Abstract
The status of the three retinoic acid receptors (RARs) α, β and γ in human colorectal cancer (CRC) has not as yet been examined. RARs are in part responsible for the actions of the retinoids (vitamin A and its derivatives), which are essential for human health and survival due to their extensive involvement in numerous cellular processes, in particular in epithelial morphology. The present study examined the expression of the three RARs in CRC using immunohistochemical analysis of paraffin-embedded tissue sections. RAR expression in tumor (T) and adjacent non-tumor (NT) specimens from stage I (n=6), stage II (n=34), stage III (n=26) and stage IV (n=14) CRC patients was compared with that in normal mucous membranes (n=10) from control individuals. The findings were correlated with tumor grade, treatment response (progression during treatment, remission, chemoresistance) and survival as clinicopathological parameters. RARα and γ expression was decreased with CRC stage in the T tissues (P=0.016 and P=0.052, respectively), suggesting that they may be used as predictive markers. RARβ expression in the NT tissues was associated with a more favorable prognosis (P=0.04). These results provide important information on the tumor microenvironment (the area adjacent to tumor cells).
Keywords: colorectal cancer, biomarkers, response to treatment
Introduction
With its first description in 1925, Wolbach and Howe implicated vitamin A and its derivatives (retinoids) in epithelial development and tumorigenesis (1). The activity of vitamin A, apart from that involved in vision, is mediated by retinoids. Many years later, De Luca further implicated the retinoids in differentiation and embryogenesis (2) by showing their effects on limb development, epithelial integrity and tumorigenesis. Since they are involved in numerous life processes (embryogenesis, cell growth, cell differentiation and cell death), retinoids are essential for life. One of their most important actions is their antitumor activity, whereby they inhibit tumor growth and promote apoptosis (3,4). This has led to their therapeutic application against cancer, for example in the treatment of acute promyelocytic leukemia (5,6).
Retinoids cross the cell membrane through hydrophobic interactions and/or endocytosis in order to bind to their specific receptors: the retinoic acid receptors (RARs) (7) and rexinoid receptors (RXRs) (8–10). When retinoids are present in cells, they bind the RAR/RXR heterodimer, which acts as a transcription factor. The retinoid-receptor complex binds to the retinoic acid response element sequence, located near the promoter of target genes, to induce or inhibit transcription (3).
The RAR family comprises three members: α, β and γ. RARβ expression is frequently reduced in tumor cells, probably due to the hypermethylation of its promoter (11,12), and in association with tumor progression in different organs or with pathologies in head and neck tissues (13), basal skin cells (14), breast (15), lung (16), esophagus (17), prostate (18), thyroid (19), larynx (20), endometrium (12) and oral tissues (21). All of these studies have concluded that RARβ should be considered a tumor suppressor (22). However, studies on RARα and γ expression have reported conflicting results, depending on the pathology and the technique used.
To the best of our knowledge, the expression of the RARs has not been reported in patients with colorectal cancer (CRC). In France, CRC is the second most common cancer in men and the third most common in women (23). This pathology is classified as the second leading cause of cancer-related death in industrialized countries (National Cancer Institute data), and the 5-year survival rate is very low. CRC development is characterized by four tumor stages, defined by the International Pathology Tumor Node Metastasis (pTNM) classification system (24), which is used as the diagnostic system upon which patient treatment is based. However, few proteins predictive of response have been identified.
RARs are implicated in homeostasis and, in particular, in epithelial morphology, which led us to hypothesize that the expression of these receptors is implicated in CRC development. Since few predictive proteins are available for CRC, the aim of this study was to examine the cellular distribution of the three RARs by immunohistochemical analysis of normal and pathological human colon tissues from different tumor stages. Their expression was compared to the cell proliferation rate, which is known to be associated with tumor growth, and was detected by immunostaining for Ki-67. The results revealed the importance of RAR signaling in the progression of CRC. Correlations with tumor grade, therapeutic response and survival were established.
Materials and methods
Patients and tissue samples
All cases of histologically confirmed CRC were included, regardless of whether the patients had received chemotherapy. Based on the Helsinki protocol, the exclusion criteria included juvenile patients, pregnant or breast-feeding women, rectal or colonic lesions that were not histologically confirmed to be CRCs, patients in whom follow-up was impossible and insufficient or unexploitable tissue due to inadequate preservation.
Archived formalin-fixed paraffin-embedded blocks of colon tissues were obtained from the Pathology Department of the Limoges Teaching Hospital. The specimens were from consecutive patients who underwent elective resection for CRC between January 2006 and December 2007. Eighty patients (37 women and 43 men) with a mean age of 71 years (range 41–93) were included prospectively. The first follow-up evaluation was made on October 31, 2008, with a median follow-up time of 24 months (range 11–32). The second follow-up evaluation was made on December 15, 2010, with a median follow-up time of 46 months (range 25–66). The tumors were graded according to the pTNM international classification (24). Forty patients had local disease (stage I, T1/2-N0, n=6; stage II, T3/4-N0, n=34), 26 had regional lymph-node involvement (stage III, any T-N1/2) and 14 had advanced disease (stage IV, any T, any N, presence of metastasis). Histological slides of the primary tumor were reviewed to identify the normal-appearing areas adjacent to the tumor sites and the tumor areas, excluding the central tumor zone, which was usually necrotic. The tissue blocks were sectioned (4-μm thick) and stained with H&E saffran (HES) for pathological diagnosis, TNM grading and immunostaining.
Histologically normal colon tissues from 10 patients who had been treated for benign pathologies, such as idiopathic chronic constipation (n=7) or diverticulosis (n=3), constituted the control group and were used to determine the constitutional expression.
Clinical and pathological parameters
Clinical, paraclinical (biological and imaging) and histological parameters were collected by Michelle Nouaille and technicians at the Pathology Department, Limoges Teaching Hospital, at the time of patient admission. The patients all underwent a uniform postoperative follow-up by the same team: they were examined within 1 month of resection, then every 3–4 months for the first year, every 6 months for the next 3 years and then at gradually increasing intervals. A clinical examination and quantification of serum carcinoembryonic antigen (CEA) were performed at each visit. Computed tomographic (CT) scans were performed every 6–12 months. A full colonoscopy was performed 1 year after surgery, then once every 3–5 years. Positron emission tomographic (PET) scans were selectively performed when abnormalities or axial imaging raised the possibility of recurrence. Local recurrence was defined as the first clinical, radiological and/or pathological evidence of a tumor of the same histological type within the colon. Distant recurrence was defined as clinical, radiological and/or pathological evidence of systemic disease at sites including, but not limited to, the liver, lungs, peritoneum and para-aortic region. Recurrence-free survival and disease-specific survival were analyzed. ‘Evolution’ was defined as disease progression without adjuvant therapy, ‘chemoresistance’ as disease progression during or after adjuvant therapy, and ‘remission’ as the absence of clinical signs of disease progression.
Antibodies
The antibodies used were rabbit polyclonal antibodies raised against a peptide mapping to the C-terminus of human RARα (sc-551; Santa Cruz Biotechnology, Le-Perrayen-Yvelines, France), human RARβ (sc-552) or human RARγ (sc-550). The commercially available antibody for Ki-67 (M7240; DakoCytomation SA, Trappes, France), which recognizes a 395-kDa nuclear protein expressed during cell-cycle phases (G1, S, G2 and M) (25) was used. For the isotypic controls, we used immunoglobulin G (IgG) (rabbit, I8140 and mouse, I8765; Sigma, St. Quentin Fallavier, France).
Control of antibody specificity
Total proteins from the WiDr cell line (American Type Culture Collection) were extracted using a lysis buffer following the manufacturer's recommendations (Cell Signaling Technology, Ozyme, St. Quentin Yvelines, France) in order to perform Western blotting. The results showed that RAR proteins stained at the expected molecular weights, without non-specific binding, as determined with isotypic controls for the three anti-RAR antibodies (data not shown).
Immunohistochemistry
Ki-67, RARα and RARγ were immuno-histochemically detected in paraffin-embedded tissues using the BenchMark technology (Ventana Medical Systems, Illkirch, France). The pathway RARα and RARγ staining module was used according to the Ki-67 protocol according to the manufacturer's instructions. The processing of the bar-code-labeled slides was fully automated and included the following steps: baking the slides, solvent-free deparaffinization and antigen retrieval in CC1 cell-conditioning buffer (30 min at 95°C). The samples were incubated with the primary antibody previously diluted in diluent solution (1:50 for Ki-67, 1:100 for RARα and 1:150 for RARγ) for 32 min at 37°C. Horseradish peroxidase (26)-coupled secondary antibody was added (8 min at 37°C), then the proteins were detected with the chromogenic substrate diaminobenzidine (DAB) (8 min at 37°C). The tissue sections were also counterstained with hematoxylin (12 min at 37°C) and a bluing reagent (4 min at 37°C) to increase the contrast. The slides were mounted with a non-aqueous mounting medium.
For RARβ immunohistochemistry, paraffin sections were deparaffinized in toluene and alcohol, and rehydrated with phosphate-buffered saline (PBS). Before staining, the sections were subjected to steam heat antigen retrieval in citrate buffer (200 μM citric acid, 9.8 mM sodium citrate, pH 7.0) for 5 min. This step was repeated four times in a microwave oven (750 W). After washing in PBS, the slides were incubated for 10 min with 5% H2O2 in methanol to inhibit endogenous peroxidases. Non-specific sites were blocked with PBS-3% bovine serum albumin (BSA) for 30 min. The sections were then incubated with the primary antibody (1:500) in PBS-3% BSA for 1 h at room temperature. After washing, the epitopes were labeled with the anti-rabbit HRP Envision™ plus system and visualized with liquid DAB (Dako SA). The sections were couterstained and examined with a Leica microscope, and the images were captured with a Zeiss camera.
To test the specificity of the signals, negative control experiments were performed either by omitting the primary antibody, by substituting the primary antibody with non-immune serum, or by omitting both the primary and secondary antibodies. No staining was observed in any of the negative controls (data not shown).
Quantification of immunostaining
Photomicrographs of each slide were captured with a Zeiss microscope with a magnification of x200. The most homogeneously stained tumor (T) and non-tumor (NT) areas on each slide were selected for quantification. Immunoreactivity was scored by a staining index based on the percentage of positive cells, by a semi-quantitative estimate, as follows: (−; 0), tissue with negative staining; (+; 1), tissue with staining in 25–49% of cells; (++; 2), tissue with staining in 50–74% of cells; (+++; 3), tissue with staining in ≥75% of cells. ‘Overexpression’ was defined as a staining index of ≥75%. The results were expressed as the mean of three independent quantifications made by different individuals.
Statistical analysis
The overall variations in the staining percentages for the RARs and Ki-67 and their relationships to tumor grade were evaluated by analysis of variance (ANOVA) using SYSTAT 12.0 (SPSS, 2007). Tukey's post hoc test was used to assess the significance of the differences between the stages, and P-values <0.05 were considered significant. Correlations between the parameters were visualized by cluster analysis using Spearman's ϱ as the measure of similarity, using PAST 1.83 (27). Survival curves were constructed using the free-access software of the Dartmouth-Hitchcock Norris Cotton Cancer Center (http://biostat.hitchcock.org/BSR/Analytics/CompareTwoSurvivalDistributions.asp).
Results
Baseline characteristics and overall survival
The overall 2-year survival rate was 70%, probably due to the age of the patients and the high proportion of advanced-stage cases. At the time of analysis, patient survival was 100% for stage I, 75% for stage II, 65% for stage III and 47% for stage IV. Nine patients (8 with stage II and 1 with stage III) died of causes not related to CRC (cardiac or neurological etiologies). Adjuvant chemotherapy was administered for stages III and IV. Twenty patients received no adjuvant therapy (16 stage III and 4 stage IV) due to postoperative death (n=3), age >85 years (n=14) and/or patient refusal (n=3). Cancer progression occurred in 11/20 patients and cancer recurrence in 9/11 patients during adjuvant chemotherapy.
Two years later, at the second evaluation, 77 patients had continued with the follow-up (3 had been lost). At this time, the overall 4-year survival rate was 49%; patient survival was 100% for stage I, 48% for stage II, 54% for stage III and 23% for stage IV. Apart from 5 stage II patients who died due to unrelated causes, 10 patients (5 in stage II, 1 in stage III and 4 in stage IV) succumbed to CRC during the interval between the first and the second evaluations.
Control of RAR expression in normal prostate
The use of antibodies against RARs for immunohistochemistry of chemically fixed tissues was previously tested in prostate tissue (18). When different antibody dilutions (1:50 to 1:500) were tested, the results obtained in normal prostate tissue were reproducible, with localization patterns similar to those described by Richter et al (18). As shown in Fig. 1 (arrows); for RARα, homogeneous staining in the cytoplasm with little nuclear staining was noted; for RARβ, the presence of staining in the basal nuclei was noted; for RARγ, homogeneous staining in the epithelial cytoplasm with little nuclear staining was observed. Since these results confirmed the specificity of the anti-RAR antibodies, they were used on the CRC tissues.
Figure 1.
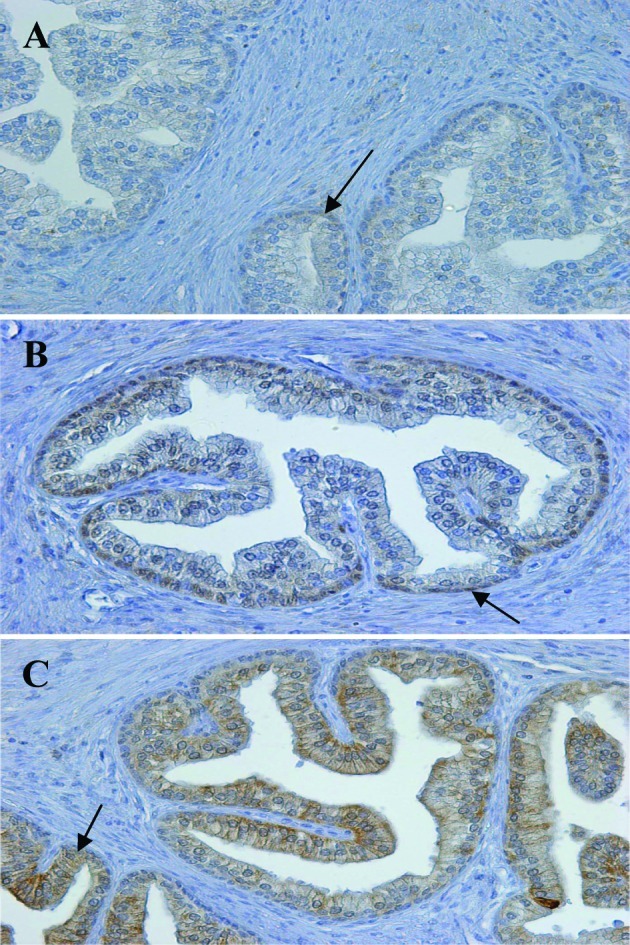
Immunohistochemical localization of RARα, β and γ in normal human prostate. Immunohistochemical staining was carried out on paraffin-embedded sections (4-μm thick), using primary antibodies as follows: (A) anti-RARα (1:100 dilution), (B) anti-RARβ (1:100 dilution) and (C) anti-RARγ (1:150 dilution). The Envision system was used as the secondary antibody. Original magnification, x200.
Ki-67 and RAR expression in different stages of CRC
The constitutional expression of the proteins was first evaluated by immunohistochemistry in the normal control group, then examined in the adjacent NT tissue of each patient, for use as an internal control. The Ki-67 and RAR staining profiles in the NT tissues were identical to those observed in the control group. Finally, the expression of the RARs was examined in the T and NT areas in the specimens from patients with different stages of CRC.
Random Ki-67 staining was detected in the nuclei of all the cells, located both inside and outside the T fields, with some differences in the percentages of labeled cells among patients (data not shown). However, ANOVA between the groups of different stages revealed no statistically significant differences (P>0.05).
RARα staining was uniformly detected in the cytoplasm of the epithelial cells in the NT and T tissues (Fig. 2). Of the 80 patients analyzed, all expressed this receptor in the NT tissues (50–75% of cells), as did in the control group (data not shown). In the T tissues, only 6 (7.5%) (stage II, n=1; stage III, n=3 and stage IV, n=2) showed no expression, 11 (13.75%) showed weak expression, 20 (25%) showed moderate expression and most (n=43; 53.75%) showed strong RARα expression. At the inital evaluation, a statistically significant difference between stages was detected with ANOVA (P=0.016) (Fig. 3). Indeed, RARα expression in the T tissues was directly correlated with tumor stage, as it was predominantly expressed in the early rather than the late stages of CRC, when its expression decreased. Reinforcing this result, this tendency was maintained at the second evaluation (P=0.0018)
Figure 2.
Immunohistochemical localization of RARα in the tumor areas of samples from patients with different stages of CRC. (A) Stage I, (B) stage II, (C) stage III and (D) stage IV. Immunohistochemical staining was carried out on paraffin-embedded sections (4-μm thick) using a primary antibody against RARα (1:100) and the Envision system as the secondary antibody. Original magnification, x200.
Figure 3.
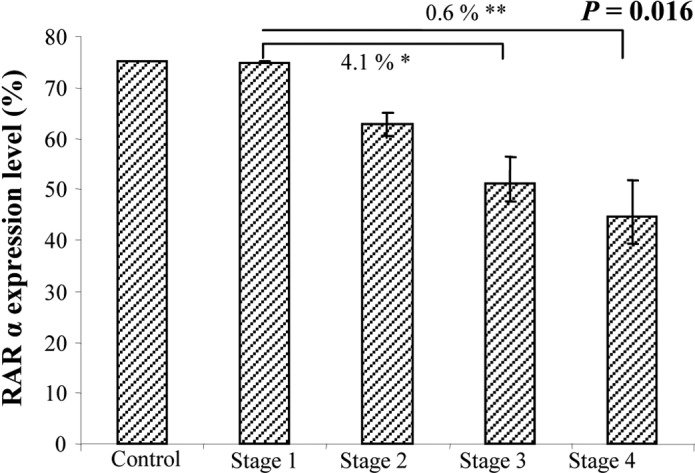
ANOVA analysis of RARα expression in the tumor areas in different CRC stages.
RARβ staining was restricted to the mucous membrane and was uniform in the cytoplasm of epithelial cells in the NT and T tissues. In the NT tissues, RARβ was expressed as a mean of 71%, comparable to that observed in the normal control group (72.5%). In the T tissues, 15 patients (18.75%) (stage II, n=7; stage III, n=4; and stage IV, n=4) showed no expression, 4 (5%) showed weak expression, 6 (7.5%) showed moderate expression and most (55; 68.75%) showed strong RARβ expression. No statistically significant differences were observed between the CRC stages as analyzed by ANOVA (P>0.4).
RARγ staining was very similar to that observed for RARβ, as it was predominant in the cytoplasm of epithelial cells in both the NT and T tissues (Fig. 4). As with the other RARs, the majority of patients expressed RARγ in the NT tissues (50–75% of cells), similar to the normal control group (data not shown). In the T tissues, only 1 (1.25%) (stage II) showed no expression, 10 (12.5%) showed weak expression, 19 (23.75%) showed moderate expression and most (50; 62.5%) showed strong RARγ expression. RARγ expression tended to differ at each CRC stage when assessed by ANOVA (P=0.052) (Fig. 5). However, this value was probably attributable to the small number of patients in stage I (only 6 patients).
Figure 4.
Immunohistochemical localization of RARγ in the tumor areas of samples from patients with different stages of CRC. (A) Stage I, (B) stage II, (C) stage III and (D) stage IV. Immunohistochemical staining was carried out on paraffin-embedded sections (4-μm thick) using a primary antibody against RARα (1:150) and the Envision system as the secondary antibody. Original magnification, ×200.
Figure 5.
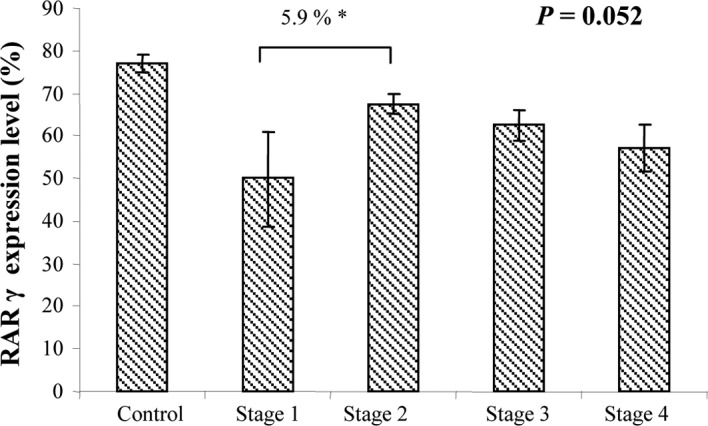
ANOVA of RARγ expression in tumor areas of samples from patients with different stages of CRC.
Correlation with patient outcome
RARα expression in the T tissues was positively correlated with CRC stage [correlation coefficient (r)=0.0011] and remission (r=0.027). However, it was negatively correlated with disease evolution (r=0.012), chemoresistance (r=0.024) and death (r=0.0047). RARβ expression may be considered a marker of CRC development, as it decreased in conjuction with disease progression (confirmed by ANOVA at the two evaluations). Moreover, strong RARβ expression in the T tissues was associated with a more favorable survival probability (P=0.0072 at the first and P=0.1 at the second evaluation) (Fig. 6A and B).
Figure 6.
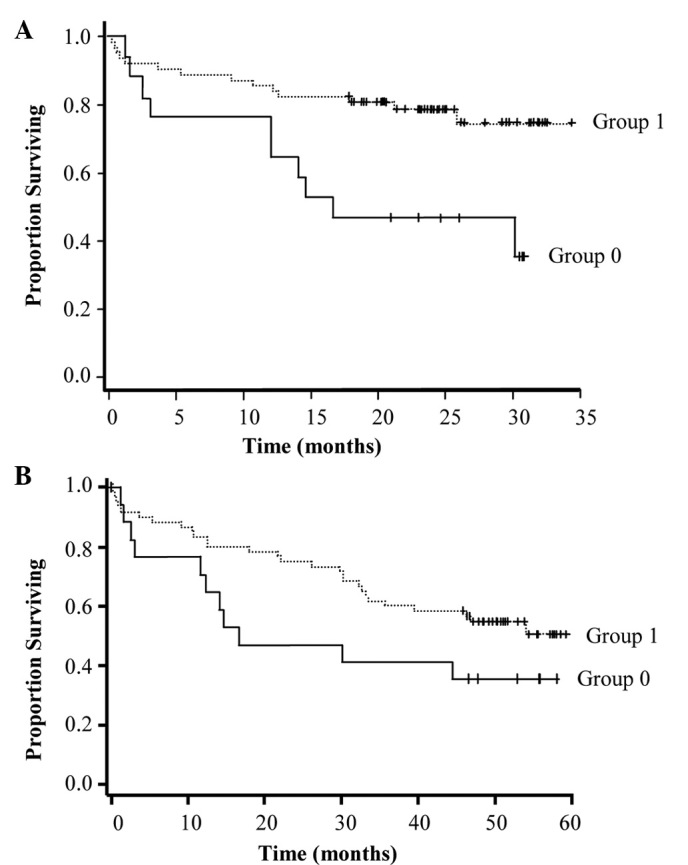
Kaplan-Meier estimation of survival probability of CRC patients based on RARα expression in the tumor tissues. (A) Evaluation carried out at the first follow-up. Group 0, patients in which 0–25% of cells expressed RARα; group 1, patients in which 75–50% of cells expressed RARα. Estimated relative risk (RR) =0.327; standard error (SE) =0.136; P =0.0072. (B) Evaluation carried out at the second follow-up. Group 0, patients in which 0–25% of cells expressed RARα; group 1, patients in which 75–50% of cells expressed RARα. RR=0.558; SE=0.199; P=0.1.
RARβ expression in the NT tissues may serve as a marker of a more favorable prognosis, as its expression was positively associated with remission (r=0.021) and negatively associated with disease evolution (r=0.022), chemoresistance (r=0.016) and death (r=0.033). Although RARβ was also expressed in the NT tissues of the patients as the internal control group, its high expression was linked to a longer survival (P=0.04) (Fig. 7).
Figure 7.
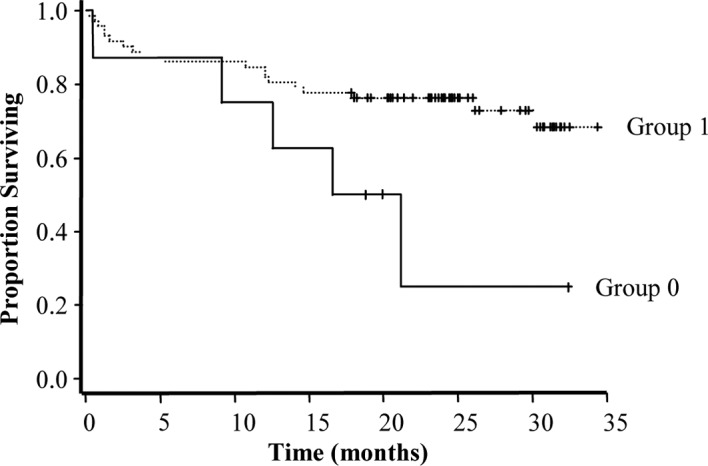
Kaplan-Meier estimation of survival probability of CRC patients based on RARβ expression in NT tissue. Group 0, patients in which 0–50% of cells expressed RARβ; group 1, patients in which 75% of cells expressed RARβ. The estimated relative risk is 0.355; the standard error is 0.179 and the P-value is 0.04.
RARγ expression in the NT tissues was negatively associated with disease evolution (r=0.033). Moreover, its expression in the T tissues was negatively associated with chemoresistance (r=0.014). Based on these results, RARγ may be used as an indicator of a more favorable prognosis for CRC, although no significant association with survival probability was found (P>0.05).
Discussion
In the present study, the expression of the proliferation marker Ki-67 and the three RARs in different stages of CRC, including the rare analysis of stage I (which is rarely operated on), was analyzed by immunohistochemistry in both the T and NT areas (the latter representing the tumor environment) of each patient.
Ki-67 immunoreactivity was present in the NT and T tissues of all of the patients analyzed, but no correlation with tumor grade or other parameters was established. Strong Ki-67 reactivity was found in the T tissues, which confirmed the high proliferative activity of CRC, but the proliferation rate was not an indicator of disease progression, as observed for prostate cancer (28).
RARβ expression is commonly lost in various tumor types (12–20). In this study, RARβ expression in the T tissues was not an indicator of tumor progression. Different RARβ isoforms, with varying biological functions, have been identified. Two known RARβ promoters and alternative splicing give rise to three major isoforms in humans (β1, β2 and β4). RARβ2 is the most abundant, and the term RARβ used in the literature frequently refers to this isoform. The loss of RARβ2 expression during cancer development is associated with tumorigenesis and retinoid resistance. The induction of its expression suppresses carcinogenesis. RARβ4 expression is also increased in various types of cancer, but induction of its expression increases the growth of tumor cells that do not express RARβ2 (29). In the present study, expression of RARβ was examined without distinguishing its different isoforms, which explains our results. Evaluating the specific expression of the various RARβ isoforms in different CRC stages and in pre-cancerous stages is of interest. RARβ expression in the NT tissues was the most significant finding of the study, as it was correlated with remission. Therefore, as a positive marker, RARβ may be an indicator of patient response to treatment and a prognostic marker of a beneficial clinical outcome.
RARα expression in the T tissues was lower than that in the NT tissues and decreased from the early to late CRC stages (first follow-up, P=0.016; second follow-up, P=0.0018), as has been shown in head and neck tumors (13), carcinogenesis of the endometrium (12) and breast tumors (30). Its expression was also positively associated with remission, which reinforces the hypothesis that RARα is a marker of disease progression.
Finally, RARγ expression in the T tissues decreased progressively with tumor stage (P=0.052), which suggests that RARγ may serve as an indicator of CRC tumor progression, as previously found in the carcinogenesis of the endometrium (12) and oral lesions (21). Its expression in the T tissues was also negatively correlated with chemoresistance. Therefore, weak RARγ expression or its loss in T tissues may be an indicator of a poor clinical outcome. In parallel, its expression was negatively correlated with disease progression in the NT tissues. Collectively, these results suggest that RARγ may be as a suitable indicator of treatment response for CRC.
Altered RAR expression is associated with the tumorigenic transformation of cells. Retinoids are potentially important due to their multi-target actions, and promising results have been obtained in different in vitro studies demonstrating the inhibition of cell growth, increased cell differentiation and the induction of apoptosis (3). Although in vitro growth inhibition of human CRC cells by retinoids or their analogues has been documented (31–33), prompting initial enthusiasm, the findings concerning their therapeutic efficacy in vivo remain controversial. Conflicting results have emerged, and retinoid resistance has been reported (34). The adverse effects of retinoids are also considerable; therefore, they must be used with caution. Further studies are warranted to clarify the mechanisms of retinoids and to improve their clinical usefulness, in particular in CRC.
In this patient cohort comprising 80 patients living in the Limousin region of France, Ki-67 and RAR expression was evaluated in tissues from patients with different stages of CRC by immunohistochemical analysis. The relationships found provide information complementary to the pTNM international classification. The mechanisms implied by the changes in RAR expression are not currently well defined. Further investigations are required to better understand the roles of retinoids in CRC carcinogenesis, in particular the corroboration of these results by a study on RXR expression. It may be useful to examine RAR expression in pre-cancerous patient tissues in order to improve patient care and the treatment of CRC, the second most common cause of death by cancer in industrialized countries.
Acknowledgments
This study was supported in part by the University of Limoges, La Ligue Contre le Cancer and the Région Limousin (to A.P.). H.A. is the recipient of a grant from the Conseil Régional du Limousin. We express our gratitude to Professor Descottes (deceased), Professor Valleix and Professor Gainant (Heads of the Departments of Surgery), to the technical experts of the Pathology Department, Limoges Teaching Hospital, for the helpful assistance with the immunohistochemistry, to ‘La Tumorothèque du Limousin’ for providing the samples and to Dr J. Cook-Moreau for the editorial assistance.
References
- 1.Wolbach B, Howe PR. Tissue changes following deprivation of fat soluble A vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 3.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 4.Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 5.Asou N. All-trans retinoic acid in the treatment of acute promyelocytic leukemia. Intern Med. 2007;46:91–93. doi: 10.2169/internalmedicine.46.1780. [DOI] [PubMed] [Google Scholar]
- 6.Degos L, Wang ZY. All trans retinoic acid in acute promyelocytic leukemia. Oncogene. 2001;20:7140–7145. doi: 10.1038/sj.onc.1204763. [DOI] [PubMed] [Google Scholar]
- 7.Nagy L, Thomazy VA, Shipley GL, et al. Activation of retinoid X receptors induces apoptosis in HL-60 cell lines. Mol Cell Biol. 1995;15:3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 9.Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LX Retinoic acid receptors. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 10.Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LXIII Retinoid X receptors. Pharmacol Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 11.Sun SY. Retinoic acid receptor beta and colon cancer. Cancer Biol Ther. 2004;3:87–88. doi: 10.4161/cbt.3.1.686. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Saito T, Tanaka R, et al. Hypermethylation in promoter region of retinoic acid receptor-beta gene and immunohistochemical findings on retinoic acid receptors in carcinogenesis of endometrium. Cancer Lett. 2005;219:33–40. doi: 10.1016/j.canlet.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54:3580–3587. [PubMed] [Google Scholar]
- 14.Kamradt J, Reichrath J. Expression of retinoic acid receptor proteins in basal cell carcinomas: an immunohistochemical analysis. J Histochem Cytochem. 1996;44:1415–1420. doi: 10.1177/44.12.8985133. [DOI] [PubMed] [Google Scholar]
- 15.Widschwendter M, Berger J, Daxenbichler G, et al. Loss of retinoic acid receptor beta expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res. 1997;57:4158–4161. [PubMed] [Google Scholar]
- 16.Picard E, Seguin C, Monhoven N, et al. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Rashid A, Wu H, Xu XC. Differential expression of retinoic acid receptors and p53 protein in normal, premalignant, and malignant esophageal tissues. J Cancer Res Clin Oncol. 2001;127:237–242. doi: 10.1007/s004320000183. [DOI] [PubMed] [Google Scholar]
- 18.Richter F, Joyce A, Fromowitz F, et al. Immunohistochemical localization of the retinoic acid receptors in human prostate. J Androl. 2002;23:830–838. [PubMed] [Google Scholar]
- 19.Haugen BR, Larson LL, Pugazhenthi U, et al. Retinoic acid and retinoid X receptors are differentially expressed in thyroid cancer and thyroid carcinoma cell lines and predict response to treatment with retinoids. J Clin Endocrinol Metab. 2004;89:272–280. doi: 10.1210/jc.2003-030770. [DOI] [PubMed] [Google Scholar]
- 20.Karamouzis MV, Sotiropoulou-Bonikou G, Vandoros G, Varakis I, Papavassiliou AG. Differential expression of retinoic acid receptor beta (RARβ) and the AP-1 transcription factor in normal, premalignant and malignant human laryngeal tissues. Eur J Cancer. 2004;40:761–773. doi: 10.1016/j.ejca.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Ralhan R, Chakravarti N, Kaur J, et al. Clinical significance of altered expression of retinoid receptors in oral precancerous and cancerous lesions: Relationship with cell cycle regulators. Int J Cancer. 2006;118:1077–1089. doi: 10.1002/ijc.21483. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez S, Germain P, Alvarez R, Rodriguez-Barrios F, Gronemeyer H, de Lera AR. Structure, function and modulation of retinoic acid receptor beta, a tumor suppressor. Int J Biochem Cell Biol. 2007;39:1406–1415. doi: 10.1016/j.biocel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Bossard N, Veltenc M, Remonteta L, et al. Survival of cancer patients in France: A population-based study from The Association of the French Cancer Registries (FRANCIM) Eur J Cancer. 2007;43:149–160. doi: 10.1016/j.ejca.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Greene FL, Page DL, Fleming I, et al. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 6th edition. Springer-Verlag; New York: 2002. p. 113. [Google Scholar]
- 25.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 26.Du L, Wang H, He L, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 27.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4:9. [Google Scholar]
- 28.Gyftopoulos K, Perimenis P, Sotiropoulou-Bonikou G, Sakellaropoulos G, Varakis I, Barbalias GA. Immunohistochemical detection of retinoic acid receptor-alpha in prostate carcinoma: correlation with proliferative activity and tumor grade. Int Urol Nephrol. 2000;32:263–269. doi: 10.1023/a:1007126332651. [DOI] [PubMed] [Google Scholar]
- 29.Xu XC. Tumor-suppressive activity of retinoic acid receptor-beta in cancer. Cancer Lett. 2007;253:14–24. doi: 10.1016/j.canlet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Leede BM, Geertzema J, Vroom TM, et al. Immunohistochemical analysis of retinoic acid receptor-alpha in human breast tumors: retinoic acid receptor-alpha expression correlates with proliferative activity. Am J Pathol. 1996;148:1905–1914. [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MO, Han SY, Jiang S, Park JH, Kim SJ. Differential effects of retinoic acid on growth and apoptosis in human colon cancer cell lines associated with the induction of retinoic acid receptor beta. Biochem Pharmacol. 2000;59:485–496. doi: 10.1016/s0006-2952(99)00355-x. [DOI] [PubMed] [Google Scholar]
- 32.Nicke B, Kaiser A, Wiedenmann B, Riecken EO, Rosewicz S. Retinoic acid receptor alpha mediates growth inhibition by retinoids in human colon carcinoma HT29 cells. Biochem Biophys Res Commun. 1999;261:572–577. doi: 10.1006/bbrc.1999.1086. [DOI] [PubMed] [Google Scholar]
- 33.Kim EJ, Kang YH, Schaffer BS, Bach LA, MacDonald RG, Park JH. Inhibition of Caco-2 cell proliferation by all-trans retinoic acid: role of insulin-like growth factor binding protein-6. J Cell Physiol. 2002;190:92–100. doi: 10.1002/jcp.10045. [DOI] [PubMed] [Google Scholar]
- 34.Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]




