INTRODUCTION
“Screening enters the political context as soon as someone must pay for services not previously provided.”(1)
The Hemochromatosis and Iron Overload Study (HEIRS)(2, 3) was designed as a genotypic and phenotypic screening study of abnormalities in iron metabolism. To our knowledge, there have been no published estimates based on objective data of the potential magnitude of ambiguous gene test results on subsequent physician utilization. The purpose of this study was to conduct exploratory analyses of physician utilization before and after people received genetic and phenotypic test results that differed in terms of estimated future disease risk and follow-up advice.
METHODS
Selection of subjects
In the HEIRS study, 99,711 participants aged 25 years or older were recruited from waiting rooms of primary care practices and blood-drawing laboratories. The present investigation was an ancillary study approved by the HEIRS Steering Committee and by the local institutional review board. It was a secondary analysis of the 20,306 participants from the HEIRS Canadian site.
Exclusion criteria
All (n = 85) C282Y homozygotes were eligible for a follow-up clinical examination, but they were omitted from the present analysis. An additional 582 participants (2.9% of total) were excluded because they had declined further participation at the screening phase. All 19,639 remaining participants were mailed a letter informing them of the results of their gene and iron tests.
Genotypic and phenotypic test result groups
Two mutations result in six genotypes: two homozygote groups (C282Y/C282Y and H63D/H63D); two heterozygote groups (C282Y/+, H63D/+), one group of compound heterozygotes (C282Y/H63D), and ‘wild types’ who had neither mutation (+/+). As well, two iron tests, Serum Ferritin, (SF), and Transferrin Saturation, (TS) were used to create a phenotypic dichotomy of ‘elevated’ versus ‘normal’. Six genotypes subdivided by two iron values results in 12 groups.
Participant notification of test results
Notification letters contained different information about future risk of developing disease, and advice about whether the participant was ‘recommended’, ‘encouraged’, or merely ‘welcome’ to share their test results with their physician (Figure 1). Results letters were available in English, Mandarin, Spanish or Vietnamese depending on participants’ stated preferences.
Figure 1.
A group of particular interest for examining the possible effect of ambiguous gene test results on physician utilization was the H63D heterozygotes with normal iron values (Group 5). The message in their letter was that their estimated disease risk was low, and that their genotype was ‘very common’ in healthy people. Like Group 1, participants in Group 5 were ‘welcome’ to share their results with their MD, because there was insufficient evidence to encourage or recommend a medical consultation. Thus, the sole difference from Group 1 was that Group 5 had a common mutation of unknown but presumed low risk to future health.
Primary outcome variable: physician claims
All residents of Ontario are covered for physician and hospital services under a single-payer scheme, the Ontario Health Insurance Plan (OHIP). The primary outcome variable comprised the total number of claims per patient per day submitted by general and specialist physicians for office visits, laboratory tests, and genetic counseling. In addition, we examined claims separately for laboratory tests and genetic counseling.
For each participant, two utilization windows were examined: the 12 months before and after they were mailed their results (the latter beginning one month after mailing date to allow mail to be received and appointments booked). We chose to analyze claims rather than costs because costs vary widely between countries and health care systems, and over time.
Statistical Analysis
Because number of physician claims is a discrete (count) variable, between-group differences in the number of claims in the 12 month post-results period were modeled using multiple Poisson regression. The primary ‘exposure’ variable was letter group, which differed on whether no abnormality, phenotypic only, genotypic only, or both abnormalities had been detected, and the corresponding risk and advice messages in the different letters. Covariates included in the model were number of physician claims in the 12 month pre-results period, age, gender, self-rated health, history of diagnosis of arthritis, diabetes, heart failure, and impotence, and baseline ln Serum Ferritin level. Using this method, each participant served as his or her own control. The reported risk ratios are adjusted for the effects of all main effects and interactions.
RESULTS
Exploratory analyses of claims specifically for laboratory tests and genetic counseling indicated that laboratory tests were a very small component of total claims. The median number of post-results genetic counseling claims was zero in all study groups (Table 1).
Table 1.
Letter groups formed by genotypic (HFE) and phenotypic (SF/TS) results, and key risk message, and follow-up advice. HEIRS Study.
| Letter Group | HFE result | Elev. SF/TS | Key risk message | Key follow-up advice |
|---|---|---|---|---|
| 1 | +/+ (reference category) | No | No genetic variation; “your iron tests are within the usual range” | “You are welcome to share this info with your MD” |
| 2 | +/+ | Yes | No genetic variation; “you do not have the type of iron levels that we are investigating in this study. However, the result of at least one of your iron tests was outside the usual range” | “We recommend you share this info with your MD” |
| 3 | C282Y/+ H63D/H63D C282Y/H63D |
No | “Iron tests are within the usual range”; you have “one or more” genetic variations that may “slightly increase your risk to develop iron overload in the future” | “We encourage you to 1) share results with your MD, and 2) talk to a genetics counselor about the risk to your family members” |
| 4 | C282Y/+ H63D/H63D C282Y/H63D |
Yes | “You do not have the type of iron levels that we are investigating in this study. However, the result of at least one of your iron tests was outside the usual range”; you have “one or more” genetic variations that experts are not sure exactly how much these changes increase your risk to develop iron overload in the future”. |
“We recommend that you 1) share results with your MD, and 2) talk to a genetics counselor about the risk to your family members” |
| 5 | H63D/+ | No | “Your iron tests are within the usual range”; “you have a variation in one of the genes that has been observed in people with iron overload. However, this variation is also very common in healthy people” | “You are welcome to 1) share this info with your MD” and 2) discuss with MD the possibility that others in your family could be at risk” |
| 6 | H63D/+ | Yes | “You do not have the type of iron levels that we are investigating in this study. However, the result of at least one of your iron tests was outside the usual range”; “you have a variation in one of the genes that has been observed in people with iron overload… It is unlikely that the genetic variation identified is contributing to the iron test results that are described above” |
“We recommend that you 1) share this info with your MD” and 2) discuss with MD the possibility that others in your family could be at risk” |
Notes: Letter group 1 was the reference category for all analyses. Some letter groups with the same risk and advice messages (Groups 4 and 6) were combined to increase sub-sample sizes. Group 3 was excluded from analysis to minimize the number of hypothesis tests.
Genotypic abnormality only
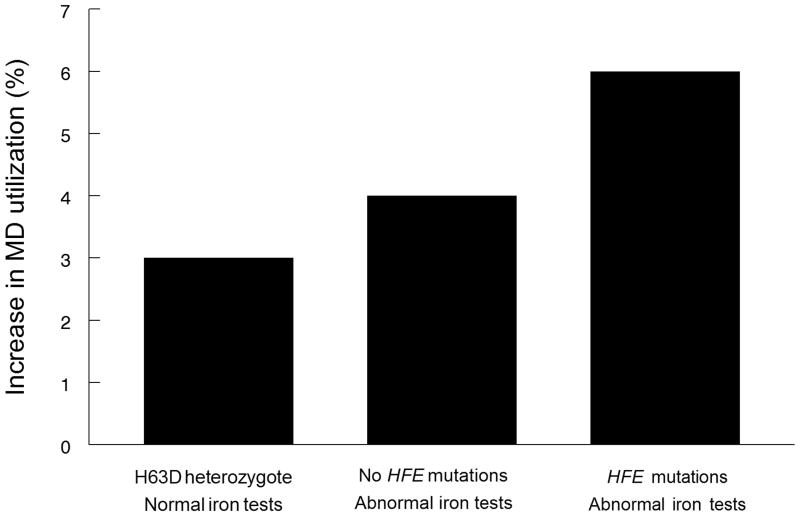
Compared to the reference group, who were also ‘welcome’ to share their results with their MD, H63D heterozygotes with normal SF/TS (Fig. 1, Group 5) had statistically significantly more post-results utilization than controls (RR = 1.03; 95%CI 1.02–1.04, P<0.001) (Table 2).
Table 2.
Baseline characteristics, and the mean (standard deviation) and median (Interquartile range) number of pre- and post-letter physician claims, for control and comparison groups.
| VARIABLE | Control (Group 1) N=10,370 |
Comparison (Groups 2–6) N=8,119 |
TOTAL N=18,489 |
|---|---|---|---|
| Age | |||
| Mean ± SD | 50.19 ± 13.7 | 49.65 ± 13.93 | 49.96 ± 13.84 |
| Median (IQR) | 49 (39–60) | 48 (39–59) | 49 (39–60) |
| Female | 6,099 (58.81%) | 5,080 (62.57%) | 11,179 (60.46%) |
| Initial Serum Ferritin | |||
| Mean ± SD | 134.92 ± 108.86 | 157.06 ± 191.16 | 144.64 ± 151.04 |
| Median (IQR) | 100 (54–184) | 91 (37–201) | 97 (47–190) |
| Pre-results physician claims* | |||
| Total: | |||
| Mean ± SD | 16.41 ± 14.87 | 17.43 ± 15.81 | 16.86 ± 15.30 |
| Median (IQR) | 12 (7–21) | 13 (8–22) | 13 (7–22) |
| Genetic Counselling: | |||
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Laboratory Tests: | |||
| Median (IQR) | 3 (2–6) | 4 (2–6) | 4 (2–6) |
| Post-results Physician Claims* | |||
| Total: | |||
| Mean ± SD | 15.09 ± 15.66 | 16.23 ± 17.56 | 15.59 ± 16.53 |
| Median (IQR) | 11 (5–20) | 12 (6––21) | 11 (5–20) |
| Genetic Counselling: | |||
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Laboratory Tests: | |||
| Median (IQR) | 2 (0–5) | 2 (0–5) | 2 (0–5) |
Total claims submitted by generalist and specialist physicians include office/clinic visits, laboratory tests, and genetic counseling
Phenotypic abnormality only
Those with no genetic abnormalities, an out-of-range iron test, and a recommendation to share their results with their MD (Fig. 1, Group 2) were compared to the reference group (Table 2). This combination of disease risk and follow-up advice was associated with approximately four percent relative difference in post-results utilization (RR= 1.04; 95%CI 1.02–1.05, p < 0.001).
Both genotypic and phenotypic abnormalities
Those with both genotypic and phenotypic abnormalities, who were also recommended to share their results with their MD (Fig. 1, Groups 4 & 6) had approximately six percent relative difference in post results utilization (RR = 1.06; 95%CI 1.04–1.08, p < 0.001) (Table 2).
DISCUSSION
It is plausible that people will seek medical advice in response to uncertainty, and even slightly elevated utilization among individuals can sum to large effects in populations.
Investigators have examined the psychological impacts of receiving ambiguous genetic test results, notably in the case of breast and ovarian cancer.(7) In a previous HEIRS study, only 2.1 percent of those with neither genotypic or phenotypic abnormality incorrectly believed that they had hemochromatosis (with 4.5 percent unsure), suggesting that the risk message was clear for the vast majority. By contrast, 9.7 percent of those with a low-risk gene abnormality and normal iron values (similar to our Group 5) incorrectly believed they had hemochromatosis (with 15.3 percent unsure). Similarly, 8 percent of those with a normal gene test and mild iron elevations (i.e. our Group 2) incorrectly believed that they had hemochromatosis (with 18% unsure). Taken together, these findings suggest some confusion about the meaning of iron or gene abnormalities in about one quarter of people. In addition, those with one or two low risk HFE mutations or mild TS/SF abnormalities were more likely to report diminished perception of general health and mental wellbeing, and more health worries, and this effect was pronounced if the person also believed they had hemochromatosis.(5) In companion studies Harrison found that those with no identified mutations understood their results and recommendations better than those with one or two mutations.(6) Walker found that, while nearly 90% of participants in the clinical examination phase of HEIRS felt the results letters contained sufficient information, nearly half (47.5%) of the respondents across all genotype/phenotype combinations still had questions after receiving the results.(7)
The study of behavioral responses to gene test results lags behind psychological outcomes and we could find no studies of behavioral responses to receiving ambiguous genetic test results. Indirect evidence that heightened genetic awareness leads to increased utilization was seen in a study from a US Health Maintenance Organization which showed that referrals for genetic counseling increased by 240% in the year following an intensive direct-to-consumer advertising campaign for a commercially available BRCA1/2 test.(8)
In the present study, participants who were mailed letters indicating normal iron results and a common ambiguous gene test with a mild risk and the same follow-up advice message as the control group had statistically significantly higher average physician utilization of approximately three percent, with the lower bound of the confidence interval suggesting at least a two percent increase. H63D heterozygosity may be a suitable example for exploration of the potential utilization effects of large numbers of people receiving ambiguous gene test results. This mutation is common, and has been found to occur in 24% of Caucasians, 20% of native Americans, and 18% of Hispanics. (3) There was no significant difference in transferrin saturation or serum ferritin between H63D heterozygotes (n = 15,289) and wild type participants in the HEIRS study (n = 75,615)(3).
An increase in physician utilization of three percent (or one physician claim) possibly due to ambiguous gene test results does not seem large. At the population level, however, any increase is concerning because there is no evidence that medical follow-up for a mutation such as H63D heterozygosity has positive effects on physical health status. In the HMO study referenced above, Mouchawar found that the proportion of women with a high probability of a mutation who were referred to genetic counseling after the ad campaign actually decreased (61% to 48%), which suggests an opportunity cost of ambiguous or low risk findings.(8)
To put a three percent increase in context, post-results utilization in those with elevated Serum Ferritin (Group 2), who might benefit from repeat testing, was only slightly higher at four percent, which in turn was only slightly lower than the six percent difference seen in those with both genotypic and phenotypic abnormalities (Groups 4 and 6).
We chose participants with normal genetic and phenotypic results as our controls. We did not choose controls from the general population because they would not have had serum ferritin results or the diagnostic histories or self-rated health to include in the multivariable models. In addition, by choosing an internal control group who were all in the same large genetic screening study, any “Hawthorne” effect (changes in behavior due to participation in research) was constant across groups, which would not be the case if general population controls were used.
Our ancillary study did not allow us to determine if the utilization was appropriate or even related to the different risk and follow-up advice. Descriptive comparisons of claims data for laboratory tests indicated that these were not large components of overall utilization before or after participation in the HEIRS study and that the observed differences in utilization across groups are overwhelmingly due to office visits to general and specialist physicians. In particular, median number of claims for genetic counseling were zero in all study groups and thus do not explain any differences in physician utilization in the Ontario context. This may not generalize to health care systems with more established and widespread genetic counseling services.
Table 3.
Results of Poisson regression models comparing post-results physician claims between participants with genotypic only, phenotypic only, or both abnormalities, versus (Group 1).
| Parameter | Relative Risk* | 95% Confidence Interval | P value |
|---|---|---|---|
| Reference (Group 1) | - | - | - |
| Abnormality: | |||
| Genotypic only (Group 2) | 1.03 | 1.02–1.04 | < 0.0001 |
| Phenotypic only (Group 5) | 1.04 | 1.02–1.05 | < 0.0001 |
| Both abnormalities (Groups 4 and 6) | 1.06 | 1.04–1.08 | < 0.0001 |
Adjusted for age, gender, history of diagnosis for arthritis, diabetes, heart failure, or impotence, self rated health (poor, fair, good, excellent), baseline (ln)serum ferritin, number of physician claims in 12 months before receiving results, and all statistically significant first-order interactions.
References
- 1.Foltz AM, Kelsey JL. The annual pap test: A dubious policy success. Milbank Mem Fund Q Health Soc. 1978 Fall;56(4):426–62. [PubMed] [Google Scholar]
- 2.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005 Apr 28;352(17):1769–78. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 3.McLaren CE, Barton JC, Adams PC, Harris EL, Acton RT, Press N, et al. Hemochromatosis and iron overload screening (HEIRS) study design for an evaluation of 100,000 primary care-based adults. Am J Med Sci. 2003 Feb;325(2):53–62. doi: 10.1097/00000441-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vadaparampil ST, Miree CA, Wilson C, Jacobsen PB. Psychosocial and behavioral impact of genetic counseling and testing. Breast Dis. 2006–2007;27:97–108. doi: 10.3233/bd-2007-27106. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RT, Wenzel L, Walker AP, Ruggiero A, Acton RT, Hall MA, et al. Impact of hemochromatosis screening in patients with indeterminate results: The hemochromatosis and iron overload screening study. Genet Med. 2006 Nov;8(11):681–7. doi: 10.1097/01.gim.0000245631.07117.ac. [DOI] [PubMed] [Google Scholar]
- 6.Harrison HF, Harrison BW, Walker AP, Lohman K, Ellis SD, Hall MA, et al. Screening for hemochromatosis and iron overload: Satisfaction with results notification and understanding of mailed results in unaffected participants of the HEIRS study. Genet Test. 2008 Dec;12(4):491–500. doi: 10.1089/gte.2008.0004. [DOI] [PubMed] [Google Scholar]
- 7.Walker AP, Tucker DC, Hall MA, Lohman K, Harrison H, Harrison BW, et al. Results communication and patient education after screening for possible hemochromatosis and iron overload: Experience from the HEIRS study of a large ethnically and linguistically diverse group. Genet Med. 2007 Nov;9(11):778–91. doi: 10.1097/gim.0b013e318159a303. [DOI] [PubMed] [Google Scholar]
- 8.Mouchawar J, Hensley-Alford S, Laurion S, Ellis J, Kulchak-Rahm A, Finucane ML, et al. Impact of direct-to-consumer advertising for hereditary breast cancer testing on genetic services at a managed care organization: A naturally-occurring experiment. Genet Med. 2005 Mar;7(3):191–7. doi: 10.1097/01.gim.0000156526.16967.7a. [DOI] [PubMed] [Google Scholar]