and safety monitoring board are as follows: E. Orwoll (chair), A. Newman, K. Schechtman, W. Meikle, and M. Litwin.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E. Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology. 2006;52:359–365. doi: 10.1159/000094985. [DOI] [PubMed] [Google Scholar]
- 4.Metter EJ, Schrager M, Ferrucci L, Talbot LA. Evaluation of movement speed and reaction time as predictors of all-cause mortality in men. J Gerontol A Biol Sci Med Sci. 2005;60:840–846. doi: 10.1093/gerona/60.7.840. [DOI] [PubMed] [Google Scholar]
- 5.Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R. The prevalence of functional limitations and disability in older persons in the US: data from the National Health and Nutrition Examination Survey III. J Am Geriatr Soc. 2000;48:1132–1135. doi: 10.1111/j.1532-5415.2000.tb04791.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelly-Hayes M, Jette AM, Wolf PA, D’Agostino RB, Odell PM. Functional limitations and disability among elders in the Framingham Study. Am J Public Health. 1992;82:841–845. doi: 10.2105/ajph.82.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardener EA, Huppert FA, Guralnik JM, Melzer D. Middle-aged and mobility-limited: prevalence of disability and symptom attributions in a national survey. J Gen Intern Med. 2006;21:1091–1096. doi: 10.1111/j.1525-1497.2006.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaap LA, Pluijm SM, Smit JH, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63:152–160. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 9.Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010 Apr 9; doi: 10.1210/jc.2009-2680. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter EJ. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–E294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 12.Orwoll E, Lambert LC, Marshall LM, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166:2124–2131. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 13.Araujo AB, Travison TG, Bhasin S, et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56:2000–2008. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 15.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 16.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 17.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 18.Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 19.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 20.Storer TW, Magliano L, Woodhouse LJ, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 21.Sih R, Morley JE, Kaiser FE, Perry HM, III, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 22.LeBrasseur NK, Lajevardi N, Miciek R, Mazer N, Storer TW, Bhasin S. Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (the TOM Trial): design and methods. Contemp Clin Trials. 2009;30:133–140. doi: 10.1016/j.cct.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 24.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56:2118–2123. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salameh WA, Redon-Goldman MM, Clarke NJ, Reitz RE, Caulfield M. Validation of a total testosterone assay using high turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75:165–175. doi: 10.1016/j.steroids.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74:512–519. doi: 10.1016/j.steroids.2009.01.008. [Erratum, Steroids 2010;75:517.] [DOI] [PubMed] [Google Scholar]
- 27.Bhasin S, Storer TW, Javanbakht M, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000;283:763–770. doi: 10.1001/jama.283.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennekens CH, Demets D. The need for large-scale randomized evidence without undue emphasis on small trials, meta-analyses, or subgroup analyses. JAMA. 2009;302:2361–2362. doi: 10.1001/jama.2009.1756. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 31.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 32.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Balsells MM, Murad MH, Lane M, et al. Clincal review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 34.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khaw K-T, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 36.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the Aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menke A, Guallar E, Rohrmann S, et al. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010;171:583–592. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 39.Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 40.Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure: a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 41.Kenny AM, Prestwood KM, Gruman CA, Fabregas G, Biskup B, Mansoor G. Effects of transdermal testosterone on lipids and vascular reactivity in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M460–M465. doi: 10.1093/gerona/57.7.m460. [DOI] [PubMed] [Google Scholar]
- 42.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58:618–625. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 43.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–1098. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 44.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 45.Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- 46.Brockenbrough AT, Dittrich MO, Page ST, Smith T, Stivelman JC, Bremner WJ. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. Am J Kidney Dis. 2006;47:251–262. doi: 10.1053/j.ajkd.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–2361. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- 48.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 49.Lundh B, Gardner FH. The effect of testosterone in pharmacological doses on plasma volume and on some serum proteins in patients with sickle cell anemia and in sexually impotent men. Scand J Clin Lab Invest. 1971;28:72–78. [PubMed] [Google Scholar]
- 50.Johannsson G, Gibney J, Wolthers T, Leung KC, Ho KK. Independent and combined effects of testosterone and growth hormone on extracellular water in hypo-pituitary men. J Clin Endocrinol Metab. 2005;90:3989–3994. doi: 10.1210/jc.2005-0553. [DOI] [PubMed] [Google Scholar]
- 51.Quan A, Chakravarty S, Chen JK, et al. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004;287:F452–F459. doi: 10.1152/ajprenal.00188.2003. [DOI] [PubMed] [Google Scholar]
- 52.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 53.D’Andrea A, Caso P, Salerno G, et al. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br J Sports Med. 2007;41:149–155. doi: 10.1136/bjsm.2006.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karila TA, Karjalainen JE, Mäntysaari MJ, Viitasalo MT, Seppälä TA. Anabolic androgenic steroids produce dose-dependent increase in left ventricular mass in power athletes, and this effect is potentiated by concomitant use of growth hormone. Int J Sports Med. 2003;24:337–343. doi: 10.1055/s-2003-40702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.