Abstract
We previously reported that cyclin E (CCNE1) amplification is strongly associated with resistance to treatment in serous ovarian cancer by high-resolution oligonucleotide copy number analysis. Dysregulation of cell cycle control has been implicated as the key event in human oncogenesis, and aberrant expression of G1-S phase-related genes in particular has been reported in epithelial ovarian cancer (EOC). Nevertheless, there are conflicting results concerning the prognostic values of these abnormalities in EOC. This study focused on advanced serous EOC cases and investigated the association between the expression of G1-S phase-regulatory proteins and clinicopathological parameters. The utility of these proteins as prognostic factors was assessed, and whether these targets reflect chemoresistance of advanced serous EOC was investigated. A total of 66 patients treated by primary surgery were evaluated in this study. Immunohistochemical analysis for cyclin D1, pRb, p16, p53, p27Kip1, p21Waf1/Cip1 and cyclin E was performed on formalin-fixed tissue sections collected from primary surgical specimens. The correlations between the expression of these proteins and the clinicopathological parameters, including progression-free survival (PFS), overall survival (OS) and chemosensitivity, were examined. Upon univariate analysis, overexpression of cyclin D1 was positively correlated with reduced PFS (p=0.00062) and OS (p=0.00037). Reduced expression of p27Kip1 was associated with shorter OS (p=0.064). Upon multivariate analysis, overexpression of cyclin D1 (p=0.0019), reduced expression of p27Kip1 (p=0.042) and residual tumor volume (p=0.0092) were identified as independent predictors of OS. Overexpression of cyclin D1 (p=0.011) as well as residual tumor volume (p=0.006) were significantly associated with first-line chemosensitivity. In advanced serous EOC, overexpression of cyclin D1 contributed largely to poor prognosis, and this may have been in part mediated by chemoresistance. Cyclin D1 is a possible target for overcoming the refractory nature of advanced serous EOC.
Keywords: ovarian cancer, cyclin D1, p27, prognosis, immunohistochemistry
Introduction
Ovarian cancer is the most lethal gynecological malignancy in developed countries and is the 9th most common cancer in Japanese females. An estimated 8,304 new cases and 4,467 mortalities occurred in 2005 (Center for Cancer Control and Information Services, National Cancer Center, Japan). Although ovarian cancer patients respond to cytoreductive surgery and combination chemotherapy satisfactorily, advanced cases exhibit a high level of recurrence, and the overall survival (OS) rate has not significantly changed for decades. However, clinical trials have been undertaken to improve prognosis. Since there are different clinical behavior patterns for certain histopathological subgroups, separate trials have been developed for clear cell (1) and mucinous carcinomas (2). The alteration of dose/schedule and the use of intraperitoneal therapy have been shown to be superior in at least one trial (3,4).
A number of clinicopathological factors, including the volume of residual tumor after primary surgery, FIGO stage and tumor grade, are reported to be the key prognostic factors (5,6). Cytoreduction to a non-macroscopic residual tumor is the ultimate goal, and it improves prognosis (7–9). However, in order to improve the prognosis of advanced epithelial ovarian cancer (EOC) cases, other predictive biomarkers should also be elucidated.
We previously described that cyclin E (CCNE1) amplification was strongly associated with resistance to treatment in serous ovarian cancer by high-resolution oligonucleotide copy number analysis (10). Therefore, the amplification status of cyclin E has potential for therapeutic exploitation, whereby patients exhibiting cyclin E amplification may benefit from novel, cyclin-related targeted treatments. Dysregulation of cell cycle control has been implicated as the key event in human oncogenesis, and aberrant expression of G1-S phase-related genes in particular has been reported in a number of human cancers, including EOC (5,11). Aberrant expression of the p16-cyclin D1-CDK4/6-pRb and p21-p27-cyclin E-CDK2 pathways have been reported to correlate with prognosis (5,11–14).
Barbieri et al reported in their series of 70 EOC cases that overexpression of cyclin D1 was associated with a shorter OS. In particular, among patients with stage III/IV tumors and residual disease greater than 2 cm, cyclin D1 expression significantly influenced clinical outcome (5). Bali et al reported on 134 serous ovarian cancers for which molecular markers predicted reduced OS in univariate analysis, which included overexpression of cyclin D1 and p53, and reduced expression of p27Kip1 and p21Waf1/Cip1 (11). In contrast, it was reported that low nuclear p27 expression was associated with improved 3-year OS and progression-free survival (PFS) in 150 advanced stage (FIGO stages II, III and IV) EOC patients (12). Kommoss et al carried out immunohistochemical analysis of p16Ink4a and pRb expression levels and found that they correlated with survival in a series of 300 patients with FIGO stage II–IV ovarian carcinoma. They reported that p16Ink4a-negative tumors had a significantly worse prognosis in both univariate and multivariate analyses. High expression levels of pRb were associated with an incremental deterioration of prognosis, which was also the case in the subgroup of optimally debulked patients (15). Meanwhile, Khouja et al, using immunohistochemistry, evaluated 171 primary stage III ovarian carcinoma tumors for expression of Ki-67, p16, p14 and p57. High expression of p16 was correlated with poor differentiation and survival in univariate analysis. However, in multivariate analysis, p16 expression was not significantly associated with shorter survival (13). Some of the results are contradictory, probably due to the variety of histotypes and stages of EOC as well as disease heterogeneity, different research methodologies or the sample sizes of the studies. As serous ovarian cancer is the most common histological type of EOC and the prognosis of advanced cases remains poor, we limited our analysis to the serous histotype and advanced cases to eliminate such bias.
We focused on advanced serous EOC (stage III/IV) cases in particular and investigated the association between the expression of G1-S phase-regulatory proteins and the clinicopathological parameters. We aimed to identify the utility of these proteins as prognostic factors and to evaluate whether these targets reflect chemoresistance of advanced serous EOC.
Patients and methods
Patients and tumor specimens
The Jikei University School of Medicine Ethics Review Committee approved the study protocol, and informed consent was obtained from the patients. The tumor specimens were surgically obtained from a group of 66 patients with advanced primary ovarian serous adenocarcinoma who were treated at the Department of Obstetrics and Gynecology, The Jikei University School of Medicine, and Jikei University Kashiwa Hospital. The tumors were staged in accordance with the International Federation of Gynecology and Obstetrics (FIGO) system (1988). The clinical and pathological characteristics of the patient cohort are shown in Table I. The age at diagnosis, volume of postoperative residual disease, FIGO stage, presence of intraoperative ascites and patient outcome were obtained retrospectively from patient records as shown. The median follow-up time for the cohort was 15.5 months (range 3–72). The 66 patients received first-line platinum-based chemotherapy. Among them, 62 cases received taxane simultaneously as T-C chemotherapy following primary surgery (93.9%).
Table I.
Clinical and pathological characteristics of the serous epithelial ovarian cancer patient cohort (n=66).
| Clinicopathological parameters | No. of patients (%) |
|---|---|
| Age | |
| ≤65 years | 55 (83.3) |
| >65 years | 11 (16.7) |
| FIGO stage | |
| III | 52 (78.8) |
| IV | 14 (21.2) |
| Residual disease | |
| ≤2 cm | 28 (42.4) |
| >2 cm | 38 (57.6) |
| Ascites | |
| ≤500 ml | 25 (37.9) |
| >500 ml | 41 (62.1) |
| Disease progression | |
| No | 9 (13.6) |
| Yes | 57 (86.4) |
Immunohistochemistry
Immunostaining was performed on buffered formalin-fixed, paraffin-embedded tissue sections. The sections were deparaffinized, and standard immunohistochemical techniques were performed using Ventana XT system (BenchMark® XT; Ventana Medical Systems, Inc., Tuscon, AZ, USA) in accordance with the manufacturer’s instructions. Antigen epitopes were retrieved using Ventana Benchmark CC1 standard program. The primary antibodies used in this study were: anti-cyclin D1 (rabbit monoclonal clone SP4; Ventana Medical Systems, Inc.), anti-pRb (mouse monoclonal clone 13A10; Novocastra Laboratories Ltd., UK; 1:100), anti-p16 (mouse monoclonal clone 16P04; Ventana Medical Systems, Inc.), anti-p53 (mouse monoclonal clone DO-7; Ventana Medical Systems, Inc.), anti-p21Waf1 (mouse monoclonal clone EA10; Calbiochem, Darmstadt, Germany; 1:50), anti-p27Kip1 (mouse monoclonal clone SX53G8; Dako, Glostrup, Denmark; 1:20) and anti-cyclin E (mouse monoclonal clone HE12; Medical and Biological Laboratories Co., Ltd., Japan; 1:500). Antibodies from Ventana Medical Systems, Inc. were pre-diluted. The antibodies were incubated at 37°C for 32 min (60 min for p21, Rb and cyclin E). The slides were counterstained with hematoxylin and mounted for microscopic examination. Positive and negative controls were tested in parallel for each staining.
Immunostaining evaluation
At least 500 tumor cells were evaluated for immunostaining, and the percentage of stained cells was calculated. The evaluation of immunostaining was conducted in a blinded manner by two independent screeners, without knowledge of the clinical and pathological characteristics of the cases. Standardization of scoring was achieved by comparison of scores between screeners, and discrepancies were resolved by consensus. The percentage of positive nuclear immunostaining in cells of the tumor sections was calculated. Scores are expressed as a percentage of positive nuclear staining within representative areas of the tumor sample. The percentage score above, whose staining is considered representative of overexpression, is based on published reports (11). The range and the median percentage of immunostaining, percentage values regarded as overexpression (cutoff value) and the numbers of specimens displaying positive staining are shown in Table II. Representative photomicrographs of tumor tissue showing positive and negative staining for the specific antigens are presented in Fig. 1.
Table II.
Immunohistochemical analysis of cell cycle gene expression in advanced serous epithelial ovarian cancer.
| Cyclin D1 | pRb | p16Ink4a | p53 | p27Kip1 | p21Waf1/Cip1 | Cyclin E | |
|---|---|---|---|---|---|---|---|
| Range of staining (% positive) | 0–50 | 0–90 | 0–90 | 0–90 | 0–80 | 0–70 | 0–90 |
| Median staining (%) | 10 | 50 | 80 | 60 | 50 | 5 | 50 |
| Cutoff value (%) (positive staining) | >20 | >50 | >50 | >40 | >40 | >5 | >70 |
| Positive tumors (%) | 11 (16.7) | 26 (39.4) | 42 (63.6) | 37 (56.1) | 37 (56.1) | 20 (30.3) | 11 (16.7) |
Range of staining indicates the proportion of positive nuclear staining within representative areas of the tumor samples. Cut-off value is based on published reports. Number of specimens showing positive staining is provided in the bottom row.
Figure 1.
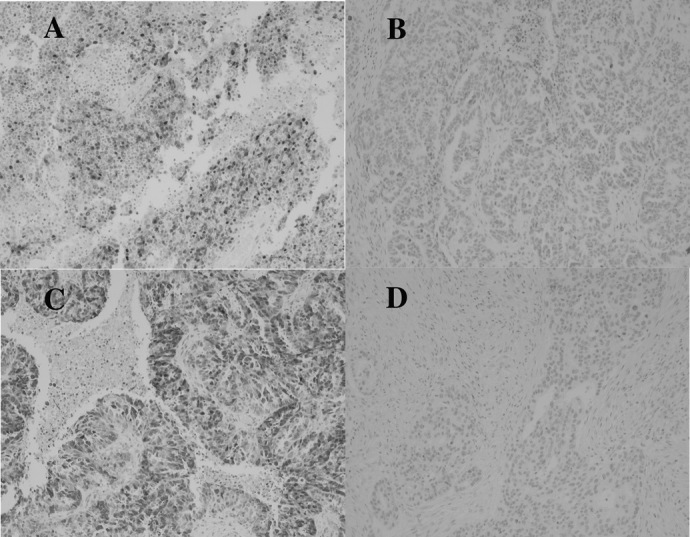
Immunohistochemistry on primary tumor tissue. Representative stainings for cyclin D1 and p27Kip1 expression are shown. Magnification, x100. (A) Cyclin D1-positive staining. (B) Cyclin D1-negative staining. (C) p27Kip1-positive staining. (D) p27Kip1-negative staining.
Statistical analysis
The associations between clinicopathological parameters and the immunostaining scores were analyzed. The correlations between the expression of each gene and the clinicopathological parameters were analyzed using the Chi-square test. p≤0.05 was considered to be statistically significant. For survival analysis, event time distributions were evaluated using the Kaplan-Meier method, and differences in survival rates were compared using the log-rank test for univariate analysis and Cox proportional hazards regression model for multivariate analysis. PFS was calculated from the date of primary surgery to the date of disease progression. The duration of OS was defined from the date of primary surgery to the date the patient succumbed to the disease or to the date of last follow-up. The treatment-free interval (TFI) was defined as being from the last date of first-line chemotherapy to the date of recurrence or last follow-up without recurrence.
Results
Expression of G1-S phase-regulatory proteins and the association with clinicopathological parameters
The expression of G1-S phase-regulatory proteins was analyzed by immunohistochemistry in advanced serous EOC. Overexpression of cyclin D1, pRb, p16, p53, p27Kip1, p21Waf1/Cip1 and cyclin E was detected with incidences of 16.7, 39.4, 63.6, 56.1, 56.1, 30.3 and 16.7%, respectively. Associations of the expression of each protein and clinicopathological parameters are shown in Table III. The volume of postoperative residual disease and the presence of ascites were not correlated with the expression pattern of any of the studied proteins. The expression of p53 appeared to be positively correlated with that of p16Ink4a (Table III). No other significant association among the gene expressions was observed.
Table III.
Association of gene expression and clinicopathological parameters in serous epithelial ovarian cancer.
| Cyclin D1 | pRb | p16Ink4a | p53 | p27Kip1 | p21Waf/Cip1 | Cyclin E | |
|---|---|---|---|---|---|---|---|
| Residual disease | 0.15 | 0.99 | 0.14 | 0.7300 | 0.097 | 0.17 | 0.91 |
| Ascites | 0.82 | 0.10 | 0.12 | 0.3000 | 0.310 | 0.81 | 0.82 |
| Cyclin D1 | 0.43 | 0.30 | 0.6600 | 0.820 | 0.19 | 0.77 | |
| pRb | 0.45 | 0.0820 | 0.420 | 0.95 | 0.14 | ||
| p16Ink4a | 0.0049 | 0.190 | 0.34 | 0.73 | |||
| p53 | 0.380 | 0.51 | 0.37 | ||||
| p27Kip1 | 0.67 | 0.82 | |||||
| p21Waf/Cip1 | 0.90 |
Significant p-values are indicated in boldface type.
Correlation between G1-S phase-regulatory protein expression and patient outcome in advanced serous epithelial ovarian cancer
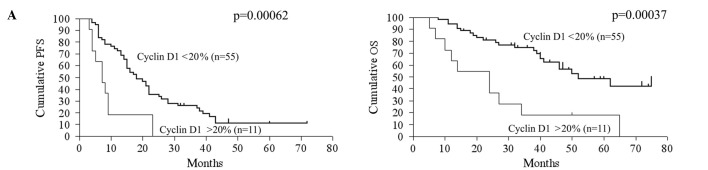
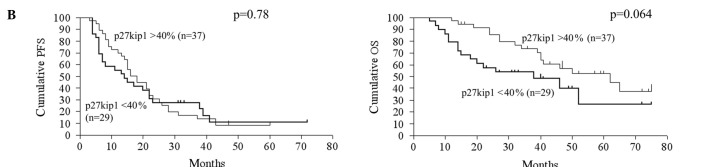
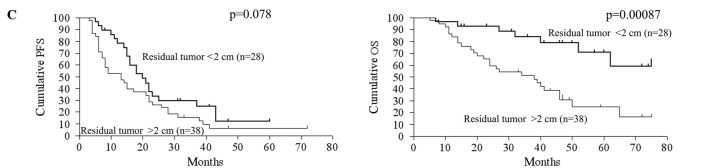
The relationship between gene expression and patient prognosis was assessed. Upon univariate analysis, the clinicopathological determinants of reduced OS included age and volume of residual disease >2 cm (Table IV). A molecular marker predictive of reduced OS upon univariate analysis was overexpression of cyclin D1 (p=0.00037, RR=0.28, 95% CI 0.044-0.40). Reduced expression of p27Kip1 had a trend of association with a shorter OS (p=0.064, RR=1.88, 95% CI 0.97–4.21). Regarding PFS, overexpression of cyclin D1 (p=0.00063, RR=0.34, 95% CI 0.054-0.43) was significantly correlated with reduced PFS, but reduced expression of p27Kip1 had no statistically significant correlation with PFS. The CA125 level, volume of intraoperative ascites, pRb, p16, p53, p21Waf1/Cip1 and cyclin E expression exhibited no statistically significant correlation with either OS or PFS. Kaplan-Meier curves and log-rank p-values according to cyclin D1 expression, p27Kip1 expression and residual tumor volume are shown in Fig. 2.
Table IV.
Univariate analysis for the association of clinicopathological parameters and gene expression with clinical outcome in serous epithelial ovarian cancer.
| Parameters | PFS
|
OS
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | |
| Age (≤65 vs. >65 years) | 0.71 | 0.300-1.46 | 0.32000 | 0.42 | 0.100-0.87 | 0.02900 |
| Residual disease (≤2 vs. >2 cm) | 0.62 | 0.360-1.04 | 0.07800 | 0.27 | 0.150-0.60 | 0.00087 |
| CA125 (≤500 vs. >500) | 0.83 | 0.440-1.54 | 0.56000 | 1.10 | 0.470-2.65 | 0.81000 |
| Cyclin D1 (≤20 vs. >20%) | 0.34 | 0.054-0.43 | 0.00063 | 0.28 | 0.044-0.40 | 0.00037 |
| pRb (≤50 vs. >50%) | 1.00 | 0.580-1.73 | 0.99000 | 0.90 | 0.440-1.81 | 0.76000 |
| p16Ink4a (≤50 vs. >50%) | 1.13 | 0.650-2.00 | 0.65000 | 0.97 | 0.480-1.96 | 0.93000 |
| p53 (≤40 vs. >40%) | 1.43 | 0.850-2.59 | 0.17000 | 1.38 | 0.690-2.83 | 0.35000 |
| p27Kip1 (≤40 vs. >40%) | 1.08 | 0.630-1.87 | 0.78000 | 1.88 | 0.970-4.21 | 0.06400 |
| p21Waf1/Cip1 (≤5 vs. >5%) | 0.82 | 0.440-1.45 | 0.48000 | 1.48 | 0.710-3.00 | 0.31000 |
| Cyclin E (≤70 vs. >70%) | 0.97 | 0.460-2.04 | 0.94000 | 0.86 | 0.330-2.19 | 0.75000 |
| Ascites (≤500 vs. >500 ml) | 0.81 | 0.470-1.37 | 0.43000 | 0.63 | 0.320-1.28 | 0.21000 |
Data census was at 75 months. Significant p-values are indicated in boldface type. PFS, progression-free survival; OS, overall survival; RR, relative risk; CI, confidence interval.
Figure 2.
Kaplan-Meier curve. Kaplan-Meier curves and log-rank p-values for 5-year PFS and OS in the context of cyclin D1 expression (A), p27Kip1 expression (B) and residual tumor volume (C) are shown. PFS, progression-free survival; OS, overall survival.
In the multivariate analysis using the Cox proportional hazards model, overexpression of cyclin D1 was identified as the key determinant of OS (p=0.0019, RR=3.61, 95% CI 1.61–8.12) and PFS (p=0.0052, RR=2.70, 95% CI 1.35–5.41) (Table V). The volume of residual disease and reduced expression of p27Kip1 were found to be independent predictors of OS (p=0.0092 and p=0.042, respectively), but not of PFS when incorporated into a multivariate model (Table V).
Table V.
Multivariate Cox regression analysis of PFS and OS of patients with serous EOC.
| Clinical parameter | PFS
|
OS
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | |
| Residual disease (≤2 vs. >2 cm) | 1.53 | 0.89–2.64 | 0.1200 | 3.06 | 1.32–7.12 | 0.0093 |
| Cyclin D1 (≤20 vs. >20%) | 2.70 | 1.35–5.41 | 0.0052 | 3.61 | 1.61–8.12 | 0.0019 |
| p27Kip1 (≤40 vs. >40%) | 1.01 | 0.59–1.72 | 0.9700 | 2.15 | 1.03–4.51 | 0.0420 |
Significant p-values are indicated in boldface type. EOC, epithelial ovarian cancer; PFS, progression-free survival; OS, overall survival; RR, relative risk; CI, confidence interval.
Association between chemosensitivity and G1-S phase-regulatory protein expression
In order to assess whether the clinicopathological parameters reflect the chemosensitivity, the cohort was divided into two groups: patients who relapsed within 6 months after the last date of first-line chemotherapy; and patients who had no disease progression within 6 months after the last date of first-line chemotherapy. Using the Chi-square test, overexpression of cyclin D1 (p=0.011) as well as residual tumor volume >2 cm (p=0.006) were found to be significantly associated with TFI, suggesting that these parameters are correlated with first-line chemosensitivity (Table VI). In contrast, expression of pRb, p16, p53, p27Kip1, p21Waf1/Cip1 and cyclin E had no statistical correlation with chemosensitivity.
Table VI.
Association of the TFI and clinicopathological parameters in serous EOC.
| TFI <6 months, n (%) | TFI ≥6 months, n (%) | p-value | |
|---|---|---|---|
| Residual disease | |||
| ≤2 cm (n=28) | 4 (6.0) | 24 (36.4) | 0.006 |
| >2 cm (n=38) | 19 (28.8) | 19 (28.8) | |
| Cyclin D1 | |||
| ≤20% (n=55) | 15 (22.7) | 40 (60.6) | 0.011 |
| >20% (n=11) | 8 (12.1) | 3 (4.6) | |
| pRB | |||
| ≤50% (n=40) | 12 (18.2) | 28 (42.4) | 0.305 |
| >50% (n=26) | 11 (16.7) | 15 (22.7) | |
| p16Ink4a | |||
| ≤50% (n=24) | 7 (10.6) | 17 (25.8) | 0.464 |
| >50% (n=42) | 16 (24.3) | 26 (39.4) | |
| p53 | |||
| ≤40% (n=29) | 11 (16.7) | 18 (27.3) | 0.642 |
| >40% (n=37) | 12 (18.2) | 25 (37.9) | |
| p27Kip1 | |||
| ≤40% (n=29) | 13 (19.7) | 16 (24.3) | 0.132 |
| >40% (n=37) | 10 (15.2) | 27 (40.9) | |
| p21Waf/Cip1 | |||
| ≤5% (n=46) | 19 (28.8) | 27 (40.9) | 0.165 |
| >5% (n=20) | 4 (6.0) | 16 (24.3) | |
| Cyclin E | |||
| ≤70% (n=55) | 18 (27.3) | 37 (56.1) | 0.644 |
| >70% (n=11) | 5 (7.6) | 6 (9.1) |
p-values are for TFI <6 months vs. TFI ≥6 months (Chi-square test, Yates correlation). Significant p-values are indicated in boldface type. EOC, epithelial ovarian cancer; TFI, treatment-free interval.
Discussion
Various studies exist concerning the association between G1-S phase-related genes and EOC prognosis, however, the results are conflicting. Amplification of cyclin E in high-resolution oligonucleotide microarrays was previously found to be associated with poor response to primary treatment in serous ovarian cancer (10), but in the present study, cyclin E expression in immunohistochemical analysis revealed no significant correlation with patient outcome of advanced serous EOC. It is considered that the variety of histological types of EOC, different tumor stages, tumor heterogeneity, racial backgrounds of patients, research methodologies and sample sizes may contribute to inconsistent results. In this study, we focused on advanced serous cases (limited to stage III/IV cases) at a single institution with similar surgical and chemotherapeutic procedures administered in order to eliminate such bias.
Cyclin D1, a regulatory kinase subunit that is selectively associated with cyclin-dependent kinase 4 (CDK4), is a crucial modulator of G1 progression in the cell cycle (16). In our analysis, overexpression of cyclin D1 was detected in 11% of the cases (Table II). The overexpression of cyclin D1 was previously observed in 14–89% of EOC cases (11,17–19), but the underlying mechanism has yet to be elucidated. Amplification of cyclin D1 in ovarian tumors occurs infrequently (20). Furthermore, the mostly small cyclin D1 copy gains are not associated with an increase in detectable cyclin D1 protein by immunohistochemistry (21). These findings suggest that the post-transcriptional regulation of cyclin D1 protein production is complex. Recently, Jiang et al performed a systematic validation of the predicted microRNAs for cyclin D1 and revealed that microRNAs suppressed the endogenous cyclin D1 protein and mRNA levels in vitro (22). microRNAs may aid in determining the mechanism of cyclin D1 expression.
Barbieri et al reported that cyclin D1 overexpression significantly influenced the clinical outcome in advanced EOC cases with residual disease greater than 2 cm. They identified cyclin D1 overexpression as an independent prognostic factor in multivariate analysis (5). Similarly, Bali et al identified cyclin D1 overexpression as an independent prognostic factor in the multivariate analysis of 134 serous EOC cases (11). In our study, overexpression of cyclin D1 was significantly correlated with reduced OS and PFS in both univariate and multivariate analyses, suggesting that overexpression of cyclin D1 actually contributes to the prognosis of advanced serous EOCs; therefore, its application to clinical practice is expected.
We found that both overexpression of cyclin D1 and residual tumor volume were significantly associated with TFI, suggesting that these parameters are correlated with first-line chemosensitivity (Table VI). Zhou et al showed that inhibition of cyclin D1 expression by siRNA in oral squamous cell carcinoma cells resulted in a decrease in cisplatin IC50 level. In vivo transplantation models also confirmed a cisplatin-sensitizing effect of cyclin D1 knockdown in these cell lines (23). In addition, it was reported that overexpression of cyclin D1 was associated with reduced chemosensitivity and a higher survival rate upon cisplatin administration in a pancreatic cancer model (24). Moreover, inhibition of cyclin D1 expression rendered cells more susceptible to cisplatin-mediated apoptosis in the same model (24). Taken together, these findings indicate that cyclin D1 expression may contribute to chemoresistance in a number of cancers, although further investigation is required. Therefore, we speculate that overexpression of cyclin D1 contributes to poor prognosis, which may in part be mediated by chemoresistance in ovarian cancer.
p27Kip1 is a cyclin-dependent kinase (cdk) inhibitor that regulates cell cycle progression from the G1 to S-phase. In non-cycling cells, p27Kip1 binds to cyclin E-cdk2 complexes and inhibits their activation. In contrast, p27Kip1 binding to catalytically active cyclin D-cdk4/6 complexes results in p27Kip1 degradation and the subsequent release of cdk2 from inhibition in proliferating cells (12,25). Therefore, p27Kip1 helps to coordinate a balance between proliferation and arrest (12,25). In the present study, reduced expression of p27Kip1 was detected in 43.9% of the cases. In previous immunohistochemical studies of ovarian tumors, 36.2-100% exibited low expression of p27Kip1 (26). We found that reduced expression of p27Kip1 was associated with shorter OS (p=0.064), but had no statistically significant correlation with PFS (p=0.78). When incorporated into a multivariate model, reduced expression of p27Kip1 was found to be an independent predictor of OS (p=0.042) (Table V). The relationship between p27Kip1 expression levels and prognosis is controversial. Conflicting data regarding the possible prognostic role of p27Kip1 status also exist for ovarian cancer. Psyrri et al evaluated subcellular localization and protein levels of p27Kip1 in 150 advanced EOCs and found that low nuclear p27Kip1 expression was associated with improved prognosis, suggesting its potential as a strong predictor of outcome in advanced EOCs (12). On the other hand, Shigemasa et al reported that negative p27Kip1 expression was significantly correlated with poor survival in serous EOC patients, suggesting that the underexpression of p27Kip1 caused by a post-translational mechanism may contribute to development and progression and may result in poor prognosis of serous EOCs (27). It is hypothesized that different methodologies of immunohistochemical grading may account for these discrepancies. Our result suggests that p27Kip1 is associated with the prognosis of this disease, as previously reported.
In conclusion, overexpression of cyclin D1 contributed markedly to poor prognosis in advanced serous EOC; this may in part be mediated by chemoresistance. Cyclin D1 may be a target for overcoming the refractory nature of advanced serous EOC.
Acknowledgments
We thank Dr Miho Takao and Dr Hideo Shinozaki for the assistance in this research. This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology to N.Y., A.O. and S.T., and by the Japanese Ministry of Health, Labour and Welfare to K.O.
References
- 1.Takakura S, Takano M, Takahashi F, Saito T, Aoki D, Inaba N, Noda K, Sugiyama T, Ochiai K, Japanese Gynecologic Oncology Group Randomized phase II trial of paclitaxel plus carboplatin therapy versus irinotecan plus cisplatin therapy as first-line chemotherapy for clear cell adenocarcinoma of the ovary: a JGOG study. Int J Gynecol Cancer. 2010;20:240–247. doi: 10.1111/igc.0b013e3181cafb47. [DOI] [PubMed] [Google Scholar]
- 2.Shimada M, Kigawa J, Ohishi Y, Yasuda M, Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T, Kaku T. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009;113:331–334. doi: 10.1016/j.ygyno.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K, Japanese Gynecologic Oncology Group Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri F, Lorenzi P, Ragni N, Schettini G, Bruzzo C, Pedullà F, Alama A. Overexpression of cyclin D1 is associated with poor survival in epithelial ovarian cancer. Oncology. 2004;66:310–315. doi: 10.1159/000078332. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Conference Ovarian cancer. Screening, treatment, and follow-up. JAMA. 1995;273:491–497. [PubMed] [Google Scholar]
- 7.Terauchi F, Nishi H, Moritake T, et al. Prognostic factor on optimal debulking surgery by maximum effort for stage IIIC epithelial ovarian cancer. J Obstet Gynaecol Res. 2009;35:315–319. doi: 10.1111/j.1447-0756.2008.00928.x. [DOI] [PubMed] [Google Scholar]
- 8.Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 9.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Etemadmoghadam D, deFazio A, Beroukhim R, Mermel C, George J, Getz G, Tothill R, Okamoto A, Raeder MB, Harnett P, Lade S, Akslen LA, Tinker AV, Locandro B, Alsop K, Chiew YE, Traficante N, Fereday S, Johnson D, Fox S, Sellers W, Urashima M, Salvesen HB, Meyerson M, Bowtell D, AOCS Study Group Integrated copy number and expression analysis of chemoresistant ovarian carcinomas. Clin Cancer Res. 2009;15:1417–1427. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bali A, O’Brien PM, Edwards LS, Sutherland RL, Hacker NF, Henshall SM. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004;10:5168–5177. doi: 10.1158/1078-0432.CCR-03-0751. [DOI] [PubMed] [Google Scholar]
- 12.Psyrri A, Bamias A, Yu Z, et al. Subcellular localization and protein levels of cyclin-dependent kinase inhibitor p27 independently predict for survival in epithelial ovarian cancer. Clin Cancer Res. 2005;11:8384–8390. doi: 10.1158/1078-0432.CCR-05-1270. [DOI] [PubMed] [Google Scholar]
- 13.Khouja MH, Baekelandt M, Nesland JM, Holm R. The clinical importance of Ki-67, p16, p14, and p57 expression in patients with advanced ovarian carcinoma. Int J Gynecol Pathol. 2007;26:418–425. doi: 10.1097/pgp.0b013e31804216a0. [DOI] [PubMed] [Google Scholar]
- 14.Milde-Langosch K, Riethdorf S. Role of cell-cycle regulatory proteins in gynecological cancer. J Cell Physiol. 2003;196:224–244. doi: 10.1002/jcp.10286. [DOI] [PubMed] [Google Scholar]
- 15.Kommoss S, du Bois A, Ridder R, Trunk MJ, Schmidt D, Pfisterer J, Kommoss F, AGO-OVAR Independent prognostic significance of cell cycle regulator proteins p16(INK4a) and pRb in advanced-stage ovarian carcinoma including optimally debulked patients: a translational research subprotocol of a randomised study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. Br J Cancer. 2007;96:306–313. doi: 10.1038/sj.bjc.6603531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 17.Dhar KK, Branigan K, Parkes J, et al. Expression and subcellular localization of cyclin D1 protein in epithelial ovarian tumour cells. Br J Cancer. 1999;81:1174–1181. doi: 10.1038/sj.bjc.6690826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worsley SD, Ponder BA, Davies BR. Overexpression of cyclin D1 in epithelial ovarian cancers. Gynecol Oncol. 1997;64:189–195. doi: 10.1006/gyno.1996.4569. [DOI] [PubMed] [Google Scholar]
- 19.Barbieri F, Cagnoli M, Ragni N, Foglia G, Bruzzo C, Pedullà F, Alama A. Increased cyclin D1 expression is associated with features of malignancy and disease recurrence in ovarian tumors. Clin Cancer Res. 1999;5:1837–1842. [PubMed] [Google Scholar]
- 20.Masciullo V, Scambia G, Marone M, et al. Altered expression of cyclin D1 and CDK4 genes in ovarian carcinomas. Int J Cancer. 1997;74:390–395. doi: 10.1002/(sici)1097-0215(19970822)74:4<390::aid-ijc5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Diebold J, Mösinger K, Peiro G, et al. 20q13 and cyclin D1 in ovarian carcinomas. Analysis by fluorescence in situ hybridization. J Pathol. 2000;190:564–571. doi: 10.1002/(SICI)1096-9896(200004)190:5<564::AID-PATH569>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Q, Feng MG, Mo YY. Systematic validation of predicted microRNAs for cyclin D1. BMC Cancer. 2009;9:194. doi: 10.1186/1471-2407-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Zhang Z, Yang X, Chen W, Zhang P. Inhibition of cyclin D1 expression by cyclin D1 shRNAs in human oral squamous cell carcinoma cells is associated with increased cisplatin chemosensitivity. Int J Cancer. 2009;124:483–489. doi: 10.1002/ijc.23964. [DOI] [PubMed] [Google Scholar]
- 24.Biliran H, Jr, Wang Y, Banerjee S, et al. Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin Cancer Res. 2005;11:6075–6086. doi: 10.1158/1078-0432.CCR-04-2419. [DOI] [PubMed] [Google Scholar]
- 25.Blain SW, Scher HI, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3:111–115. doi: 10.1016/s1535-6108(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 26.Nam EJ, Kim YT. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:1169–1182. doi: 10.1111/j.1525-1438.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 27.Shigemasa K, Shiroyama Y, Sawasaki T, et al. Underexpression of cyclin-dependent kinase inhibitor p27 is associated with poor prognosis in serous ovarian carcinomas. Int J Oncol. 2001;18:953–958. doi: 10.3892/ijo.18.5.953. [DOI] [PubMed] [Google Scholar]