Abstract
The precise mechanism of prolapse uteri is not fully understood. There is evidence to suggest that abnormalities of collagen, the main component of extracellular matrix, or its repair mechanism, may predispose women to prolapse. To investigate the characteristic structure of human uterine cervix of patients with prolapse uteri, various types of collagen expression in the uterine cervix tissues of the prolapse uteri were compared to those of normal uterine cervix. After informed consent, 36 specimens of uterine cervical tissues were obtained at the time of surgery from 16 postmenopausal women with prolapse uteri (stage III–IV by the Pelvic Organ Prolapse Quantification examination) and 20 postmenopausal women without prolapse uteri (control group). Collagens were extracted from the uterine cervix tissues by salt precipitation methods. The relative levels of various collagens were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The uterine cervix was longer in the patients with prolapse uteri than those of postmenopausal controls without prolapse uteri. The ratios of type III to type I collagen in the uterine cervical tissues were significantly decreased in the prolapse uteri, as compared to those of the postmenopausal uterine cervix without prolapse. These results suggest that decreased type III collagen expression may play an important role in determing the physiology and structure of the uterine cervix tissues of prolapse uteri.
Keywords: collagen, cervix, uterine prolapse
Introduction
The extracellular matrix (ECM) is considered to play an important role in the stability of tissues and in regulating the growth and differentiation of cells (1,2). Synthesis, accumulation and catabolism of the ECM occur during wound healing and during the initiation and progression of numerous diseases (3).
Moreover, it is generally acknowledged that the ECM does not function as a mere passive scaffold for connective tissue within organ architecture. It also plays an ‘informational’ role through a network of interactions between cells and signal molecules which is of primary importance in the control of cellular proliferation and motility during histogenesis for the maintenance of tissue homeostasis and in cancer development.
Prolapse of the pelvic organs is a common disease affecting the lives of millions of women. Women with prolapse suffer from chronic pelvic pain and pressure, urinary and fecal incontinence, sexual dysfunction and social isolation. Despite the high prevalence of this disease and the devastating impact on the lives of women, very little is known about its pathophysiology.
Recently, it has been suggested that a structural defect in the vagina and its supportive tissues, such as a decrease in collagen content or a change in collagen subtypes, is one of the mechanisms that predisposes a woman to prolapse. Thus, there have been many studies throughout the literature in which collagen is analyzed in vaginal and uterine-supporting ligament biopsies procured at the time of a repair of prolapse in patients with prolapse or at the time of a hysterectomy in patients without prolapse. The comparison of these samples has provided conflicting results (4–13).
Trauma or pathology may lead to altered responses to mechanical stresses placed on connective tissue, producing changes in the ECM. Alterations in collagen synthesis and collagen types are causally related to connective tissue disorders, such as inguinal hernia (14), genito-urinary prolapse and urinary stress incontinence (15,16). The uterine cervices of patients with prolapse are dramatically altered when compared to those without prolapse. Therefore, the precise control of ECM metabolism in the uterine cervix is critical for the pathophysiology of prolapse uteri.
In the present study, we investigated the composition of the various types of collagen, the major component of ECM, in human uterine cervical tissues of prolapse uteri by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Materials and methods
This project was approved by the Committee on Investigations Involving Human Subjects of Wakayama Medical College. Informed consent was obtained from each subject after the purpose and nature of the study had been fully explained.
Patients and tissues
Sixteen uterine cervical samples were obtained from postmenopausal Japanese women (59–85 years of age) with uterine prolapse, who had undergone a vaginal hysterectomy and colpoperineoplasty, and were defined as the prolapse group. A Pelvic Organ Prolapse Quantification examination (17) was performed on the patients with uterine prolapse to define the stage of prolapse. All patients with uterine prolapse showed stage II or greater prolapse (POP-Q).
As the control group, 20 uterine cervical samples were obtained from postmenopausal Japanese women (58–82 years of age) without uterine prolapse, who had undergone a abdominal hysterectomy for a benign indication other than prolapse. Variables, including age, postmenopausal period, parity and BMI, were not significantly different in the two groups. None of the patients received any hormone therapy before surgery.
The uterine cervical length was measured from the internal to external os along the cervical canal, and values are expressed in millimeters.
SDS-PAGE of pepsin-solubilized collagens from the uterine cervical tissues
Minced samples of human uterine cervical tissues were washed overnight in cold distilled water and freed of blood. Tissues were homogenized with a Polytron homogenizer in 50 volumes of 0.5 M acetic acid that contained 1 mg/ml pepsin (Sigma Chemical Co., St. Louis, MO, USA). Collagens were extracted with constant stirring for 24 h at 4°C. Each solution was centrifuged at 39,000 × g for 1 h at 4°C. Collagens were reextracted from the pellet under the same conditions as described above for 48 h.
The supernatants corresponding to individual samples were then combined, and collagens were precipitated by addition of 4.0 M NaCl to a final concentration of 2.0 M. Each precipitate was dissolved in 0.5 M acetic acid, and the solution was dialyzed against 0.02 M Na2HPO4. Precipitated collagens were redissolved in 0.5 M acetic acid, dialyzed exhaustively against 0.05 M acetic acid and finally lyophilized.
The solubility of the tissue collagen from each uterine cervical tissue sample was estimated by comparing the hydroxyproline content of the initial homogenate to that of the final solution of collagen (18). Type V collagen was isolated by salt precipitation from pepsin digests of human uterine cervical tissues by the methods described elsewhere (19,20). The extracted type V collagen was also lyophilized.
Estimation of the relative abundance of the α1(III) chain and α1(V) chain was performed by interrupted gel electrophoresis (21). Electrophoresis was performed in an 8% polyacrylamide slab gel (Sigma Chemical Co.). The gel and electrode buffers consisted of 0.1 M phosphate buffer, pH 7.2, containing 0.1% SDS (Nacalai Tesque, Inc., Kyoto, Japan), as previously described (22). Lyophilized samples of collagen and type V collagen were dissolved at a concentration of 0.2 mg/ml and denatured by heating in the gel buffer that contained 1% SDS at 60°C for 30 min.
Aliquots of 25 μl of each solution of denatured collagens and 5 μl of denatured type V collagen were applied to the gel and subjected to electrophoresis at 80 mA. After 1.5 h, the current was switched off and sample wells were filled with a solution of 20% β-mercaptoethanol (Wako Chemical Co., Osaka, Japan) in gel buffer, and the β-mercaptoethanol was allowed to diffuse into the gel for 1 h to cleave the intramolecular disulfide bonds of type III collagen, [α1(III)]3. Then, electrophoresis was resumed and allowed to continue for another 1 h. Each collagen α chain was stained with Coomassie brilliant blue (Sigma Chemical Co.) and quantitated by densitometry.
The relative amounts of α1(III) or α1(V) chains were calculated by dividing the intensities of the band areas under densitometric peaks of α1(III) and of α1(V) by that of α1(I). In this method, the α chains derived from type IV collagen could not be evaluated because of degradation of this collagen by pepsin.
Statistical analysis
The uterine cervical length was expressed as the mean ± SD. Densitometric data were expressed as the mean ± SEM. Mean values were compared by the Student’s t-test or analysis of variance using a StatView software program on a Macintosh computer. Two-tailed P-values <0.05 were considered statistically significant.
Results
The uterine cervix of prolapse uteri were significantly longer compared to those of the postmenopausal uterus (Table I).
Table I.
Relative abundance of the α1(III) and α1(V) chains of collagen as compared to the α1(I) chain in the human uterine cervix tissues of the postmenopausal uterus as control and the prolapse uteri.
| No. of patients | Cervical length (mm) | Relative ratio of collagen α chain
|
||
|---|---|---|---|---|
| α1(III)/α1(I) | α1(V)/α1(I) | |||
| Control | 16 | 28±5 | 0.18±0.05 | 0.02±0.01 |
| Prolapse uteri | 20 | 37±8a | 0.06±0.03b | 0.01±0.01 |
Data are presented as the means ± SEM.
P<0.05;
P<0.01.
Although the relative levels of α1(I) and α1(V) were similar in the uterine cervical tissues in the two groups (Fig. 1), those of α1(III) were decreased in the uterine cervical tissues of the prolapse uteri compared to those of the normal postmenopausal uterine cervix (Fig. 1). The mean ratio of the intensity of the band of α1(III) to that of α1(I) in the uterine cervix tissues of prolapse uteri was significantly lower than that in the normal postmenopausal uterine tissues (P<0.01). The data are summarized in Table I.
Figure 1.
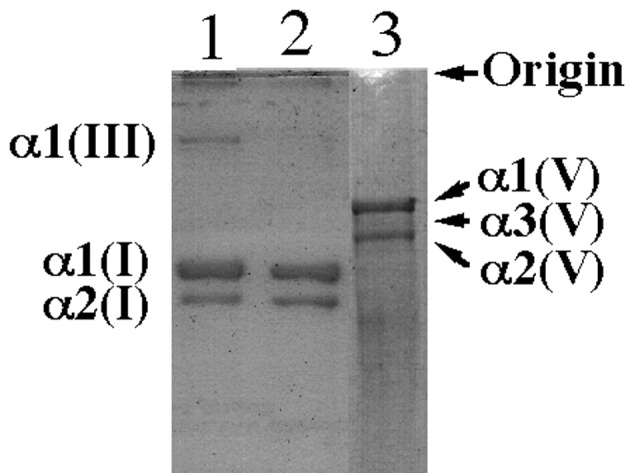
SDS-PAGE of pepsin-solubilized collagens from the human uterine cervical tissues. Normal postmenopausal uterine cervical tissues (lane 1), uterine cervical tissues with prolapse uteri (lane 2) and type V collagen extracted from the human uterine cervical tissues (lane 3). Samples of heat-denatured collagen (5 μg, lanes 1 and 2; 1.0 μg, lane 3) were subjected to electrophoresis on a slab gel for 1 h. After reduction in situ with β-mercaptoethanol for 30 min, electrophoresis was resumed for 1 h.
Discussion
In the present study, we investigated changes in the composition of the ECM, including type I, III and V collagens, in the human uterine cervical tissues obtained from the women with or without uterine prolapse. We were able to solubilize 70–85% of collagen in the human cervical tissues, as measured by reference to levels of hydroxyproline (data not shown). Therefore, it was postulated that the extracted collagen may accurately reflect the entire complement of collagen in the tissues.
Human cervix is made up mainly of fibrous connective tissues in which collagen (23) and glycosaminoglycans (24) predominate. The physiological properties of the cervix depend on the interplay between collagen and glycosaminoglycan molecules (25). Therefore, collagen may play a pivotal role in the structure and function of human uterine cervix.
Although type I and type III collagens are commonly found in combination, the ratios of type III to type I collagen in the tissues of human cervix of the patients with uterine prolapse were significantly lower than those of the patients without prolapse. Our findings suggest at least two possible mechanisms for decreased type III collagen expression in the uterine cervices with uterine prolapse. First, the reduced type III collagen expression in the pelvic organ supporting system involving uterine cervix may induce genital prolapse. However, these present findings conflict with those of previous reports (4–13).
Second, the pelvic supporting system is relaxed in patients with uterine prolapse. Therefore, a progressively increasing mechanical load may reduce focal type III collagen expression in uterine cervical tissues.
It has been suggested that the ECM in human cervical tissues may be controlled by collagenases, hormones, such as estrogens (26–28), dehydroepiandrosterone (29) and prostaglandins (30,31), and/or cytokines, such as IL-I (32) and IL-8 (33–37).
Recent studies have shown that nitric oxide (NO) may participate in the biochemical and anatomical changes in human and animal cervical tissues (38–42). In addition, it has been suggested that NO directly controls various metalloproteinases (43). A low estrogen level is commonly recognized in postmenopausal women with or without uterine prolapse. Therefore, in regards to the causes of uterine prolapse, we may suggest not only lack of estrogen, but also other factors, such as NO.
The ECM of the uterine cervix is a fiber-reinforced compositive viscoelastic material made up of fibrillar collagen (two thirds type I, one third type III) and proteoglycans (hyaluronic acid, chondroitin sulfate, keratan sulfate and dermatan sulfate) (44,45). Thus, our study suggests that a decreased ratio of type III collagen and an increased ratio of type I collagen in the uterine cervix may induce elongation of the cervix in terms of uterine prolapse. Type III collagen belongs to a family of fibril-forming collagens that are found throughout the body. Each of the fibrillar collagens has a unique structural profile that in turn imparts tissues with distinct physical properties (46,47). This collagen, forming smaller fibers, predominates in tissues that require increased flexibility and distensibility and are subject to periodic stress, such as the vasculature. Therefore, decreased expression of type III collagen, the main macromolecular component of the ECM of the cervix, may be a key event in the development of uterine prolapse.
In conclusion, these changes in the composition of collagens may result in the morphologic and functional characteristics of human uterine cervix with uterine prolapse.
This study furthers the understanding of the pathophysiology of human uterine cervix in terms of ECM metabolism. Further research is required to elucidate the mechanisms regulating the expression of genes of other types of collagen or metalloproteinases in the uterine cervix during the premenopausal period and pathological status.
References
- 1.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 2.Madri JA, Basson MD. Extracellular matrix-cell interactions: dynamic modulators of cell, tissue and organism structure and function. Lab Invest. 1992;66:519–521. [PubMed] [Google Scholar]
- 3.Haralson MA. Extracellular matrix and growth factors: an integrated interplay controlling tissue repair and progression to disease. Lab Invest. 1993;69:369–372. [PubMed] [Google Scholar]
- 4.Goepel C, Heller L, Methfessel HD, Koelbl H. Periurethral connective tissue stastus of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet Gynecol Scand. 2003;82:659–664. doi: 10.1034/j.1600-0412.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 5.Soderberg MW, Falconer C, Bystrom B, Malmstrom A, Ekman G. Young women with genital prolapse have a low collagen concentration. Acta Obstet Gynecol Scand. 2004;83:1193–1198. doi: 10.1111/j.0001-6349.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in the metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658–1661. doi: 10.1016/s0140-6736(96)91489-0. [DOI] [PubMed] [Google Scholar]
- 7.Liapis A, Bakas P, Pafiti A, Frangos-Plemenos M, Arnoyannaki N, Creatsas G. Changes of collagen type III in female patients with genuine stress incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod Biol. 2001;97:76–79. doi: 10.1016/s0301-2115(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 8.Makinen J, Kahari VM, Soderstrom KO, Vuorio E, Hirvonen T. Collagen synthesis in the vaginal connective tissue of patients with and without uterine prolapse. Eur J Obstet Gynecol Reprod Biol. 1987;24:319–325. doi: 10.1016/0028-2243(87)90157-2. [DOI] [PubMed] [Google Scholar]
- 9.Kokcu A, Yanik F, Cetinkaya M, Alper T, Kandemir B, Malatyalioglu E. Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. 2002;226:75–78. doi: 10.1007/s004040100194. [DOI] [PubMed] [Google Scholar]
- 10.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:80–87. doi: 10.1007/s001920200020. [DOI] [PubMed] [Google Scholar]
- 11.Takano CC, Girao MJ, Sartori MG, et al. Analysis of collagen in parametrium and vaginal apex of women with and without uterine prolapase. Int Urogynecol J Floor Dysfunct. 2002;13:342–345. doi: 10.1007/s001920200076. [DOI] [PubMed] [Google Scholar]
- 12.Ewies A, Al-Azzawi F, Thompson J. Changes in extracellular matrix proteins in the cardinal ligaments of post-menopausal women with or without prolapse: a computerized immunohistomorphometric analysis. Hum Reprod. 2003;18:2189–2195. doi: 10.1093/humrep/deg420. [DOI] [PubMed] [Google Scholar]
- 13.Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106:953–963. doi: 10.1097/01.AOG.0000182584.15087.dd. [DOI] [PubMed] [Google Scholar]
- 14.Friedman D, Boyd C, Norton P, Deak S. Altered type III collagen synthesis in patients with inguinal hernia. Am Surg. 1993;218:754–760. doi: 10.1097/00000658-199312000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulmsten U, Ekman G, Giertz G, Malmstrom A. Different biochemical composition of connective tissue in continent and stress incontinent women. Acta Obstet Gynecol Scand. 1987;66:455–457. doi: 10.3109/00016348709022054. [DOI] [PubMed] [Google Scholar]
- 16.Versi E, Cardozo L, Bribcat M, Cooper D, Montgomery J, Studd J. Corelation of urethral physiology and skin collagen in postmenopausal women. Br J Obstet Gynecol. 1988;95:147–152. doi: 10.1111/j.1471-0528.1988.tb06844.x. [DOI] [PubMed] [Google Scholar]
- 17.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 18.Kivirikko KI, Prockop DJ. Hydroxylation of proline in synthetic polypeptides with purified protocollagen hydroxylase. J Biol Chem. 1967;242:4009–4012. [PubMed] [Google Scholar]
- 19.Furuto DK, Miller EJ. Isolation of a unique collagenous fraction from limited pepsin digests of human placental tissue. J Biol Chem. 1980;255:290–295. [PubMed] [Google Scholar]
- 20.Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82:33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- 21.Sykes B, Puddle B, Francis M, Smith R. The estimation of two collagen from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976;72:1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Danforth DN. The fibrous nature of the human cervix, and its relation to isthmic segment in gravid and non gravid uteri. Am J Obstet Gynecol. 1974;53:541–560. doi: 10.1016/0002-9378(47)90273-1. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura K, Ito A, Mori Y, Hirakawa S. Glycosaminoglycans of human uterine cervix: heparan sulfate increase with reference to cervical ripening. Biochem Med. 1980;23:159–167. doi: 10.1016/0006-2944(80)90067-8. [DOI] [PubMed] [Google Scholar]
- 25.Wallis RM, Hiller K. Regulation of collagen dissolution in human cervix by oestradiol-17b and progesterone. J Reprod Fertil. 1981;62:55–61. doi: 10.1530/jrf.0.0620055. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Ito A, Mori Y, Yamashita K, Hayakawa T, Nagase H. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor metalloproteinases (TIMP) by progesterone and oestradiol 17 b Biochem J. 1991;275:645–650. doi: 10.1042/bj2750645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajabi MR, Dodge GR, Solomon S, Poole AR. Immunochemical and immunohistochemical evidence of estrogen-mediated collagenolysis as a mechanism of cervical dilatation in the guinea pig at partrition. Endocrinology. 1991;128:371–378. doi: 10.1210/endo-128-1-371. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki M, Honda T, Deguchi M, Morikawa H, Tojo S. A study of the effect of dehydroepiandrosterone sulfate on so-called cervical ripening. Acta Obstet Gynecol Scand. 1978;57:397–401. doi: 10.3109/00016347809156518. [DOI] [PubMed] [Google Scholar]
- 29.Uldbjerg N, Forman A, Petersen L, et al. Biochemical changes of the uterus and cervix during pregnancy. In: Reece EA, Hobbins JC, Mahoney MJ, Petrie RH, editors. Medicine of the Fetus and Mother. JB Lippincott Co; Philadelphia: 1992. pp. 849–868. [Google Scholar]
- 30.Kelly RW. Pregnancy maintenance and partrition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev. 1994;15:684–706. doi: 10.1210/edrv-15-5-684. [DOI] [PubMed] [Google Scholar]
- 31.El Maradney E, Kanayama N, Halim A, Maehara K, Sumimoto K, Terao T. The effect of interleukin-1 in rabbit cervical ripening. Eur J Obstet Gynecol Reprod Biol. 1995;60:75–80. doi: 10.1016/0028-2243(95)02085-3. [DOI] [PubMed] [Google Scholar]
- 32.Barclay CG, Brennad JE, Kelly RW, Calder AA. Interleukin-8 production by human cervix. Am J Obstet Gynecol. 1993;169:625–632. doi: 10.1016/0002-9378(93)90634-u. [DOI] [PubMed] [Google Scholar]
- 33.El Maradney E, Kanayama N, Kobayashi H, et al. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod. 1997;12:1080–1088. doi: 10.1093/humrep/12.5.1080. [DOI] [PubMed] [Google Scholar]
- 34.Osmers R, Blaser J, Kuhn W, Tshesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 35.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with cytokine interleukin-8, interleukin-1 beta and tumour necrosis factor alpha in guinea-pigs. Hum Reprod. 1994;9:2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- 36.El Maradney E, Kanayama N, Halim A, Maehara K, Sumimoto K, Terao T. Interleukin-8 induces cervical ripening in rabbits. Am J Obstet Gynecol. 1994;171:77–83. doi: 10.1016/s0002-9378(94)70081-8. [DOI] [PubMed] [Google Scholar]
- 37.Garfield RE, Saade G, Buhimschi C, et al. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4:673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- 38.Tschugguel W, Schneeberger C, Lass H, et al. Human cervical ripening is associated with an increase in cervical inducible nitric oxide synthase expression. Biol Reprod. 1999;60:1367–1372. doi: 10.1095/biolreprod60.6.1367. [DOI] [PubMed] [Google Scholar]
- 39.Buhimschi I, Ali M, Jain V, Chwalisz K, Garfield RE. Differential regulation of nitric oxide in the rat uterus and cervix during pregnancy and labour. Hum Reprod. 1996;11:1755–1766. doi: 10.1093/oxfordjournals.humrep.a019481. [DOI] [PubMed] [Google Scholar]
- 40.Chwalisz K, Shao-Qing S, Garfield RE, Beier HM. Cervical ripening in guinea pigs after a local application of nitric oxide. Hum Reprod. 1997;12:2093–2101. doi: 10.1093/humrep/12.10.2093. [DOI] [PubMed] [Google Scholar]
- 41.Ali M, Buhimschi I, Chwalisz K, Garfield RE. Changes in expression of the nitric oxide synthase isoforms in rat uterus and cervix during pregnancy and parturition. Mol Hum Reprod. 1997;3:995–1003. doi: 10.1093/molehr/3.11.995. [DOI] [PubMed] [Google Scholar]
- 42.Thomson AJ, Lunan CB, Cameron AD, Cameron IT, Greer IA, Norman JE. Nitric oxide donors induce ripening of the human uterine cervix: a randomised controlled trial. Br J Obstet Gynaecol. 1997;104:1054–1057. doi: 10.1111/j.1471-0528.1997.tb12066.x. [DOI] [PubMed] [Google Scholar]
- 43.Drapier JC, Bouton C. Modulation by nitric oxide of metalloproteinase regulatory activities. Bioessays. 1996;18:549–556. doi: 10.1002/bies.950180706. [DOI] [PubMed] [Google Scholar]
- 44.Aspeden RM. Collagen organization in the cervix and its relation to mechanical functions. Collagen Relat Res. 1988;8:103–112. doi: 10.1016/s0174-173x(88)80022-0. [DOI] [PubMed] [Google Scholar]
- 45.Kleissel HP, van der Rest M, Naftolin F, Glorieux FH, de Leon A. Collagen changes in the human uterine cervix at partruition. Am J Obstet Gynecol. 1978;130:748–753. doi: 10.1016/0002-9378(78)90003-0. [DOI] [PubMed] [Google Scholar]
- 46.Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M. Hierarchical structures in fibrillar collagen. Micron. 2002;33:587–596. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 47.Oxlund H. Relationships between the biomechanical properties, composition and molecular structure of connective tissues. Connect Tissue Res. 1986;15:65–72. doi: 10.3109/03008208609001974. [DOI] [PubMed] [Google Scholar]


