Abstract
Although the mechanism involved in acute liver failure (ALF) has not yet been clarified, microcirculatory disturbance in the liver appears to play a pivotal role in the progression of this disease. To confirm the existence of hepatic hypoxic conditions, we evaluated the amounts of lactate dehydrogenase (LDH) in hepatocytes, since its production increases under low oxygen concentrations. Histological examination was performed in 7 patients with ALF. All 7 patients underwent a liver biopsy during the acute phase of ALF, and 4 of them underwent a second biopsy during the recovery phase. The obtained samples were immunohistochemically stained with anti-LDH5 and anti-CD-68 antibodies. As controls, we examined samples from patients with acute hepatitis, chronic hepatitis and liver cirrhosis. The production of LDH by hepatocytes and the number of CD-68 positive macrophages were markedly increased at the acute phase of ALF, and both of these effects abruptly decreased during the recovery phase. By contrast, most of the samples from the patients with chronic hepatitis and acute hepatitis showed slightly any increase in LDH staining. In cirrhotic patients, partially elevated LDH production was observed mainly around the central vein, but the staining intensity was less compared to that in ALF patients. Our findings indicate that hepatic hypoxic conditions exist in ALF at the acute phase and seem to closely correlate with macrophage overactivation in the liver. We speculate that microcirculatory disturbance may be a key process in the development and progression of ALF.
Keywords: lactate dehydrogenase, acute liver failure
Introduction
Acute liver failure (ALF) is a disease associated with high mortality and characterized by massive hepatocyte necrosis. Once liver atrophy has progressed, liver transplantation is the only promising treatment option (1,2). Even in such cases, it is difficult to determine the indications for liver transplantation, since some patients recover successfully without it. The difficulties associated with predicting the prognosis of ALF are mainly caused by the fact that the mechanism of the disease has not yet been fully clarified.
Recently, several authors have reported that products derived from macrophages are increased in the serum of patients with ALF, suggesting that overactivated macrophages in the liver may play an important role in the progression of ALF (3–5). However, the issue of how activated macrophages are involved in the development of massive necrosis remains to be clarified. On the other hand, earlier studies have revealed deposition of fibrin in the sinusoid using rat models of ALF, suggesting that microcirculatory disturbance may be a final process in the development of massive liver necrosis in ALF (6–8).
Collectively, considering these previous findings, we subsequently hypothesized that overactivated macrophages damage endothelial cells of the sinusoid, either directly or indirectly via their release of cytokines, which may cause microcirculation disturbance and lead to massive liver necrosis. Although proving the existence of anaerobic conditions in the liver would support our theory, it is difficult to directly measure oxygen concentrations in the hepatic microcirculation. Therefore, we focused on the production of lactate dehydrogenase (LDH) in hepatocytes. LDH is an essential enzyme for anaerobic respiration, and its production has been shown to be increased under hypoxic conditions in various cell lines (9–12).
In the present study, we examined the amounts of LDH production in hepatocytes using biopsy samples from patients with ALF in comparison to samples from patients with acute hepatitis, chronic hepatitis and liver cirrhosis.
Patients and methods
Patients
Between April 2005 and March 2007, 15 patients suffering from ALF were admitted to our hospital. The patients showed a prolonged prothrombin time (PT) [PT-international normalized ratio (INR) >1.5] and encephalopathy of more than grade 2 that occurred within 1 week of onset. Ultrasonography guided liver biopsy was performed in 7 patients for the purpose of the following: i) an examination for etiology, particularly to evaluate the participation of autoimmune hepatitis (AIH); ii) exclusion of the pre-existence of chronic liver disease; iii) evaluation of the clinical course of the liver disease. The etiologies of the patients were varied: 2 were hepatitis B virus (HBV), 1 was hepatitis A virus (HAV) and 4 were undetermined. For all patients, liver-supporting procedures, such as plasma exchange and brain edema inhibition, were performed. Lamivudine was also administered when HBV was detected in the serum.
To clarify the pathological characteristics of the ALF patients, we compared their samples to liver biopsy samples obtained from patients with other liver diseases [7 acute hepatitis (AH) patients, 8 chronic hepatitis (CH) patients and 6 liver cirrhosis (LC) patients] during the same period. The causes of AH were HBV (n=3), AIH (n=2) and undetermined (n=2). These patients maintained a PT-INR of <1.5 throughout the clinical course and recovered within 1 month. Regarding the patients with chronic liver diseases, the etiologies of CH were HCV (n=2), HBV (n=3) and AIH (n=3), and those of LC were HCV (n=2), AIH (n=2), primary biliary cirrhosis (n=1) and non-alcoholic steatohepatitis (n=1). All of the patients with LC were classified as Child-Pugh A. The laboratory data at the time of liver biopsy are shown in Table I.
Table I.
Laboratory findings of the patients at the time of liver biopsy.
| ALF (n=7) | AH (n=7) | CH (n=8) | LC (n=6) | |
|---|---|---|---|---|
| Albumin (g/dl) | 3.3±0.3 | 3.8±0.3 | 3.8±0.6 | 3.6±0.6 |
| Bilirubin (mg/dl) | 10.2±8.2 | 7.7±7.4 | 2.0±1.9 | 1.1±0.6 |
| D/T ratio | 0.69±0.02 | 0.58±0.16 | 0.34±0.19 | 0.31±0.06 |
| AST (U/l) | 3,660.3±2,553.5 | 1,815.4±1,294.1 | 248.0±208.8 | 96.3±59.2 |
| ALT (U/l) | 3,921.9±2,249.3 | 3,079.0±2,613.0 | 310.5±238.4 | 132.2±145.0 |
| LDH (U/l) | 1,849.7±1,799.9 | 731.3±394.2 | 249.9±70.8 | 257.0±89.5 |
| PT-INR | 2.92±2.36 | 1.32±0.12 | 1.23±0.23 | 1.13±0.06 |
| Platelets (×104/μl) | 15.2±6.5 | 23.4±12.0 | 16.0±4.7 | 12.6±3.9 |
Data are shown as the mean ± SD. ALF, acute liver failure; AH, acute hepatitis; CH, chronic hepatitis; LC, liver cirrhosis. D/T ratio, direct bili-rubin/total bilirubin ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PT-INR, prothrombin time-international normalized ratio.
Pathological examination
The biopsy samples were fixed with 20% formalin and embedded in paraffin blocks. Serial sections (3 μm) were cut from the blocks, deparaffinized and subjected to H&E staining or immunohistochemical staining by an indirect immunoperoxidase method using Histofine Simple Stain (Nichirei Corporation, Tokyo, Japan). The primary antibodies used were a monoclonal antibody against CD-68 (Abcam Inc., Cambridge, MA, USA) and a polyclonal antibody against LDH-5 (Abcam Inc.). As normal controls for the immunohistochemical staining, three biopsy samples from liver transplant (LT) donors were stained using the same procedure. The intensity of staining for LDH and the number of CD-68-positive cells were classified into three grades: 1+, weak; 2+, moderate; and 3+, intense/markedly increased. When the staining result did not differ from that in the normal control samples, the sample was graded as 1+.
Results
The H&E-stained sections from ALF patients on admission revealed that >50% of the hepatocytes were necrotized. In serial sections stained with anti-LDH and anti-CD-68 antibodies, LDH was strongly and diffusely detected in the remaining hepatocytes, and a marked increase in the number of CD-68-positive cells was mainly observed in the sinusoid (Fig. 1). In 4 patients who survived without LT, a second biopsy was performed during their recovery phase at 7–10 days after the first biopsy. These second biopsies exhibited significant decreases in LDH production in hepatocytes and the number of CD-68-positive cells accompanied by the regeneration of hepatocytes.
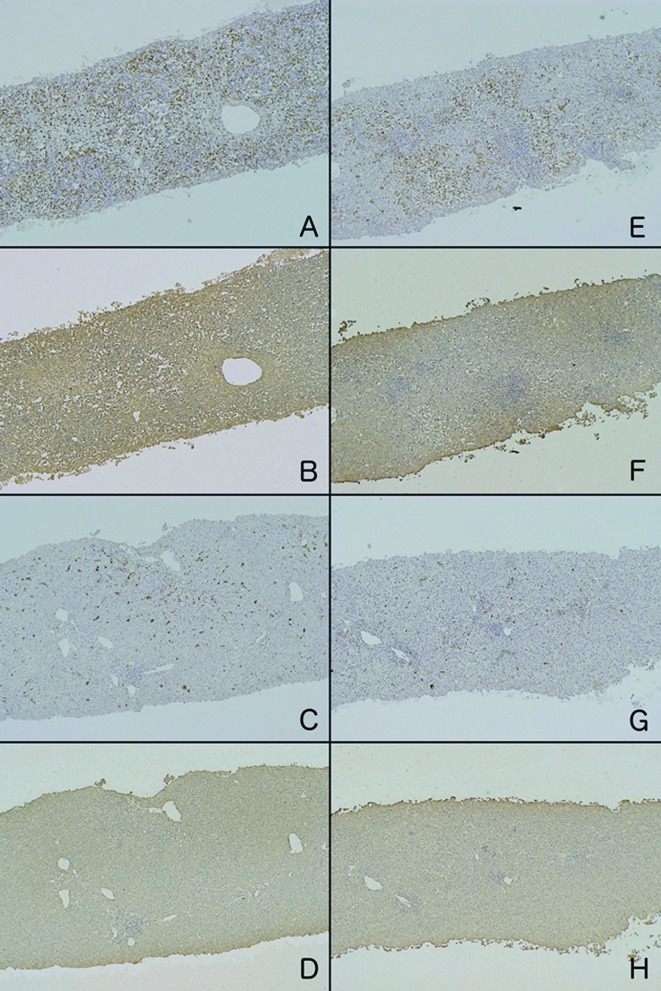
Figure 1.
Immunohistochemical staining of 2 patients (A/B/C/D and E/F/G/H) with ALF. On admission, CD-68-positive cells were markedly increased (A and E), and the residual hepatocytes were diffusely stained with an anti-LDH antibody (B and F). In the recovery phase, significant decreases in CD-68-positive cells (C and G) and LDH production in hepatocytes (D and H) were observed.
In patients with AH, the results of CD-68 immunostaining were varied (Fig. 2). Two patients exhibited a marked increase in CD-68-positive cells, similar to the case for the ALF patients, whereas the other patients showed less intense staining. In contrast to the CD-68 staining, the intensity of LDH staining was generally weak. None of the CD-68-stained samples from the patients with AH were comparable to those from the ALF patients. Regarding the patients with CH, the staining intensities of both LDH and CD-68 were similar to those in the normal control samples (Fig. 3).
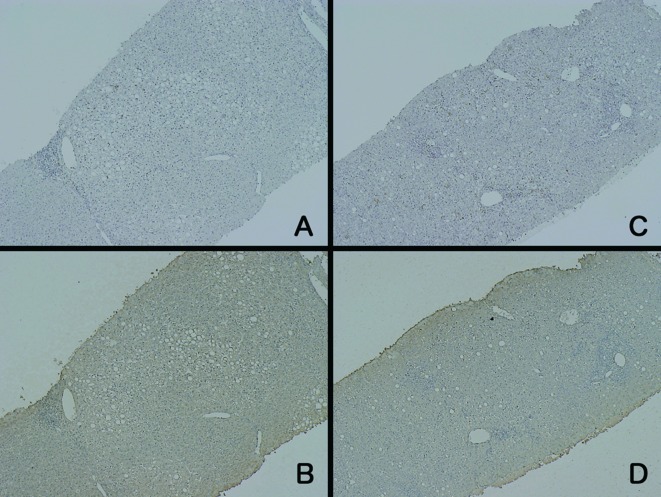
Figure 2.
Immunohistochemical staining of 2 patients (A/B and C/D) with AH. The increase in the number of CD-68-positive cells varied (A and C). The intensity of LDH staining (B and D) was weaker than that in the patients with ALF.
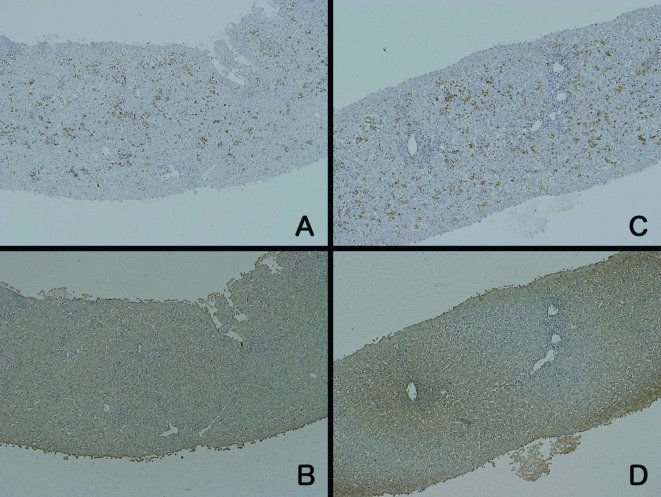
Figure 3.
Immunohistochemical staining of 2 patients (A/B and C/D) with CH. The staining intensities for CD-68 (A and C) and LDH (B and D) were similar to those of the normal control samples.
The samples from the LC patients showed different findings compared to the samples from the patients with the other diseases (Fig. 4). Regarding LDH staining, unevenly stained hepatocytes were observed, and the staining intensity was weaker around the portal area. The hepatocytes in the zone around the central vein showed relatively strong staining for LDH, but the intensity was not as strong as that in the samples from the ALF patients at the acute phase. On the other hand, the number of CD-68-positive cells was equal or slightly increased compared to the normal control samples. When the number of CD-68-positive cells was increased, they were distributed evenly throughout the liver. The grading results for LDH and CD-68 staining are summarized in Table II.
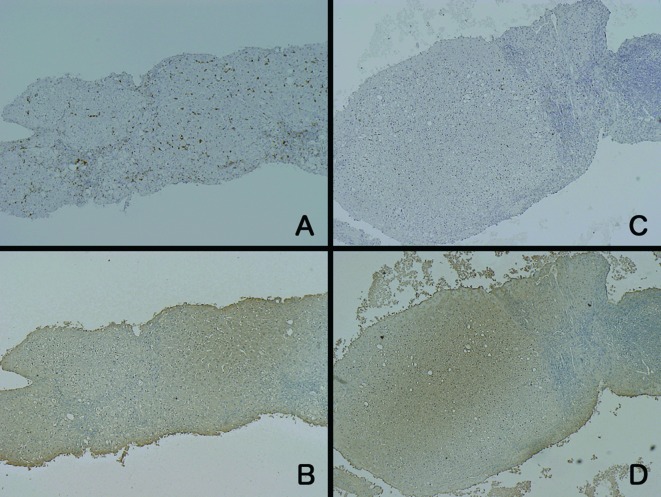
Figure 4.
Immunohistochemical staining of 2 patients (A/B and C/D) with LC. The intensity of CD-68 staining (A and C) was weaker than that in the patients with ALF or AH. On the other hand, LDH-positive hepatocytes were unevenly distributed (B and D) and the intensity was strong around the central vein.
Table II.
Grading of staining intensity.
| Anti-LDH5
|
Anti-CD-68
|
|||||
|---|---|---|---|---|---|---|
| 1+ | 2+ | 3+ | 1+ | 2+ | 3+ | |
| ALF (n=7) | 0 | 1 | 6 | 0 | 1 | 6 |
| AH (n=7) | 3 | 2 (2) | 0 | 1 | 3 | 3 |
| CH (n=8) | 7 | 1 | 0 | 7 | 1 | 0 |
| LC (n=6) | 0 | (5) | (1) | 3 | 3 | 0 |
The patient numbers in parentheses indicate that the obtained samples showed uneven staining, while the patient numbers without parentheses indicate that the samples exhibited diffuse staining. ALF, acute liver failure; AH, acute hepatitis; CH, chronic hepatitis; LC, liver cirrhosis.
Discussion
Although various ‘triggers’ have the potential to elicit ALF, most of them are divided into two major categories, namely direct hepatotoxic agents, such as acetaminophen, and immune response-related causes, including hepatitis viruses (2,13). With the former, the degree of liver damage generally depends on the ingested dose of the toxin. With the latter, however, it remains unclear why some patients proceed to ALF, while most patients with the same etiology suffer no more than self-limiting AH. The main ‘triggers’ of the latter category are hepatitis viruses, such as HBV, HAV and hepatitis E virus (HEV), in which hepatocyte death has been considered to be induced by cytotoxic T cells that recognize virus-related antigens on the cell surface (14). However, this cytotoxic mechanism cannot explain the reason why a limited proportion of patients develop ALF.
Previous studies using ALF animal models with immune response processes indicated that hepatic microcirculation disturbance may play a pivotal role in disease progression, which may be caused by cytotoxic mediators from activated sinusoidal-lining cells (6–8). However, it is difficult to directly prove that the hepatocytes of patients suffering from ALF exist under hypoxic conditions. Before beginning the present study, we hypothesized that the amount of intracellular LDH represents the intracellular oxygen concentration as it is well known to exhibit increased transcription under anaerobic conditions (9–12). This phenomenon is widely observed, regardless of the organ or species. Considering that LDH is only a crucial enzyme for anaerobic respiration, its increased transcription appears to be a reasonable response.
In the past, elevated serum levels of LDH in patients with hepatitis have merely been regarded as a result of enzyme leakage following destruction of hepatocytes. Furthermore, the diagnostic value of serum LDH in liver disease has been thought to be low compared to alanine aminotransferase due to the widespread distribution of LDH-producing cells in the whole body (15,16). The only exceptions are congestive liver and shock liver, which are both associated with decreases in the hepatic oxygen concentration and distinct increases in the serum LDH level (17). Taking these findings for hypoxic liver diseases together with the observation that LDH transcription increases under hypoxic conditions, we consider that the degree of LDH production can be used as a marker of hypoxia in immunohistochemical analysis.
In patients with ALF, we found that LDH production in the remaining hepatocytes was distinctly increased compared to that in other liver diseases. Considering that our biopsy samples were obtained within 1 week of onset, this result indicates that microcirculation disturbance exists from the early stage of the disease. Another important point is that the biopsy samples simultaneously showed marked proliferation of macrophages. In patients who survived without liver transplantation, decreases in the number of macrophages and in the amount of LDH production occurred during the recovery phase. Notably, some of the patients with AH showed similar macrophage proliferation as the ALF patients, but their intensity of LDH staining was not strong. Although it remains unclear what finally drives AH patients with macrophage overactivation towards ALF, we suggest that a transient hyper-inflammatory response results in self-limiting AH, while a persistent and uncontrolled response leads to ALF.
Another important finding of the present study was the elevated LDH production in patients with LC. Although the intensity of this staining was not comparable to that in samples from the ALF patients, it was significantly stronger than that in samples from CH patients. Although hepatocyte hypoxia in cirrhosis has been well established in experimental models, it has been difficult to demonstrate that hepatocytes in cirrhotic patients exist under hypoxic conditions (18,19). Our findings suggest that hepatocytes in patients with LC are under chronic hypoxic conditions, which may be caused by accumulated collagens that interfere with oxygen diffusion to hepatocytes.
In our preliminary study, we were unable to enroll any cases of acetaminophen-induced ALF as such cases are scarce in Japan. Therefore, it remains unclear whether microcirculation disturbance contributes to the progression of ALF caused by agents exhibiting direct hepatotoxicity. We presume that the process of macrophage overactivation and consequent microcirculation disturbance may have lower contributions to ALF caused by acetaminophen. Further studies, including animal model experiments, are required.
In conclusion, we demonstrated that LDH production in hepatocytes varies among ALF, AH, LC and CH patients, and we consider that this variation reflects the degree of intracellular oxygen concentration. Elevated macrophage proliferation and increased LDH production were observed in ALF, suggesting that overactivation of macrophages and subsequent microcirculation disturbance in the liver play pivotal roles in the progression of ALF.
References
- 1.Sass DA, Shakil AO. Fulminant hepatic failure. Liver Transpl. 2005;11:594–605. doi: 10.1002/lt.20435. [DOI] [PubMed] [Google Scholar]
- 2.Vaquero J, Blei AT. Etiology and management of fulminant hepatic failure. Curr Gastroenterol Rep. 2003;5:39–47. doi: 10.1007/s11894-003-0008-8. [DOI] [PubMed] [Google Scholar]
- 3.Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, Onji M. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol. 2005;40:52–56. doi: 10.1007/s00535-004-1493-8. [DOI] [PubMed] [Google Scholar]
- 4.Matsui A, Mochida S, Ohno A, Nagoshi S, Hirose T, Fujiwara K. Plasma osteopontin levels in patients with fulminant hepatitis. Hepatol Res. 2004;29:202–206. doi: 10.1016/j.hepres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Møller HJ, Gronbaek H, Schiodt FV, Holland-Fischer P, Schilsky M, Munoz S, Hassanein T, Lee WM, the US Acute Liver Failure Study Group Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671–676. doi: 10.1016/j.jhep.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka Y, Yamada S, Sato Y, Masaki N, Oka H. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut. 1988;29:1103–1108. doi: 10.1136/gut.29.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirata K, Ogata I, Ohta Y, Fujiwara K. Hepatic sinusoidal cell destruction in the development of intravascular coagulation in acute liver failure of rats. J Pathol. 1989;158:157–165. doi: 10.1002/path.1711580211. [DOI] [PubMed] [Google Scholar]
- 8.Takenaka K, Sakaida I, Yasunaga M, Okita K. Ultrastructural study of development of hepatic necrosis induced by TNF-alpha and D-galactosamine. Dig Dis Sci. 1998;43:887–892. doi: 10.1023/a:1018898905478. [DOI] [PubMed] [Google Scholar]
- 9.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay HH, Zhu S, Tsoi S. Hypoxia and lactate production in trophoblast cells. Placenta. 2007;28:854–860. doi: 10.1016/j.placenta.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Shi LB, Huang JH, Han BS. Hypoxia inducible factor-1alpha mediates protective effects of ischemic preconditioning on ECV-304 endothelial cells. World J Gastroenterol. 2007;28:2369–2373. doi: 10.3748/wjg.v13.i16.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen BS, Alsner J, Overgaard J, Horsman MR. Hypoxia induced expression of endogenous markers in vitro is highly influenced by pH. Radiother Oncol. 2007;83:362–366. doi: 10.1016/j.radonc.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Losser MR, Payen D. Mechanisms of liver damage. Semin Liver Dis. 1996;16:357–367. doi: 10.1055/s-2007-1007249. [DOI] [PubMed] [Google Scholar]
- 14.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 15.Rosalki SB, McIntyre N. Biochemical investigations in the management of liver disease. In: Bircher J, editor. Oxford Textbook of Clinical Hepatology. 2nd edition. Oxford University Press; Oxford: 1999. pp. 503–521. [Google Scholar]
- 16.Kaplan MM. Laboratory tests. In: Shiff L, editor. Diseases of the Liver. 7th edition. J.B. Lippincott Company; Philadelphia: 1993. pp. 108–144. [Google Scholar]
- 17.Cassidy WM, Reynolds TB. Serum lactic dehydrogenase in the differential diagnosis of acute hepatocellular injury. J Clin Gastroenterol. 1994;19:118–121. doi: 10.1097/00004836-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 18.DeLeve LD. Hepatic microvasculature in liver injury. Semin Liver Dis. 2007;27:390–400. doi: 10.1055/s-2007-991515. [DOI] [PubMed] [Google Scholar]
- 19.Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol. 2007;6:208–213. [PubMed] [Google Scholar]