Abstract
Integrins mediate the interaction of cells with the extracellular matrix and are believed to be involved in tumor cell survival and metastasis, and in tumor angiogenesis. We used immunohistochemistry of fresh-frozen human tumor tissues to analyze the presence of integrins αvβ3, αvβ5 and α5β1, which are believed to be involved in tumor growth and migration, together with integrin ligands, vitronectin, osteopontin, fibronectin and fibrinogen, in human oral squamous cell carcinomas. Samples of squamous cell carcinomas and control tissues from patients without cancer undergoing oral or maxillofacial surgery were frozen in liquid nitrogen within 30 min of removal. Frozen sections were prepared, and the presence of integrins or ligands was visualized using standard immunohistochemistry (APAAP) with a blinded evaluation. Comparison of samples from the 40 oral cancer patients and the 20 controls revealed increased staining in tumors compared with the controls, and staining was demonstrated for αvβ3 in endothelia. αvβ5 staining was increased in the tumor samples, but this was associated with increased expression in stroma rather than in endothelia. Modestly increased expression of α5β1 was observed in the tumor samples, and this was associated with tumor cells, endothelia and stroma. Expression of ligands for the integrins varied between tissue types, with increased fibrinogen and fibronectin expression in tumor endothelia. Confirmation of the presence of these integrins and their association with tumor cells, endothelia or stroma suggests their potential for these integrins in human oral tumors. Overall, the increased expression of integrins within tumors, particularly expression associated with endothelial cells, supports the principle of selective integrin blockade as a novel anticancer strategy.
Keywords: integrins, cancer, squamous cell carcinoma of the head and neck, αvβ3, αvβ5, α5β1, immunohistochemistry, alkaline phosphatase-anti-alkaline phosphatase, frozen sections
Introduction
Worldwide, the 5-year survival rate for patients with squamous cell carcinoma of the head and neck (HNSCC) has not significantly increased for many years (1–5). HNSCC is diagnosed predominantly at the age range of 50–70 years, but is also observed in younger patients (6–8). Despite aggressive initial management of the primary tumor, locoregional recurrence occurs in some 60% of cases, and distant metastasis is observed in some 25%. Therefore, innovative therapeutic concepts are urgently required.
Angiogenesis is essential for tumor progression and metastasis. Tumor angiogenesis is complex and involves crosstalk between tumor-derived growth factors, the modified extracellular matrix that develops around tumors, and endothelial receptors for extracellular matrix and growth factors (9,10). Inhibition of angiogenesis often suppresses the tumor growth of model tumors, and the suppression and eradication of malignant tumors by targeting angiogenetic endothelial cells is a rapidly evolving approach to cancer therapy (10,11). Such therapies might influence highly vascularized head and neck cancers (12–17). Integrin antagonists are good candidates for such antiangiogenic strategies (9,18–23). In particular, the integrins, αvβ3, αvβ5 and α5β1, have been implicated in tumor angiogenesis. Inhibitors of these integrins are being investigated in clinical trials (9,19–21,24–26), and we previously reported a signal in an HNSCC patient when using an αvβ3/αvβ5 inhibitor (27).
Integrin action depends on the presence of complementary ligands. While αvβ5 and α5β1 are conservative in their ligand binding, being essentially monospecific for vitronectin and fibronection, respectively, αvβ3 binds promiscuously to numerous matrix components. The ligands fibrinogen and osteopontin rather monospecifically target αvβ3 (28). Vitronectin is a common serum component activated by conformational change (29); the activated molecule is detected immunologically (30). In the present study, we evaluated the expression of integrins, αvβ3, αvβ5 and α5β1, and their ligands, fibrinogen (αvβ3, α5β1), fibronectin (αvβ3, α5β1), osteopontin (αvβ3) and activated vitronectin (αvβ3, αvβ5), in head and neck cancer and control tissues.
Materials and methods
Patients
Samples of squamous cell carcinomas from 40 patients (32 male, 8 female) were obtained during oral or maxillofacial surgery. Control non-cancerous tissues containing squamous epithelium were obtained from 20 patients undergoing outpatient surgical procedures (Tables I and II). Patients provided informed consent for the collection of samples, and all tissues examined were taken from the head and neck area with previous consent of the patients in our clinic in the context of diagnostics and therapy.
Table I.
Characteristics of the 40 patients with head and neck squamous cell carcinoma (HNSCC), localization and TNMa classification of the tumors.
| No. | Gender/Agea | Localization | TNMb | Stage | Grade |
|---|---|---|---|---|---|
| 1 | M/39 | Floor of mouth | pT3 pN1 | 3 | 3 |
| 2 | M/38 | Floor of mouth | pT4 pN2 | 4 | 2 |
| 3 | M/52 | Floor of mouth | pT4 pN0 | 4a | 3 |
| 4 | M/59 | Floor of mouth | pT1 pN2 | 4 | 2 |
| 5 | M/50 | Floor of mouth | pT2 pN2b | 4a | 2 |
| 6 | M/50 | Floor of mouth | pT4 pN1 | 4 | 2 |
| 7 | M/61 | Floor of mouth | pT2 pN2 | 4a | 3 |
| 8 | M/62 | Floor of mouth | pT4 pN2 | 4a | 2 |
| 9 | M/50 | Floor of mouth | pT4 pN1 | 4a | 3 |
| 10 | M/48 | Floor of mouth | pT4 pN2 | 4a | 2 |
| 11 | M/52 | Floor of mouth | pT4 pN2 | 4a | 1 |
| 12 | M/63 | Floor of mouth | pT2 pN0 | 2 | 2 |
| 13 | M/52 | Floor of mouth | pT1 pN0 | 1 | 2 |
| 14 | M/60 | Floor of mouth | pT3 pN2 | 4a | 3 |
| 15 | M/46 | Floor of mouth | pT4 pN0 | 4a | 3 |
| 16 | M/53 | Floor of mouth | pT2 pN0 | 2 | 2 |
| 17 | M/57 | Floor of mouth | pT4 pN3 | 4b | 2 |
| 18 | F/50 | Floor of mouth | pT4 pN2 | 4a | 2 |
| 19 | M/58 | Floor of mouth/Tongue | pT3 pN2 | 4a | 3 |
| 20 | M/57 | Floor of mouth/Tongue | pT4 pN0 | 4a | 2 |
| 21 | F/48 | Floor of mouth/Tongue | pT4 pN2 | 4a | 2 |
| 22 | F/65 | Floor of mouth/Tongue | pT2 pN0 | 2 | 2 |
| 23 | M/52 | Oropharynx | pT2 pN2 | 4 | 3 |
| 24 | M/59 | Oropharynx | pT3 pN1 | 3 | 2 |
| 25 | M/57 | Oropharynx | pT2 pN1 | 3 | 2 |
| 26 | F/62 | Planum buccale | pT4 pN3 | 4 | 3 |
| 27 | F/76 | Planum buccale | pT3 pN1 | 3 | 2 |
| 28 | F/71 | Planum buccale | pT3 pN0 | 3 | 1 |
| 29 | M/53 | Processus alveolaris | pT4 pN2 | 4 | 2 |
| 30 | M/58 | Processus alveolaris | pT4 pN3 | 4b | 2 |
| 31 | M/59 | Processus alveolaris | pT4 pN2c | 4a | 2 |
| 32 | F/61 | Processus alveolaris | pT4 pN0 | 4a | 2 |
| 33 | F/64 | Processus alveolaris | pT4 pN0 | 4a | 1 |
| 34 | M/56 | Tongue | pT1 pN0 | 1 | 3 |
| 35 | M/58 | Tongue | pT2 pN1 | 3 | 2 |
| 36 | M /49 | Tongue | pT2 pN0 | 2 | 2 |
| 37 | M/53 | Tongue/Floor of mouth | pT4 pN0 | 4a | 3 |
| 38 | M/55 | Tongue/Floor of mouth | pT4 pN0 | 4a | 3 |
| 39 | M/55 | Tongue/Floor of mouth | pT4 pN0 | 4a | 3 |
| 40 | M/56 | Tongue/Floor of mouth | pT1 pN1 | 3 | 3 |
Age at tissue harvesting in years.
Wittekind et al (68), TNM classification. M, male; F, female.
Table II.
Characteristics of the 20 patients without tumors and localization of the control tissues.
| No. | Gender/Agea | Localization |
|---|---|---|
| 1 | M/20 | Gingiva |
| 2 | M/58 | Gingiva |
| 3 | M/23 | Gingiva |
| 4 | M/64 | Gingiva |
| 5 | M/33 | Gingiva |
| 6 | F/56 | Gingiva |
| 7 | M/16 | Oral mucosa |
| 8 | M/36 | Oral mucosa |
| 9 | F/36 | Oral mucosa |
| 10 | F/30 | Oral mucosa |
| 11 | F/61 | Oral mucosa |
| 12 | F/30 | Oral mucosa |
| 13 | F/22 | Oral mucosa |
| 14 | M/58 | Oropharynx |
| 15 | F/64 | Oropharynx |
| 16 | F/1 | Oropharynx |
| 17 | F/48 | Planum buccale |
| 18 | M/61 | Tongue |
| 19 | M/60 | Tongue |
| 20 | F/60 | Tongue |
Age at tissue harvesting in years. M, male; F, female.
Tumor samples and sample preparation
The tissue samples were stored in isotonic saline for 15–30 min immediately following removal from patients. All tissues were cut into pieces with an edge length of ∼4 mm, embedded in freezing medium (Leica Instrument, Nussloch) in a plastic tube, shock-frozen for 2 min in liquid nitrogen, and cryopreserved at −80°C until sectioning. A cryomicrotome (CM3000; Leica Instrument) was used to prepare 4- to 6-μm sections, which were placed on coated slides (SuperFrost Plus, Menzel, Braunschweig or Dako, Denmark), air-dried for ∼12 h at 20°C, and stored frozen in a dry atmosphere usually at −80°C (occasionally −20°C).
Frozen sections were thawed, air-dried, and fixed for 15 min in fresh dry acetone at −20°C. Experience revealed that this method provides clearer and stronger staining compared to fixing with methyl alcohol-acetone (9 min methanol and 1 min acetone at −20°C). All fixed sections were incubated with blocking buffer X0909 (ready-to-use; Dako) for 20 min to reduce non-specific staining. Samples were incubated with primary antibodies for 60 min. Table III lists the antibodies and dilution used. Optimal dilutions of antibodies were identified in preliminary experiments and were then used throughout the study.
Table III.
Antibodies.
| Antibody | Antibody type | Target antigen | Dilution | Author | Refs. |
|---|---|---|---|---|---|
| Clone LM609a,f | Monoclonal (IgG1) | αvβ3 integrin | 1:300 | Cheresh and Spiro | 69 |
| Clone P1F6a,f | Monoclonal (IgG3) | αvβ5 integrin | 1:300 | Weinacker et al | 70 |
| Clone P1D6a,f | Monoclonal (IgG3) | α5β1 integrin | 1:30 | Wayner et al | 71 |
| A0080c,e,g | Polyclonal (IgG) | Fibrinogen | 1:10.000 | ||
| RB-9097-P1d,e,g | Polyclonal (IgG) | Osteopontin | 1:30 | ||
| 153b,f | Monoclonal | Vitronectin | 1:200 | Seiffert et al | 72 |
| A0245c,e,g | Polyclonal (Ig) | Fibronectin | 1:30 | ||
| M0823 clone JC70Ac,f | Monoclonal (IgG1κ) | CD31 | 1:30 | ||
| N1698c | Negative control (Ig) | Negative control mouse | 1:1 | ||
| N1699c | Negative control (Ig) | Negative control rabbit | 1:1 |
Suppliers of the antibodies were
Chemicon/Millipore (USA),
Merck (Darmstadt, Germany);
Dako (Denmark);
NeoMarkers (UK).
Polyclonal antibodies (others were monoclonal antibodies);
murine antibody;
rabbit antibody.
An alkaline phosphatase-anti-alkaline phosphatase (APAAP) system was used to visualize the bound antibody (31). Slides were rinsed three times with Tris-wash buffer, pH 7.6, (Dako S3001) and incubated for 40 min with a bridging antibody diluted 1:40. Sections incubated with monoclonal antibodies (Table III) were incubated with polyclonal rabbit anti-mouse bridging antibody (Dako Z02259), and sections incubated with polyclonal antibodies were incubated with monoclonal mouse anti-rabbit bridging antibody (Dako M0737), diluted with the antibody diluent (Dako S2022) plus 5% AB serum (Biotest AG, cat. no. 805135) in each case. Sections were washed again three times in TBS buffer and then incubated for 40 min with the monoclonal APAAP complex (Dako D0651) diluted 1:100 in antibody diluent plus 5% inactivated fetal calf serum (Biochrom S0115). After thorough rinsing, the subsequent substrate development was carried out for over 20 min with the substrate (Dako 070524) containing two drops of levamisole (Dako K5000). After further rinsing, counterstaining was carried out using hemalaun (Dako S2020) for 5 min followed by bluing for 5 min in tap water.
For optimum recognition of squamous cell carcinoma in the small frozen sections, we used a monoclonal antibody against proliferation marker Ki-67 (Dako, M7240, clone MIB-1) and a monoclonal antibody against the adhesion molecule CD44v6 (Bender BMS116, clone VFF-7), performing the same immunohistochemical APAAP method as previously (32–34). Although this was effective, we did not use the synopsis of score values for the expression of Ki-67 and CD44v6. Vessel densities were routinely assessed using CD31 staining including score values.
Evaluation of expression with immunoreactivity scores and number of vessels
The evaluation of immunoreactivity scores (IHS) was carried out using x200 magnification as described (32–35). Sections were evaluated three times including an evaluation by a tumor pathologist in a blinded manner. Staining intensity (SI) was assessed according to a categorical scale: 0, no staining; 1, faint staining; 2, slight staining; 3, moderate staining; and 4, strong staining. The percentage of positively stained cells (PP) was assessed as: 0, no positive cells; 1, 0–25% positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells; and 4, 76–100% positive cells. An overall IHS was derived by multiplying the staining intensity (SI) by the percentage of positive staining or the staining frequency (PP) scores (range of possible scores 0–16). Staining of glands, muscle, histiocytes and inflammatory cells was ignored. In no instances were single cells counted in the tumors or in the squamous epithelium samples.
An additional parameter was used in the third microscopic evaluation with assessment of the number of vessels. This involved quantitative estimation of the number of marked vessels using a lower magnification (x100). Using antibodies (Table III), we distinguished the estimated numbers of marked vessels in the tumors (or squamous epithelium in controls) and stroma: scale 0, no vessels; scale 1, isolated vessels; scale 2, few vessels; scale 3, numerous vessels; and scale 4, large quantities of vessels. First, the highest possible vessel density was visualized using the antibody directed at the ‘typical’ endothelial marker, CD31, followed by visualization of other antigens of interest using the antibodies described in Table III.
Statistics
PASW Statistics for Windows (version 18.0.0) was used for statistical evaluation, with a cut-off for significance of p<0.05. The t-test was used when the values were distributed normally, and most often with the Mann-Whitney U-test for non-normally distributed data (36).
Results
Samples analyzed
Tumor samples (n=40) (Table I) were from the floor of the mouth (n=18), the tongue or tongue plus the floor of the mouth (n=11), the oropharynx (n=3) and the alveolar process, gingiva, or planum buccale (n=8). According to pathologic TNM tumor staging, approximately half of the tumors were T4 (n=21) with the remainder distributed among T3 (n=6), T2 (n=9) and T1 (n=4); in each case tumors were fairly evenly distributed among N0–N3, and M status was not available. Overall stage grouping identified 27 samples as S4, 7 as S3, 4 as S2 and 2 as S1; 14 tumors were grade 3, 23 were grade 2 and 3 were grade 1. Control samples (n=20) (Table II) were from the tongue (n=3), the oropharynx (n=3) and the gingiva, oral mucosa or planum buccale (n=14).
Expression in tumor and control tissues, in endothelial cells and in stroma
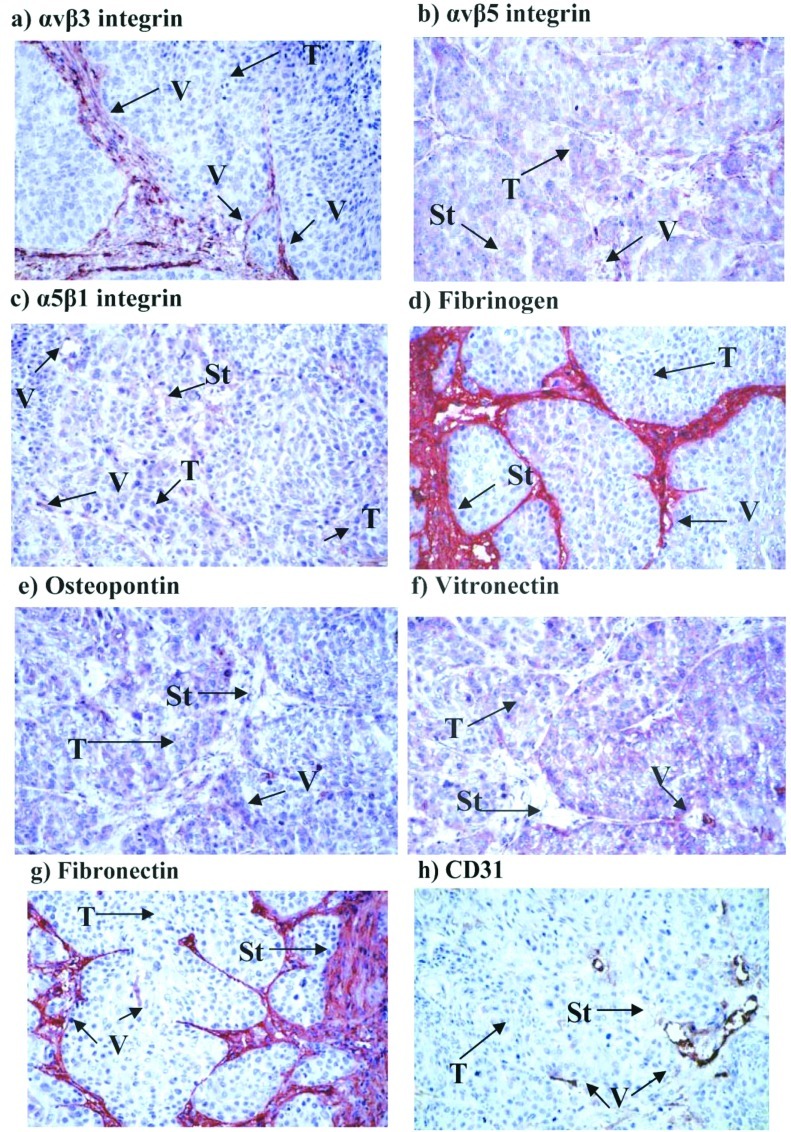
Fig. 1 compares the IHS (maximum score 16.0) for carcinoma tissue, endothelial cells and stroma in the samples from patients with oral cancer or from the control subjects. Table IV reveals the contributions of frequency (PP) and expression scores (SI) to the overall IHS. Representative examples of immunostaining for the integrins and ligands using various sections from a single patient (no. 30, Table I) are shown in Fig. 2a–h.
Figure 1.
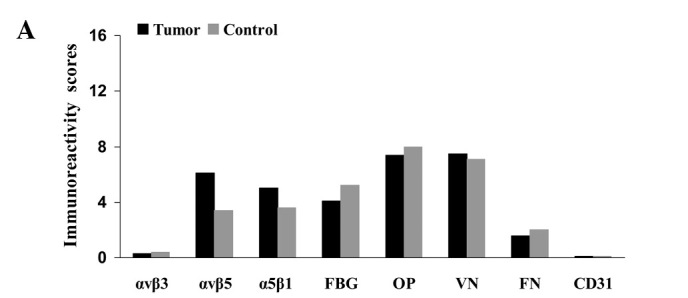
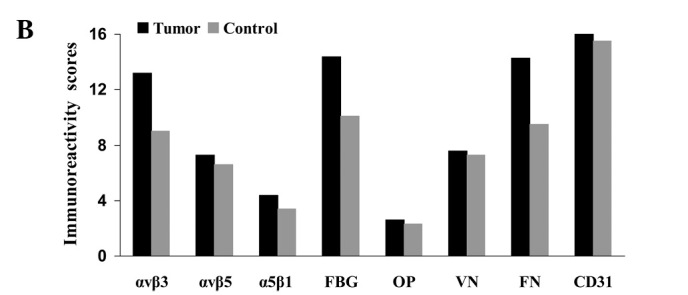
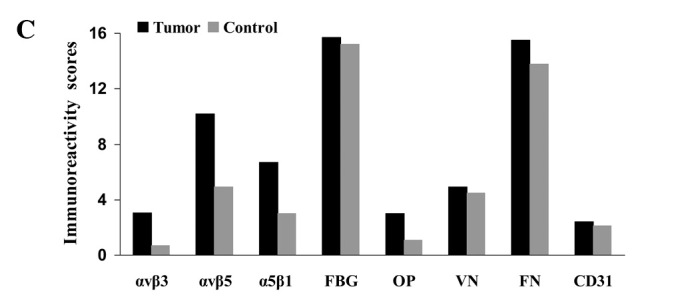
Immunoreactivity scores for integrins and their ligands in (A) tumor tissues, (B) endothelial cells and (C) stroma (see mean values, SD and significant values in Tables IV and V). Control, squamous epithelium from control samples. FBG, fibrinogen; OP, osteopontin; VN, vitronectin; FN, fibronectin.
Table IV.
Contribution of the intensity scores and frequency scores to the overall immunoreactivity scores for expression of the integrins and ligands in the tumors and squamous epithelium of the controls both in the endothelium and stroma, respectively.a
| Squamous cell carcinoma | Squamous epithelium controls | Endothelium
|
Stroma
|
||||
|---|---|---|---|---|---|---|---|
| Tumors | Controls | Tumors | Controls | ||||
| Integrin αvβ3 | Intensity | 0.21±0.57 | 0.30±0.60 | 3.60±0.77 | 3.30±0.80 | 0.80±0.67 | 0.20±0.30 |
| Frequency | 0.72±1.41 | 0.60±1.10 | 3.59±0.84 | 2.70±1.40 | 3.18±1.48 | 1.90±2.00 | |
| Immunoreactivity score | 0.29±0.62 | 0.40±0.60 | 13.18±4.37 | 9.00±5.60 | 3.06±2.60 | 0.70±0.80 | |
| Integrin αvβ5 | Intensity | 2.00±0.90 | 1.50±0.90 | 2.70±1.10 | 2.40±1.00 | 2.60±1.40 | 1.30±1.00 |
| Frequency | 3.20±0.90 | 2.60±1.10 | 2.60±1.10 | 2.60±1.30 | 3.80±0.70 | 3.50±1.40 | |
| Immunoreactivity score | 6.10±3.40 | 3.40±2.10 | 7.30±4.90 | 6.60±5.00 | 10.2±5.40 | 4.90±4.00 | |
| Integrin α5β1 | Intensity | 1.70±0.90 | 1.30±0.70 | 2.10±0.90 | 1.60±0.90 | 1.80±0.80 | 0.80±0.40 |
| Frequency | 3.00±1.00 | 2.90±0.90 | 2.10±1.20 | 2.20±1.30 | 3.80±0.50 | 3.60±1.10 | |
| Immunoreactivity score | 5.00±2.70 | 3.60±1.90 | 4.40±3.00 | 3.40±2.80 | 6.70±2.90 | 3.00±1.70 | |
| Fibrinogen | Intensity | 1.30±0.90 | 2.30±1.40 | 4.00±0.20 | 3.60±0.60 | 4.00±0.00 | 3.80±0.60 |
| Frequency | 3.20±1.10 | 2.40±0.90 | 3.60±0.80 | 2.90±1.20 | 3.90±0.60 | 4.00±0.00 | |
| Immunoreactivity score | 4.10±3.10 | 5.20±3.20 | 14.40±3.30 | 10.1±4.70 | 15.7±2.20 | 15.2±2.20 | |
| Osteopontin | Intensity | 2.30±0.80 | 2.90±1.30 | 1.80±1.20 | 1.60±1.50 | 0.80±0.70 | 0.30±0.40 |
| Frequency | 3.20±0.90 | 3.00±0.80 | 1.40±0.80 | 1.50±1.10 | 3.90±0.50 | 2.60±2.00 | |
| Immunoreactivity score | 7.40±3.60 | 8.00±4.20 | 2.60±2.10 | 2.30±2.00 | 3.00±2.80 | 1.10±1.50 | |
| Vitronectin | Intensity | 2.30±1.10 | 2.30±1.30 | 2.80±1.40 | 2.80±1.30 | 1.30±0.90 | 1.10±0.90 |
| Frequency | 3.40±0.80 | 3.00±1.10 | 2.60±1.20 | 2.50±1.30 | 3.80±0.60 | 3.90±0.60 | |
| Immunoreactivity score | 7.50±3.60 | 7.10±4.80 | 7.60±5.40 | 7.30±6.00 | 4.90±2.20 | 4.50±3.80 | |
| Fibronectin | Intensity | 0.60±0.60 | 1.20±1.30 | 3.80±0.60 | 3.30±0.80 | 3.90±0.30 | 3.40±0.90 |
| Frequency | 1.90±1.60 | 1.60±1.50 | 3.70±0.70 | 2.90±1.10 | 4.00±0.30 | 4.00±0.00 | |
| Immunoreactivity score | 1.60±2.20 | 2.90±4.00 | 14.30±3.70 | 9.50±4.80 | 15.5±1.60 | 13.8±3.80 | |
| CD31 | Intensity | 0.01±0.00 | 0.01±0.05 | 4.00±0.00 | 3.90±0.50 | 0.70±0.70 | 0.90±1.40 |
| Frequency | 0.10±0.40 | 0.20±0.90 | 4.00±0.00 | 4.00±0.20 | 3.10±1.50 | 2.10±1.90 | |
| Immunoreactivity score | 0.02±0.09 | 0.04±0.20 | 16.0±0.00 | 15.5±2.20 | 2.40±2.10 | 2.10±2.20 | |
Figure 2.
Representative samples of immunostaining for the integrins and ligands investigated using different sections from a single patient (no. 30; Table I) with a tumor of the alveolar process, x200 magnification. T, tumor; V, vessel; St, stroma.
The mean IHS for αvβ5 and α5β1 integrins in tumor cells were significantly higher than those from the control samples of squamous epithelium (Fig. 1a; Tables IV and V); this resulted from higher SI and PP scores for αvβ5 and from a higher SI score for α5β1 (Table IV). Expression of the other antigens was comparable between the tumor cells and the control samples, although there was a tendency in the control samples towards higher expression of fibrinogen (IHS 5.2 in control vs. 4.1 in tumor cells) and fibronectin (IHS 2.9 in control vs. 1.6 in tumor cells), but not significantly higher (U-test; fibrinogen, p=0.145 and fibronectin, p=0.416) (Table VI). αvβ3 expression (IHS 0.29) and CD31 (IHS 0.02) exhibited weak or no staining in the tumor cells.
Table V.
Statistical comparison between the immunoreactivity scores (IHS) in the tumors, endothelia, stroma or controls (squamous epithelia, endothelia and stroma), respectively.a
| IHS in the carcinoma cells vs. squamous epithelia in the controls | IHS in the carcinoma cells are not statistically significantly higher | IHS in carcinoma cells are statistically significantly higher. |
|
| ||
| Integrin αvβ3 | 0.568 | |
| Fibrinogen | 0.145 | |
| Osteopontin | 0.487 | |
| Vitronectin | 0.693 | |
| Fibronectin | 0.416 | |
| CD31 | 0.983 | |
| Integrin αvβ5 | 0.002 | |
| Integrin α5β1 | 0.034 | |
|
| ||
| IHS in endothelia of carcinoma tissues vs. the controls | IHS in endothelia of carcinoma tissues are not statistically significantly higher | IHS in endothelia of carcinoma tissues are statistically significantly higher. |
|
| ||
| Integrin αvβ5 | 0.490 | |
| Integrin α5β1 | 0.223 | |
| Osteopontin | 0.544 | |
| Vitronectin | 0.634 | |
| CD31 | 0.168 | |
| Integrin αvβ3 | 0.004 | |
| Fibrinogen | <0.001 | |
| Fibronectin | <0.001 | |
|
| ||
| IHS in stroma of carcinoma tissues vs. the controls | IHS in carcinoma tissues are not statistically significantly higher | IHS in stroma of carcinoma tissues are statistically significantly higher |
|
| ||
| Fibrinogen | 0.082 | |
| Vitronectin | 0.456 | |
| Integrin αvβ3 | <0.001 | |
| Integrin αvβ5 | <0.001 | |
| Integrin α5β1 | <0.001 | |
| Osteopontin | <0.001 | |
| Fibronectin | 0.001 | |
| CD31 | 0.325 | |
Table VI.
Contribution of the quantitative estimate of the number of vessels in the tumor tissues or in squamous epithelium of the controls and in stroma, respectively.a
| Vessels in
|
Vessels in stroma of
|
|||
|---|---|---|---|---|
| Tumor tissues | Control tissues | Tumor tissues | Control tissues | |
| Integrin αvβ3 | 1.3±0.9 | 0.5±0.4 | 1.7±0.7 | 1.2±0.8 |
| Integrin αvβ5 | 0.7±0.6 | 0.5±0.5 | 1.2±0.6 | 1.0±0.5 |
| Integrin α5β1 | 0.7±0.5 | 0.4±0.4 | 1.0±0.5 | 0.8±0.4 |
| Fibrinogen | 1.5±0.9 | 1.1±0.7 | 2.0±0.7 | 1.4±0.7 |
| Osteopontin | 0.5±0.4 | 0.7±0.6 | 0.9±0.4 | 0.7±0.6 |
| Vitronectin | 0.9±0.7 | 0.8±0.6 | 1.2±0.7 | 1.0±0.5 |
| Fibronectin | 1.7±0.9 | 0.9±0.6 | 1.9±0.8 | 1.5±0.5 |
| CD31 | 1.9±0.9 | 0.8±0.5 | 2.6±0.8 | 2.3±0.9 |
Integrin αvβ3 (IHS 13.2), fibrinogen (IHS 14.4) and fibronectin (IHS 14.3) were strongly expressed in the endothelia in the the tumors [along with the endothelial marker CD31 (IHS 16.0), while IHS for CD31 was significantly higher: CD31 vs. αvβ3, p<0.001; CD31 vs. fibrinogen, p=0.002; CD31 vs. fibronectin, p=0.003; U-test]. In tumors, the average IHS of integrin αvβ3, fibrinogen and fibronectin were significantly higher than those in the control tissues (p=0.004, p<0.001 and p<0.001, respectively) (Table IV; Fig. 1b). Higher average SI and PP scores contributed to these differences in intensity of expression (Table IV). Lower mean IHS were observed for integrins αvβ5 and α5β1, and osteopontin and vitronectin (Table IV and Fig. 1) with no clear differences between tumor samples and control tissues (αvβ5, p=0.590; α5β1, p=0.223; osteopontin, p=0.544; vitronectin, p=0.634; U-test) (Table V).
All three integrins were more strongly and statistically significantly expressed in tumor stroma compared to stroma of control squamous epithelia (U-test; p<0.001) (Fig. 1c; Table IV and V), mainly as a result of higher SI scores for αvβ5 and α5β1, and by higher SI and PP scores for αvβ3. However, αvβ3 was less strongly expressed than αvβ5 and α5β1, as judged by the overall IHS. Osteopontin was not strongly expressed, although the IHS was higher in tumor stroma vs. the control (IHS 3.0 vs. 1.1; p<0.001). Activated vitronectin was expressed weakly at similar levels in the normal and tumor stroma. Fibrinogen (IHS 15.7 vs. 15.2; p=0.082) and fibronectin (IHS 15.5 vs. 13.8; p=0.029) were strongly expressed in the tumor and control samples, while the expression of CD31 was low and similar between the tumors and controls (IHS 2.4 vs. 2.1; p=0.325).
Quantification of blood vessels in the tumors and control epithelia or stroma in both tissues
Integrin expression in the blood vessels of the tumor tissues and in stroma were evaluated separately with a maximal score of 4.0 (Fig. 3). Using a typical marker of endothelial cells, CD31, immunostaining revealed a higher density of endothelial cells in the tumors vs. the control tissues (1.9 vs. 0.8; p=0.099; U-test), with a higher or similar density of staining in tumor stroma and control samples (tumor stroma 2.6 vs. stroma in control tissues 2.3; p=0.173; U-test) (Fig. 3; Tables VI and VII).
Figure 3.
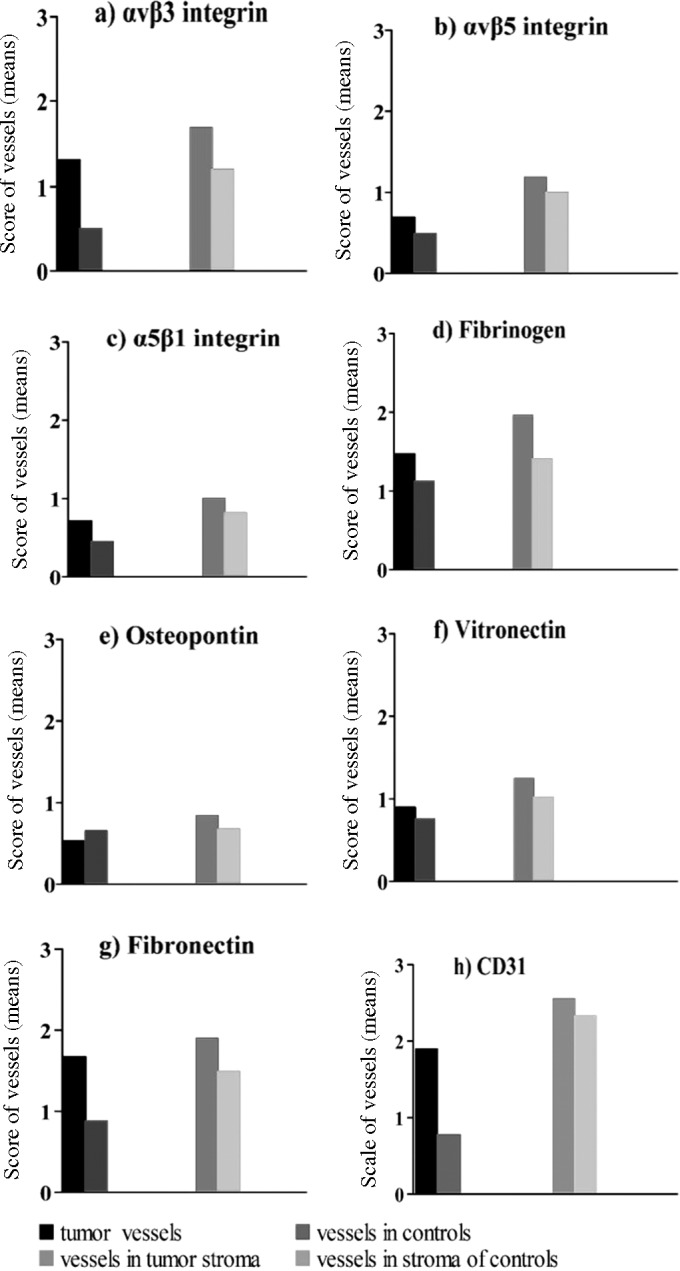
Comparison of the quantitative estimate of the number of vessels in tumors and stroma using antibodies against the integrins and ligands (mean values with standard deviations and significance values in Tables VI and VII).
Table VII.
Statistical comparison between the quantitative estimate of vascularization for squamous cell carcinomas vs. squamous epithelia of control sections and for stroma.a
| Quantitative estimate of vessels in carcinoma tissues vs. controls | Values assessed in carcinoma tissues are statistically not significantly higher | Values assessed in carcinoma tissues are statistically significantly higher |
|
| ||
| Integrin αvβ5 | 0.086 | |
| Fibrinogen | 0.145 | |
| Osteopontin | 0.792 | |
| Vitronectin | 0.312 | |
| CD31 | 0.099 | |
| Integrin α5β1 | 0.034 | |
| Fibronectin | 0.002 | |
| Integrin αvβ3 | <0.001 | |
|
| ||
| Quantitative estimate of vessels in stroma of tumor tissues vs. stroma in controls | Values assessed in stroma of tumor tissues are statistically not significantly higher | Values assessed in stroma of tumor tissues are statistically significantly higher. |
|
| ||
| Integrin αvβ5 | 0.230 | |
| Integrin α5β1 | 0.191 | |
| Osteopontin | 0.117 | |
| Vitronectin | 0.292 | |
| CD31 | 0.173 | |
| Integrin αvβ3 | 0.012 | |
| Fibrinogen | 0.009 | |
| Fibronectin | 0.025 | |
Integrins were more strongly expressed on endothelia within the tumor tissue than in the control squamous epithelium, although a clear difference between tumor and control samples was observed only for integrin αvβ3 (Table VI; Fig. 3). Endothelial cells in the stroma expressed integrins more strongly than in the tumor tissue. The number of vessels, when compared between the tumor and control samples in the stroma, was greater in the tumor tissues for αvβ3 and statistically significant (p=0.012, Table VII) compared to the other integrins (αvβ3, 1.7 vs. 1.2; αvβ5, 1.2 vs. 1.0; α5β1, 1.0 vs. 0.8) (Table VI). Fibrinogen and fibronectin were expressed strongly in the tumor tissue and tumor stroma and their respective control tissues, with mean IHS generally comparable with those for CD31 (tumor tissues vs. controls: fibrinogen, 1.5 vs. 1.1; fibronectin, 1.7 vs. 0.9; CD31, 1.9 vs. 0.8; and in tumor stroma vs. controls: fibrinogen, 2.0 vs. 1.4; fibronectin, 1.9 vs. 1.5; CD31, 2.6 vs. 2.3) (Table VI). Osteopontin was expressed less strongly with little difference in expression between the tumors and control samples for tumor tissue or stroma (tumor tissues vs. controls: 0.5 vs. 0.7 and tumor stroma vs. controls: 0.9 vs. 0.7). Tumor endothelia expressed fibronectin and fibrinogen more strongly than control endothelia, while staining for vitronectin and osteopontin expression was unchanged over the control.
Discussion
Integrins interacting with their complementary extracellular matrix targets regulate normal cellular behavior. Changes in these interactions are implicated in cancer progression (23,37–41). In this study, we used immunohistochemistry to investigate the expression of integrin-ligand combinations implicated in tumor angiogenesis within tumor material from 40 HNSSC patients compared to 20 normal controls. We investigated αvβ3, αvβ5, α5β1 and their ligands, osteopontin, vitronectin, fibronectin and fibrinogen, and found that these proteins are disregulated within the tumor environment. αvβ5 and α5β1 were overexpressed in tumor cells, αvβ3 in endothelia, and each integrin in the tumor stroma. Expression of the ligands, fibrinogen and fibronectin, was elevated in the tumor vasculature environment, fibronectin and osteopontin in the stroma, but none in the tumor cells, while activated vitronectin remained unchanged in each environment. These results support a role for αvβ3-osteopontin and fibronectin, α5β1-fibronectin interactions in influencing HNSCC angiogenesis and α5β1-fibronectin and αvβ5-vitronectin influencing tumor cell behavior. The elevated fibrinogen and fibronectin in the vasculature may be related to defective vascular patency and increased serum leakage within tumors.
Vitolo et al (39) detected an increasing frequency of α5β1 expression in oral tissues; expression in 0/7 normal epithelium, in carcinoma in situ 8/9 and in invasive carcinoma 8/13, in contrast to lack of expression of αvβ3 in the same tissues. According to Thomas and Speight (40), the integrin α5β1 was weakly expressed in oral keratinocytes, while αvβ6 was implicated in HNSCC progression (42). In the in vitro study of Reinartz et al (43), αvβ5 was expressed in human keratinocytic cells (HaCaT). In epithelia of the controls we found that each of the three integrins, αvβ3, αvβ5 and α5β1, was expressed; αvβ3 exhibited the weakest expression (Table IV). Expression of αvβ3 remained weak in normal epithelia, but was significantly higher than in the tumor tissues (Table V). However, in our study the epithelia of the controls exhibited weak expression of α5β1 and significantly lower α5β1 expression than in the tumor tissues.
Increased or inappropriate expression of integrins is believed, in coordination with their ligands, to support tumor growth and metastasis, and to promote tumor angiogenesis in head and neck carcinomas (37–41,44,45). These phenomena are of considerable scientific and clinical interest, as experimental studies indicate that disruption of integrin function may inhibit the growth, neovascularization and metastasis of some types of cancers (9,19–23). Indeed, drugs that block the interaction of integrins with the extracellular matrix are under development for the management of several clinically important tumor types. One such drug, cilengitide, is a selective blocker of ligand interaction with αvβ3 and αvβ5 integrins (9,18,24,25,27): the integrins assessed in this study.
We demonstrated marked expression of integrins and their ligands in oral tumor tissues (Table IV), and strong staining for CD31 in tumor tissues was consistent with angiogenesis and neovascularization (Table IV and Fig. 2h), thus confirming observations in oral cancer by Kurtz et al (15) and Villaret et al (46). In our study we found weak staining for αvβ3 in tumor or stromal cells (Table IV and Fig. 2a). This is in contrast to observations noted in malignant gliomas by Schnell et al (47) and in melanoma by Albelda et al (48), who found that tumors expressed higher levels of αvβ3 than normal tissues. A statistically significant increased staining vs. controls was demonstrated for αvβ3 in endothelia, but not in stroma (Tables IV and V). In the present study, αvβ5 staining was statistically significantly increased in tumor samples compared to the controls (Table V), which corroborates the findings of Jones et al (37). However, αvβ5 was markedly expressed in stroma rather than in endothelia. There was some increase in the expression of α5β1 in tumor samples associated with tumor cells, endothelia and stroma. Expression of ligands for integrins varied between the tissue types, with no clear differentiation and no statistically significant expression between tumor and control samples, with the notable exception of the αvβ3 ligand osteopontin and the αvβ3/α5β1 ligand fibronectin, which were significantly up-regulated in the tumor stroma. This complements the up-regulation of αvβ3 and α5β1 noted on the tumor vasculature. Notably, since activated vitronectin was conspicuously uniformly distributed between the normal and tumor tissues, it appears to be less involved in tumor-specific integrin-driven behaviors in HNSCC.
Previous histochemical studies identified the expression of αvβ3 in various tumors, with a particularly strong and functional association with tumor invasive blood vessels consistent with the more detailed analyses of the present study (49–52). Other studies have found increased αvβ3 expression to be correlated with greater invasive or metastatic potential (53–55). Radiotracers specific to αvβ3 have revealed this integrin in human tumor tissue in situ (47,56). αvβ5 integrin has also been implicated in tumor cell invasion and migration (57–59), and αvβ3 and αvβ5 regulate cellular responses to hypoxia in glioblastomas (60). α5β1 has also been implicated in tumor migration and angiogenesis (61–65) and may control cell migration in concert with αvβ3 (66).
Confirmation of the presence of integrins, αvβ3 and αvβ5, and their activating ligands in association with HNSCC tumors, supports a potential role for these integrins in human oral tumors. Overall, increased expression of integrins within tumors, particularly expression associated with endothelial cells, supports the emergent therapeutic concept of selective integrin blockade as a anticancer strategy (9,23,27,67).
Acknowledgments
This study was supported by a grant from Merck KGaA. The authors would like to thank Dr Andreas Eilers (Merck KGaA, Darmstadt) and Dr Mike Gwilt (supported by Merck KGaA) for editorial assistance. We thank Professor David Loskutoff (Scippts Research Institute, USA) for the kind gift of monoclonal antibody 153 and Mr. Franz Hafner (Clinic for Oral and Maxillofacial Surgery, Campus Virchow Hospital Charité-Universitätsmedizin, Berlin, Germany) for the micro-photo scanning.
Abbreviations:
- APAAP,
alkaline phosphatase-anti-alkaline phosphatase;
- FBG,
fibrinogen;
- FN,
fibronectin;
- HNSCC,
squamous cell carcinoma of the head and neck;
- Ig,
immunoglobulin;
- IHS,
immunohistochemical score;
- OP,
osteopontin;
- PP,
staining frequency, percentage of positive staining;
- SD,
standard deviation;
- SI,
staining intensity;
- St,
stroma;
- TBS,
tris buffered saline;
- TNM of malignant tumors: T,
tumor;
- N,
node;
- M,
metastasis;
- V,
vessel;
- VN,
vitronectin
References
- 1.Rapidis AD, Keramidas T, Panangiotopoulos H, Andressakis D, Angelopoulos AP. Tumours of the head and neck in the elderly: analysis of 190 patients. J Craniomaxillofac Surg. 1998;26:153–158. doi: 10.1016/s1010-5182(98)80005-x. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 3.Döbrossy L. Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev. 2005;24:9–17. doi: 10.1007/s10555-005-5044-4. [DOI] [PubMed] [Google Scholar]
- 4.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tejani MA, Cohen RB, Mehra R. The contribution of cetuximab in the treatment of recurrent and/or metastatic head and neck cancer. Biologics. 2010;4:173–185. doi: 10.2147/btt.s3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people — a comprehensive literature review. Oral Oncol. 2001;37:401–418. doi: 10.1016/s1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 7.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya KA. Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 2003;39:106–114. doi: 10.1016/s1368-8375(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 8.Rutt AL, Hawkshaw MJ, Sataloff RT. Laryngeal cancer in patients younger than 30 years: a review of 99 cases. Ear Nose Throat J. 2010;89:189–192. [PubMed] [Google Scholar]
- 9.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, Ruegg C. Integrin inhibitors reaching the clinic. J Clin Oncol. 2007;25:1637–1638. doi: 10.1200/JCO.2006.09.8376. Comment on: Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD and Grossman SA: Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 25: 1651–1657, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 12.Hasina R, Lingen MW. Angiogenesis in oral cancer. J Dent Educ. 2001;65:1282–1290. [PubMed] [Google Scholar]
- 13.Rüegg C, Dormond O, Foletti A. Suppression of tumor angiogenesis through the inhibition of integrin function and signaling in endothelial cells: which side to target? Endothelium. 2002;9:151–160. doi: 10.1080/10623320213635. [DOI] [PubMed] [Google Scholar]
- 14.Erovic BM, Neuchrist C, Berger U, El-Rabadi K, Burian M. Quantitation of microvessel density in squamous cell carcinoma of the head and neck by computer-aided image analysis. Wien Klin Wochenschr. 2005;117:53–57. doi: 10.1007/s00508-004-0298-3. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;29:354–359. doi: 10.5858/2005-129-354-PAVIIO. [DOI] [PubMed] [Google Scholar]
- 16.Walsh JE, Lathers DM, Chi AC, Gillespie MB, Day TA, Young MR. Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2007;8:227–238. doi: 10.1007/s11864-007-0032-2. [DOI] [PubMed] [Google Scholar]
- 17.Seiwert TY, Cohen EE. Targeting angiogenesis in head and neck cancer. Semin Oncol. 2008;35:274–285. doi: 10.1053/j.seminoncol.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Tucker RW, Sanford KK, Handleman SL, Jones GM. Alpha v integrin inhibitors and cancer therapy. Curr Opin Investig Drugs. 2003;4:722–731. [PubMed] [Google Scholar]
- 19.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 20.Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen X. Integrin alpha v beta 3 antagonists for anti-angiogenic cancer treatment. Recent Pat Anticancer Drug Discov. 2007;2:143–158. doi: 10.2174/157489207780832469. [DOI] [PubMed] [Google Scholar]
- 21.Paolillo M, Russo MA, Serra M, Colombo L, Schinelli S. Small molecule integrin antagonists in cancer therapy. Mini Rev Med Chem. 2009;9:1439–1446. doi: 10.2174/138955709789957404. [DOI] [PubMed] [Google Scholar]
- 22.Sheldrake HM, Patterson LH. Function and antagonism of beta3 integrins in the development of cancer therapy. Curr Cancer Drug Targets. 2009;9:519–540. doi: 10.2174/156800909788486713. [DOI] [PubMed] [Google Scholar]
- 23.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 24.Dechantsreiter MA, Planker E, Mathä B, Lohof E, Hölzemann G, Jonczyk A, Goodman SL, Kessler H. N-Methylated cyclic RGD peptides as highly active and selective alpha (V) beta (3) integrin antagonists. J Med Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 25.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O'Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ, Wittemer SM, Nippgen J, Picard M, Nabors LB. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. with comment: Chamberlain MC: Cilengitide: does it really represent a new targeted therapy for recurrent glioblastoma? J Clin Oncol 27: 1921–1922, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–1235. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raguse JD, Gath HJ, Bier J, Riess H, Oettle H. Cilengitide (EMD 121974) arrests the growth of a heavily pretreated highly vascularised head and neck tumour. Oral Oncol. 2004;40:228–230. doi: 10.1016/j.oraloncology.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 29.Preissner KT. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- 30.Seiffert D, Smith JW. The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem. 1997;272:13705–13710. doi: 10.1074/jbc.272.21.13705. [DOI] [PubMed] [Google Scholar]
- 31.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KAF, Stein H, Mason DY. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP Complexes) J Histochem Cytochem. 1984;32:219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 32.Fabricius E-M, Langford A, Bier J, Hell B, Wildner G-P, Blümcke S. Immunohistochemical characterization of E48 and CD44-v6 expression in head and neck carcinomas. Cancer J. 1997;10:325–330. [Google Scholar]
- 33.Fabricius E-M, Guschmann M, Wildner G-P, Langford A, Hell B, Bier J. Divergent immunohistochemical E48 and CD44-v6 antigen expression patterns between lymph node metastases and primary squamous cell carcinomas in the head and neck region. Cancer J. 1998;11:153–159. [Google Scholar]
- 34.Fabricius E-M, Guschmann M, Langford A, Hell B, Bier J. Immunhistochemical assessment of the tumour-associated epitopes CD44v6 and E48 in tumour-free lymph nodes from patients with squamous cell carcinoma in the head-neck region. Anal Cell Pathol. 2000;20:115–129. doi: 10.1155/2000/729537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remmele W, Stegner HE. Vorschlag zur einheitlichen Definition eines immunreaktiven Scores (IRS) für den immunhistochemischen Östrogenrezeptor-Nachweis (ER-ICA) im Mammagewebe. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 36.Sachs L. Angewandte Statistik - Anwendung statistischer Methoden. 11. Auflage Springer Verlag; Berlin: 2004. p. 889. [Google Scholar]
- 37.Jones J, Watt FM, Speight PM. Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J Oral Pathol Med. 1997;26:63–68. doi: 10.1111/j.1600-0714.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomas GJ, Jones J, Speight PM. Integrins and oral cancer. Oral Oncol. 1997;33:381–388. doi: 10.1016/s0964-1955(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 39.Vitolo D, Ciocci L, Ferrauti P, Cicerone E, Gallo A, De Vincentiis M, Baroni CD. Alpha5 integrin distribution and TGFbeta1 gene expression in supraglottic carcinoma: their role in neoplastic local invasion and metastasis. Head Neck. 2000;22:48–56. doi: 10.1002/(sici)1097-0347(200001)22:1<48::aid-hed8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Thomas GJ, Speight PM. Cell adhesion molecules and oral cancer. Crit Rev Oral Biol Med. 2001;12:479–498. doi: 10.1177/10454411010120060301. [DOI] [PubMed] [Google Scholar]
- 41.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer Metastasis Rev. 2005;24:35–45. doi: 10.1007/s10555-005-5046-2. [DOI] [PubMed] [Google Scholar]
- 42.Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 43.Reinartz J, Schäfer B, Batrla R, Klein CE, Kramer MD. Plasmin abrogates alpha v beta 5-mediated adhesion of a human keratinocyte cell line (HaCaT) to vitronectin. Exp Cell Res. 1995;220:274–282. doi: 10.1006/excr.1995.1316. [DOI] [PubMed] [Google Scholar]
- 44.Ziober BL, Silverman SS, Jr, Kramer RH. Adhesive mechanisms regulating invasion and metastasis in oral cancer. Crit Rev Oral Biol Med. 2001;12:499–510. doi: 10.1177/10454411010120060401. [DOI] [PubMed] [Google Scholar]
- 45.Lyons AJ, Jones J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int J Oral Maxillofac Surg. 2007;36:671–679. doi: 10.1016/j.ijom.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Villaret AB, Schreiber A, Facchetti F, Fisogni S, Lonardi S, Lombardi D, Cocco D, Redaelli de Zinis LO, Nicolai P. Immunostaining patterns of CD31 and podoplanin in previously untreated advanced oral/oropharyngeal cancer: prognostic implications. Head Neck. 2010;32:786–792. doi: 10.1002/hed.21256. [DOI] [PubMed] [Google Scholar]
- 47.Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, Goldbrunner RH, Wester HJ, Haubner R, Pöpperl G, Holtmannspötter M, Kretzschmar HA, Kessler H, Tonn JC, Schwaiger M, Beer AJ. Imaging of integrin {alpha} v{beta}3 expression in patients with malignant glioma by [18F] galacto-RGD positron emission tomography. Neuro Oncol. 2009;11:861–870. doi: 10.1215/15228517-2009-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 49.Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, Ruiter DJ, DeWaal RM. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–324. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. Erratum in: Int J Cancer 72: 706–707, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Sato T, Konishi K, Kimura H, Maeda K, Yabushita K, Tsuji M, Miwa A. Vascular integrin beta 3 and its relation to pulmonary metastasis of colorectal carcinoma. Anticancer Res. 2001;21:643–647. [PubMed] [Google Scholar]
- 51.Tang Y, Borgstrom P, Maynard J, Koziol J, Hu Z, Garen A, Deisseroth A. Mapping of angiogenic markers for targeting of vectors to tumor vascular endothelial cells. Cancer Gene Ther. 2007;14:346–353. doi: 10.1038/sj.cgt.7701030. [DOI] [PubMed] [Google Scholar]
- 52.Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nährig J, Watzlowik P, Wester HJ, Harbeck N, Schwaiger M. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med. 2008;49:255–259. doi: 10.2967/jnumed.107.045526. [DOI] [PubMed] [Google Scholar]
- 53.Gasparini G, Brooks PC, Biganzoli E, Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R, Cheresh DA. Vascular integrin alpha(v)beta3: a new prognostic indicator in breast cancer. Clin Cancer Res. 1998;4:2625–2634. [PubMed] [Google Scholar]
- 54.Vonlaufen A, Wiedle G, Borisch B, Birrer S, Luder P, Imhof BA. Integrin alpha(v)beta(3) expression in colon carcinoma correlates with survival. Mod Pathol. 2001;14:1126–1132. doi: 10.1038/modpathol.3880447. [DOI] [PubMed] [Google Scholar]
- 55.Schnell O, Krebs B, Wagner E, Romagna A, Beer AJ, Grau SJ, Thon N, Goetz C, Kretzschmar HA, Tonn JC, Goldbrunner RH. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18:378–386. doi: 10.1111/j.1750-3639.2008.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zannetti A, Del Vecchio S, Iommelli F, Del Gatto A, De Luca S, Zaccaro L, Papaccioli A, Sommella J, Panico M, Speranza A, Grieco P, Novellino E, Saviano M, Pedone C, Salvatore M. Imaging of alphavbeta3 expression by a bifunctional chimeric RGD peptide not cross-reacting with alphavbeta5. Clin Cancer Res. 2009;15:5224–5233. doi: 10.1158/1078-0432.CCR-08-3270. [DOI] [PubMed] [Google Scholar]
- 57.Monnier Y, Farmer P, Bieler G, Imaizumi N, Sengstag T, Alghisi GC, Stehle JC, Ciarloni L, Andrejevic-Blant S, Moeckli R, Mirimanoff RO, Goodman SL, Delorenzi M, Rüegg C. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer Res. 2008;68:7323–7331. doi: 10.1158/0008-5472.CAN-08-0841. [DOI] [PubMed] [Google Scholar]
- 58.Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, Hanks SK, Cheresh DA. Specific cross-talk between epidermal growth factor receptor and integrin alphav-beta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vocca I, Franco P, Alfano D, Votta G, Carriero MV, Estrada Y, Caputi M, Netti PA, Ossowski L, Stoppelli MP. Inhibition of migration and invasion of carcinoma cells by urokinase-derived antagonists of alphavbeta5 integrin activation. Int J Cancer. 2009;124:316–325. doi: 10.1002/ijc.23933. [DOI] [PubMed] [Google Scholar]
- 60.Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, Cohen-Jonathan Moyal E. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 61.Färber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N, Kettenmann H. An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci. 2008;39:579–585. doi: 10.1016/j.mcn.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Morozevich GE, Kozlova NI, Cheglakov IB, Ushakova NA, Preobrazhenskaya ME, Berman AE. Implication of alpha5-beta1 integrin in invasion of drug-resistant MCF-7/ADR breast carcinoma cells: a role for MMP-2 collagenase. Biochemistry. 2008;73:791–796. doi: 10.1134/s0006297908070079. [DOI] [PubMed] [Google Scholar]
- 63.Morozevich G, Kozlova N, Cheglakov I, Ushakova N, Berman A. Integrin alpha5beta1 controls invasion of human breast carcinoma cells by direct and indirect modulation of MMP-2 collagenase activity. Cell Cycle. 2009;8:2219–2225. doi: 10.4161/cc.8.14.8980. [DOI] [PubMed] [Google Scholar]
- 64.Lee MY, Huang JP, Chen YY, Aplin JD, Wu YH, Chen CY, Chen PC, Chen CP. Angiogenesis in differentiated placental multipotent mesenchymal stromal cells is dependent on integrin alpha5beta1. PLoS One. 2009;4:E6913. doi: 10.1371/journal.pone.0006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahn G, Vossmeyer D, Stragies R, Wills M, Wong CG, Löffler KU, Adamis AP, Knolle J. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch Ophthalmol. 2009;127:1329–1335. doi: 10.1001/archophthalmol.2009.265. [DOI] [PubMed] [Google Scholar]
- 66.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals-distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour. IUBMB Life. 2009;61:731–738. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter A. Integrins as target: first phase III trial launches, but questions remain. J Natl Cancer Inst. 2010;102:675–677. doi: 10.1093/jnci/djq186. [DOI] [PubMed] [Google Scholar]
- 68.Wittekind Ch, Meyer HJ, Bootz F., editors. TNM Klassifikation Maligner Tumoren. UICC International Union Against Cancer. 6. Aufl. Korr. Nachdruck. Kopf- und Halstumoren; 2005. pp. 19–52. [Google Scholar]
- 69.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 70.Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin – heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 71.Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellaular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoprotein Ic-IIa. J Cell Biol. 1988;107:1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seiffert D, Ciambrone G, Wagner NV, Binder BR, Loskutoff DJ. The somatomedin B domain of vitronectin. Structural requirements for the binding and stabilization of active type 1 plasminogen activator inhibitor. J Biol Chem. 1994;269:2659–2666. [PubMed] [Google Scholar]