Abstract
Mammalian target of rapamycin (mTOR) regulates cellular processes important for progression of human cancer. RAD001 (everolimus), an mTORC1 (mTOR/raptor) inhibitor, has broad antitumor activity in preclinical models and cancer patients. Although most tumor lines are RAD001 sensitive, some are not. Selective mTORC1 inhibition can elicit increased AKT S473 phosphorylation, involving insulin receptor substrate 1, which is suggested to potentially attenuate effects on tumor cell proliferation and viability. Rictor may also play a role because rictor kinase complexes (including mTOR/rictor) regulate AKT S473 phosphorylation. The role of raptor and rictor in the in vitro response of human cancer cells to RAD001 was investigated. Using a large panel of cell lines representing different tumor histotypes, the basal phosphorylation of AKT S473 and some AKT substrates was found to correlate with the antiproliferative response to RAD001. In contrast, increased AKT S473 phosphorylation induced by RAD001 did not correlate. Similar increases in AKT phosphorylation occurred following raptor depletion using siRNA. Strikingly, rictor down-regulation attenuated AKT S473 phosphorylation induced by mTORC1 inhibition. Further analyses showed no relationship between modulation of AKT phosphorylation on S473 and T308 and AKTsubstrate phosphorylation patterns. Using a dual pan-class I phosphatidylinositol 3-kinase/mTOR catalytic inhibitor (NVP-BEZ235), currently in phase I trials, concomitant targeting of these kinases inhibited AKT S473 phosphorylation, eliciting more profound cellular responses than mTORC1 inhibition alone. However, reduced cell viability could not be predicted from biochemical or cellular responses to mTORC1 inhibitors. These data could have implications for the clinical application of phosphatidylinositol 3-kinase/mTOR inhibitors.
Introduction
The mammalian target of rapamycin (mTOR) kinase is a key regulator of cell proliferation and survival downstream of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway (1, 2). AKT/mTOR signaling is deregulated in many cancers, suggesting mTOR as an attractive target for cancer therapy (2–4). Rapamycin exerts its action by binding to the immunophilin FK506-binding protein 12. The FK506-binding protein 12/rapamycin complex binds mTOR, preventing downstream signaling to effectors of global mRNA translation (5). The rapamycin-sensitive mTOR complex (mTORC1; containing raptor) activates protein synthesis through modulation of the 40S ribosomal protein S6 kinase (S6K) and the translational initiation factor eIF-4E binding protein 1 (4E-BP1; refs. 2, 4). The rapamycin-insensitive, rictor-containing complex (mTORC2) is implicated in actin cytoskeleton regulation, as well as phosphorylation of AKT on S473 (1–4). Rictor also regulates AKT phosphorylation events mediated by integrin-linked kinase (ILK; ref. 6). Full AKT activation requires phosphorylation on two residues: Thr308 (T308) by the pyruvate dehydrogenase kinase (PDK)-1 and Ser473 (S473) by PDK2, suggested to be mTORC2 or another candidate protein kinase (e.g., DNA-dependent protein kinase, ILK, or PKCβII; refs. 1, 4, 6). In PDK1−/− cells, AKT is phosphorylated on S473 but not T308, providing evidence that the two sites are independently regulated (reviewed in ref. 1). This was supported by analysis of cells lacking mTORC2 activity, where S473 phosphorylation was selectively lost (1).
Signaling pathways that activate mTOR (e.g., PI3K) are frequently altered in cancer. AKT is a major effector of PI3K, and its deregulation plays a pivotal role in tumor biology (7). Hyperactivation of AKT is associated with resistance to apoptosis as well as increased cell proliferation and metabolism (8). Inhibition of mTORC1 can induce AKT S473 phosphorylation in a subset of cancer cell lines and patient tumors (9–12), an event which may attenuate tumor responses (8, 13, 14). A negative feedback loop has been described, whereby mTOR/S6K1 activation attenuates PI3K signaling by suppressing insulin receptor substrate-1 (IRS1) function, a mediator of insulin receptor– dependent activation of PI3K (10, 15, 16). It has beenproposed that mTORC1 inhibition relieves inhibition of the PI3K pathway through inactivation of S6K1, thereby activating AKT (13, 17–19). However, it remains possible that other mechanisms play a role (20–22). Furthermore, there is a lack of clarity about the consequences of AKT pathway up-regulation in terms of antitumor response to mTORC1 inhibition as well as drug combinations aimed at ablating AKT activation.
Paradoxically, mTORC1 inhibition, despite inducing AKT S473 phosphorylation, suppresses the enhanced growth phenotype observed in cells expressing constitutively activated AKT (2, 23), with elevated AKT activity suggested to be associated with increased tumor cell sensitivity (2). These observations add further complexity to the question of what effect increased PI3K/AKT signaling can have on tumor cell biology.
RAD001 (everolimus) is an mTORC1 inhibitor with broad antitumor activity (2) and clinical activity in cancer patients in a number of tumor types (2, 9, 12, 24–27). Pharmacodynamic analysis of cancer patient–derived tumor material showed increased AKT S473 phosphorylation in some cases (10, 12) after treatment with doses and schedules of RAD001 defined as biologically optimal through pharmacokinetic/ pharmacodynamic modeling of preclinical and phase I data (9, 28, 29). Here, using a diverse panel of human tumor cell lines, we have shown an association between the in vitro antiproliferative response to RAD001 and basal phosphorylation levels of AKT S473 and some AKT substrates. Importantly, induction of AKT S473 phosphorylation following mTORC1 inhibition was not associated with insensitivity to RAD001 and was dependent on the maintenance of rictor expression. A dual, pan-class I PI3K/mTOR catalytic inhibitor (NVP-BEZ235; refs. 20, 30) potently inhibited the increased AKT S473 phosphorylation associated with mTORC1 inhibition. This was associated with more profound cellular responses, including loss of cell viability. However, the cellular outcome following treatment with NVP-BEZ235 could not be predicted from either the anti-proliferative or the AKT S473 phosphorylation response elicited by mTORC1 inhibition alone.
Materials and Methods
Cell Culture
A549 (ATCC CCL-185), HCT15 (ATCC CCL-225), DU145 (ATCC HTB-81), HCT116 (ATCC CCL-247), MCF7 (ATCC HTB-22), MDA-MB-231 (ATCC HTB-26), and SKBR3 (ATCC HTB-30) were obtained from American Type Culture Collection; BT474 and T47D cells were obtained from Prof. N. Hynes; LN401 and LN428 cells were obtained from Dr. M. Hegi. Tuberous sclerosis complex 2 (TSC2)+/+ and TSC2−/− mouse embryonic fibroblasts (MEF; which are p53 null) have been previously described (21, 22). All were cultured in DMEM high-glucose medium (Amimed) with 10% FCS, 2 mmol/L l-glutamine, and 100 μg/mL penicillin/streptomycin, except A549, HCT15, DU145, and HCT116, which were cultured in RPMI 1640 (Amimed). KB31 cells from Dr. R.M. Baker were cultured in RPMI 1640 with 10% FCS, 4 mmol/L l-glutamine, and 100 μg/mL penicillin/ streptomycin. PC3M cells from Dr. I.J. Fidler were cultured in MEM-EBS, 10% FCS, 2 mmol/L l-glutamine, MEM non-essential amino acids (Life Technologies, Invitrogen), 1 mmol/L sodium pyruvate (Life Technologies), MEM vitamin (Amimed), and 100 μg/mL penicillin/streptomycin.
Antibodies and Reagents
RAD001 (everolimus) and NVP-BEZ235 (Novartis Pharma AG) were prepared in DMSO. Antibodies included phospho-AKT Thr308, phospho-forkhead box O transcription factor subclasses 01/3a (FoxO1/3a) Thr24/Thr32, Fox-O3a, phospho-glycogen synthase kinase-3β (GSK3β) S9, phospho-IRS1 S636/639, IRS1, raptor, phospho-S6 S235/ 236, and phospho-TSC2 T1462 from Cell Signaling Technology; TSC2 and total AKT (MEF analysis) from Santa Cruz; phospho-AKT S473 from Dako; total AKT from Epitomics; rictor from Novus Biologics (LuBioScience); GSK3β, phospho-PRAS40 T246, and PRAS40 from Biosource (Invitrogen); S6 from J. Mestan (Novartis Pharma AG); and secondary horseradish peroxidise–conjugated antibodies from Jackson ImmunoResearch Laboratories.
Protein Extraction and Immunoblot Analysis
For protein lysates, cells were washed with ice-cold PBS containing 1 mmol/L phenylmethylsulfonyl fluoride; with ice-cold buffer containing 50 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 25 mmol/L β-glycerophosphate, 25 mmol/L NaF, 5 mmol/L EGTA, 1 mmol/L EDTA, 15 mmol/L pyrophosphate, 2 mmol/L sodium orthovana-date, 10 mmol/L sodium molybdate, leupeptin (10 μg/mL), aprotinin (10 μg/mL), and 1 mmol/L phenylmethylsulfonyl fluoride (protease inhibitors from Sigma Chemical, Buchs, Switzerland); and extracted in the same buffer containing 1% NP40 (Sigma Chemical, Buchs, Switzerland). After homogenization, cleared lysates were frozen at −80°C. Protein concentration was determined with the BCA Protein Assay (Pierce). MEFs were washed with ice-cold PBS before lysis in buffer containing 60 mmol/L Tris (pH 6.8), 2% SDS, 10% glycerol, and 100 mmol/L DTT. Immunoblotting was done as previously described (28); 20 μg of total protein were analyzed and antibody-decorated bands were quantified using Quantity One software. Spearman rank correlation coefficient analysis was done (statistical significance at P < 0.05).
Proliferation/Viability Assays
Cells were incubated in 96-well plates 24 h before compound treatment. For the methylene blue assay, glutaraldehyde (5% final) was added for 10 min at room temperature. Wells were washed with water and methylene blue (0.05% w/v in water) added for 15 min at 37°C. Wells were washed twice and 3% (v/v) HCl was added for 20 min (shaking). The absorbance (650 nm) was determined using a kinetic microplate reader (Molecular Devices). Effects on cell proliferation and loss of cell viability were assessed using the YO-PRO assay followed by statistical analysis as described previously (5). Two-way ANOVA (with Tukey test) was used to test for interactions between the compounds (statistical significance at P < 0.05).
siRNA Transfection
Cells were plated in six-well plates for 24 h, transfected with 20 nmol/L siRNA using HiPerFect (Qiagen), and cell lysates prepared 72 h later. The following siRNA target sequences were used: raptor, 5-TATTTGGTCGTCCAATCTCGT-3; rictor, 5-TTAATTGTAGCAATAGAGGGT-3; and luciferase, 5-AACGTACGCGGAATACTTCGA-3. IRS1 SmartPool siRNA was from Dharmacon.
Results
AKT Pathway Activation at Baseline but not following mTORC1 Inhibition Defines the Antiproliferative Response to RAD001
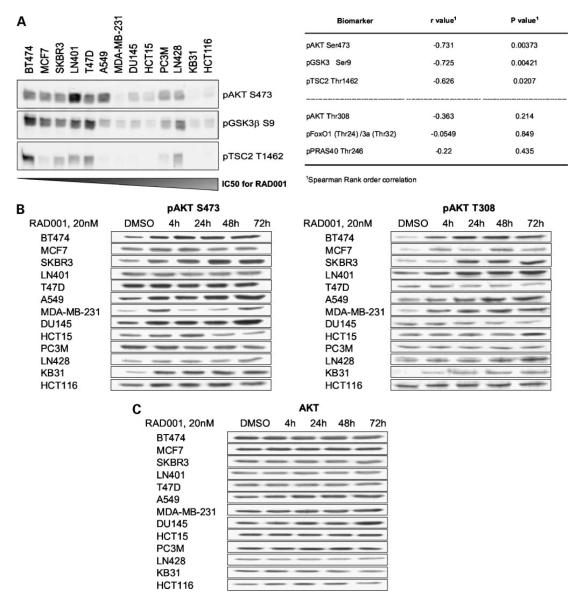
In nutrient-replete conditions, mTORC1 inhibition results in reduced cell proliferation (31), with apoptosis observed occasionally (12). A screen of the antiproliferative activity of RAD001 in 13 human cancer cell lines (representing a number of tumor histotypes) indicated that most were sensitive (nine with IC50 values ≤100 nmol/L), whereas some seemed insensitive (four with IC50 values ≥100 nmol/L; see Table 1, left). As expected from previous results (2), high levels of AKT S473 phosphorylation were associated with increased sensitivity, as was phosphorylation of the AKT substrates GSK3β and TSC2 (Fig. 1A). Indeed, there was a statistically significant correlation between AKT S473, GSK3β, and TSC2 phosphorylation and RAD001 sensitivity, which was not observed for phosphorylation of AKT T308 or the AKT substrates FoxO and PRAS40 (Fig. 1A). These data suggest that activation of some elements of the AKT pathway may indicate a selective dependence of tumor lines on mTORC1 activity.
Table 1.
The antiproliferative activity of RAD001 is associated with G1 accumulation
| RAD001 sensitivity |
Cell line | Tissue of origin |
Mean RAD001 IC50(±SD), nmol/L |
p53 status |
Treatment 24 h (20 nmol/L RAD001) |
% Cells in G1 (cell cycle) |
|---|---|---|---|---|---|---|
| Sensitive | BT474 | Breast | 0.55 ±0.12 | Mutant | DMSO | 76 |
| RAD001 | 94 | |||||
| Sensitive | MCF7 | Breast | 0.60 ± 0.10 | Wild type | DMSO | 55 |
| RAD001 | 72 | |||||
| Sensitive | SKBR3 | Breast | 0.74 ±0.34 | Mutant | DMSO | 63 |
| RAD001 | 83 | |||||
| Sensitive | LN401 | Glioblastoma | 1.5 ± 0.30 | N.d. | DMSO | 68 |
| RAD001 | 78 | |||||
| Sensitive | T47D | Breast | 1.8 (1.1; 2.5) | Mutant | DMSO | 65 |
| RAD001 | 70 | |||||
| Sensitive | A549 | Lung | 2.4 (1.2; 3.7) | Wild type | DMSO | 54 |
| RAD001 | 69 | |||||
| Sensitive | MDA-MB-231 | Breast | 7.2 ± 4.1 | Mutant | DMSO | 54 |
| RAD001 | 61 | |||||
| Sensitive | DU145 | Prostate | 10.3 ± 2.2 | Mutant | DMSO | 56 |
| RAD001 | 62 | |||||
| Sensitive | HCT15 | Colon | 65 ± 23 | Mutant | DMSO | 42 |
| RAD001 | 51 | |||||
| Insensitive | PC3M | Prostate | 149 ± 46 | Mutant | DMSO | 57 |
| RAD001 | 58 | |||||
| Insensitive | LN428 | Glioblastoma | 327 ± 206 | N.d. | DMSO | 72 |
| RAD001 | 73 | |||||
| Insensitive | KB31 | Epidermoid | 1778 ± 800 | N.d. | DMSO | 54 |
| RAD001 | 55 | |||||
| Insensitive | HCT116 | Colon | 4125 ± 1853 | Wild type | DMSO | 55 |
| RAD001 | 55 |
NOTE: The antiproliferative activity of RAD001 was measured by the methylene blue assay, defining IC50 values for RAD001 treatment in a mixed panel of human cancer cell lines (left). After 24 h treatment with optimal (20 nmol/L) RAD001, cells were subjected to cell cycle analysis by flow cytometry. A G1 accumulation was observed in RAD001-sensitive cells only (IC50 ≤ 100 nmol/L; right). There was no evidence of a sub-G1 population (not shown). Cells with known p53 status are noted.
Abbreviation: N.d., not defined.
Figure 1.
Antiproliferative response to RAD001 correlates with basal activation of the AKT pathway but not with AKT phosphorylation response following RAD001 treatment. A, left, basal expression levels of phospho-AKT S473, phospho-GSK3β S9, and phospho-TSC2 T1462 in a mixed panel of human cancer cell lines was measured by immunoblot analysis. Cell lines are listed according to their sensitivity to RAD001 treatment (antiproliferative IC50: listed in Table 1). Right, Spearman rank order correlation analysis of AKT and AKT substrate phosphorylation status was done based on the IC50 values for RAD001. R values ± 0.5 and P < 0.05 are considered statistically relevant. B, cells were treated for the indicated times with vehicle control (DMSO) or 20 nmol/L RAD001. Cell lines are listed according to ascending IC50 values for RAD001 (antiproliferative IC50: listed in Table 1). Total protein lysates were subjected to immunoblot analysis. RAD001-induced effects are shown for AKT S473 and AKT T308 phosphorylation. C, AKT levels are used as a loading control.
To assess the effect of mTORC1 inhibition on cell cycle progression, flow cytometry was also done after 24 hours of treatment with a concentration of RAD001 known to be optimal in terms of mTORC1 pathway inhibition (20 nmol/L; see Supplementary Fig. S1).3 All nine sensitive lines exhibited a clear G1 accumulation, whereas the four insensitive lines did not (Table 1, right). Furthermore, there was no evidence of apoptosis in any of the lines (data not shown).
More detailed investigation indicated that RAD001 treatment induced AKT S473 phosphorylation in approximately half of the lines tested (Fig. 1B) with no effect on AKT protein expression (Fig. 2C). This occurred with differing kinetics (in most lines persisting for up to 72 hours) and was often accompanied by increased AKT T308 phosphorylation (Fig. 1B). Examples of increased phosphorylation on both sites (e.g., BT474 and SKBR3), increased S473 phosphorylation only (e.g., DU145), and increased T308 phosphorylation only (e.g., LN428 and LN401) were observed, indicating independent regulation of these two sites following mTORC1 inhibition. Importantly, induction of AKT S473phosphorylation seemed to be independent of cellular response to RAD001, occurring in lines considered both sensitive (e.g., BT474) and insensitive (e.g., KB31) to mTORC1 inhibition and being absent in both sensitive (e.g., LN401) and insensitive (e.g., LN428) lines (Fig. 1B; Table 1). Hence, although measurement of the basal phosphorylation of AKT S473 and some AKT substrates could have application in predicting the intrinsic sensitivity of cancers to mTORC1 inhibition, induction of AKT S473 phosphorylation following treatment seems not to be a predictive pharmacodynamic biomarker.
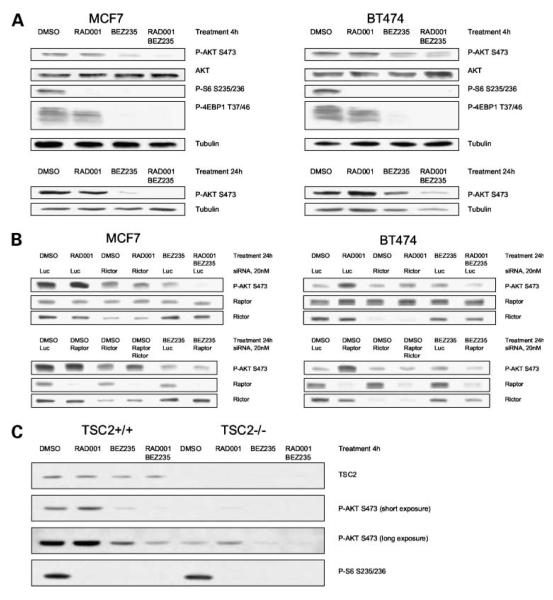
Figure 2.
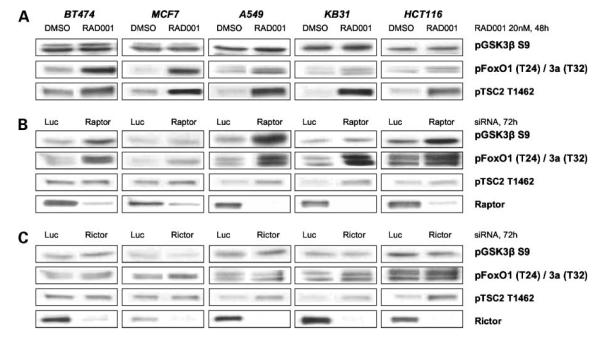
mTORC1 inhibition after treatment with RAD001 or raptor siRNA has differential effects on the phosphorylation of AKT substrates. Cells were either treated with 20 nmol/L RAD001 for 48 h (A) or transfected with raptor (B) or rictor (C) siRNA for 72 h. Total protein extracts were analyzed by immunoblot for phosphorylation of the AKT substrates—GSK3β, FoxO1/3a, and TSC2.
Induction of AKT S473 Phosphorylation after mTORC1 Inhibition Requires Rictor
Expanding on the observation that IRS1 plays a role in the up-regulation of AKT S473 phosphorylation following mTORC1 inhibition (16), an analysis of the effects of RAD001 on IRS1 indicated no clear association between changes in AKT phosphorylation and IRS1 protein expression (Supplementary Fig. S2A).3 Moreover, using three cell lines exhibiting differential IRS1 responses (Supplementary Fig. S2B),3 down-regulation of IRS1 using an siRNA approach resulted in complete (HCT116), intermediate (SKBR3), or no (A549) attenuation of phospho-AKT S473 induction after 24 hours of RAD001 treatment. Based on this observation, experiments were done to further define the molecular mechanism of RAD001-induced AKT S473 phosphorylation. Five tumor lines were analyzed, including RAD001-sensitive (BT474, MCF7, and A549) and -insensitive cells (KB31 and HCT116). As summarized in Supplementary Fig. S3A,3 RAD001 induced AKT S473 and AKT T308 phosphorylation in these lines. siRNA-mediated loss of raptor caused increased AKT phosphorylation on S473 and T308 in all lines, apart from MCF7, which seemed to be less amenable to transfection, indicating that efficient inhibition of raptor expression recapitulates the effects of RAD001 (Supplementary Fig. S3B).3 In contrast, down-regulation of rictor expression caused a slight reduction in basal AKT S473 phosphorylation levels in MCF7, A549, KB31, and HCT116 cells. In contrast, AKT T308 phosphorylation was differentially regulated, being slightly induced in BT474, A549, and HCT116 cells (Supplementary Fig. S3C).3
Analyses of AKT substrate phosphorylation suggested further complexity. Despite clear up-regulation of AKT phosphorylation on both sites, RAD001 treatment induced a dramatic increase in TSC2 T1462 phosphorylation in all lines, an effect not observed (or minimal) after down-regulation of raptor protein expression (Fig. 2A and B). Interestingly, RAD001 treatment induced FoxO1/3a phosphorylation, particularly in the RAD001-sensitive cell lines, with minimal effects on GSK3β phosphorylation; whereas substantial FoxO1/3a and GSK3β modification was observed with the raptor siRNA in both RAD001-sensitive and RAD001-insensitive cells (Fig. 2A and B). In comparison, rictor siRNA had little effect on AKTsubstrate phosphorylation. These data show that modulation of AKT phosphorylation alone does not predict effects on downstream signaling.
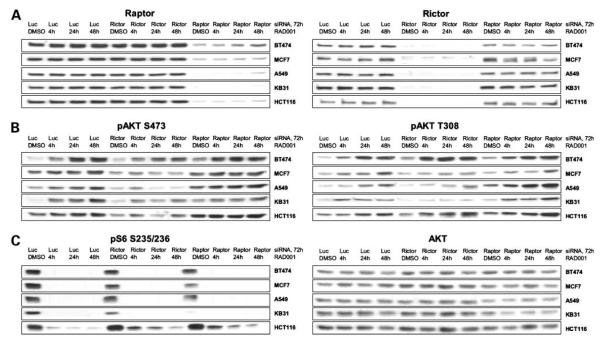
It has previously been shown that rictor regulates AKT S473 phosphorylation through the mTORC2 complex (1, 4) and potentially through interaction with ILK (6). To test the role of rictor in RAD001-induced AKT S473 phosphorylation, cells were transiently transfected with siRNA against rictor or raptor before treatment with RAD001 for up to 48 hours (Fig. 3). Reduced rictor expression clearly attenuated RAD001-induced AKT S473 phosphorylation, particularly after prolonged (24 to 48 hours) treatment (Fig. 3B, left). In contrast, reduced raptor expression had rather an enhancing effect (Fig. 3B, left). With regard to AKT T308 phosphorylation, rictor down-regulation inhibited RAD001-induced effects in some lines (MCF7, A549, and KB31) but not in others (BT474 and HCT116; Fig. 3B, right). These data show, therefore, that increases in AKT S473 phosphorylation associated with mTORC1 inhibition in human cancer cell lines are dependent on the presence of rictor. In contrast, the AKT T308 response seems to be comparatively independent, concordant with AKT S473 and T308 phosphorylation being differentially regulated.
Figure 3.
RAD001-induced AKT S473 phosphorylation is dependent on rictor. Cell lines were transfected with 20 nmol/L control siRNA or siRNA against rictor or raptor for 72 h. Treatment within these 72 h, with vehicle control (DMSO) or 20 nmol/L RAD001, was according to the indicated time points. A, siRNA transfection efficiently down-regulates raptor and rictor protein expression in all lines tested. B, rictor down-regulation attenuates RAD001-induced AKT S473 phosphorylation in all lines. Raptor down-regulation induces basal AKT S473 phosphorylation and enhances the effects of RAD001. AKT T308 phosphorylation is independently regulated, showing attenuation of RAD001-induced effects by rictor down-regulation only in some of the cell lines tested. C, dephosphorylation of S6 on S235/236 as a control for RAD001-induced inhibition of mTORC1 signaling. AKT protein levels serve as loading controls.
One point of interest is that RAD001 treatment dramatically reduced S6 S235/236 phosphorylation in all lines tested. However, although down-regulation of raptor also reduced phospho-S6 levels, most cell lines still exhibited S6 phosphorylation (Fig. 3C, left), suggesting that residual raptor protein expression is sufficient to maintain some aspects of mTORC1 signaling, a phenomenon observed and discussed by others (32, 33).
Finally, it should be noted that the above-described siRNA-mediated biochemical responses were also observed with two additional raptor and rictor siRNA sequences (Supplementary Fig. S4),3 confirming the specificity of these effects.
Targeting mTORC2 and PI3K in Tumor Cells Efficiently Inhibits AKT S473 Phosphorylation
It has been suggested that PI3K/AKT inhibition may be sufficient to prevent the induction of AKT phosphorylation following mTORC1 inhibition (34–37). To further investigate this question, an extended analysis was done with MCF7 and BT474 breast tumor cells using siRNA approaches as well as a specific, dual pan-class I PI3K and mTOR catalytic inhibitor (NVP-BEZ235), currently in phase I clinical trials in cancer patients (20, 30, 38). Cells were treated with an optimal antiproliferative concentration of RAD001 (20 nmol/L; see Table 1 and Supplementary Fig. S1)3 or NVP-BEZ235 (50 nmol/L, defined by titration; see Fig. 5C and D) for 4 or 24 h. As expected (32, 33), both drugs potently inhibited mTORC1 signaling as shown by reduced S6 phosphorylation, with NVP-BEZ235 having a more dramatic effect on 4E-BP1 T37/46 phosphorylation (Fig. 4A). Whereas RAD001 induced AKT S473 phosphorylation in BT474 cells, particularly after 24 hours, with only minor effects in MCF7 cells, NVP-BEZ235 reduced basal AKT phosphorylation in both lines. Interestingly, combining RAD001 and NVP-BEZ235 resulted in a more profound inhibition of AKT S473 phosphorylation, particularly at 24 hoursin MCF7 cells (Fig. 4A and B). The reason for this is currently unclear, but could be an indirect effect reflecting the more profound effect of the combination on cell biology at the concentrations used (see below and Fig. 5).
Figure 5.
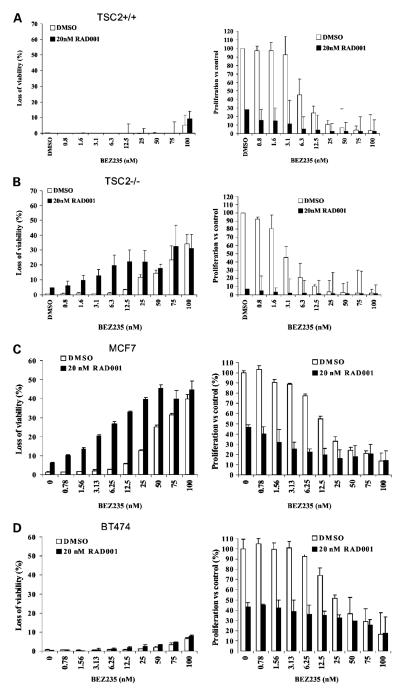
Effects of RAD001 and NVP-BEZ235 on cell proliferation and viability in MEF and tumor cell lines. The effect of RAD001 and NVP-BEZ235 treatment (alone and in combination) on relative cell proliferation and viability was evaluated using the YO-PRO assay in TSC2+/+ (A) and TSC2−/− (B) MEFs and MCF7 (C) and BT474 (D) breast tumor cells. Cells were treated for 72 h with the indicated concentrations of either RAD001 and/or NVP-BEZ235. Effects on proliferation and cell viability were statistically evaluated using two-way ANOVA (with Tukey test). In all cases, a representative experiment is shown.
Figure 4.
Targeting mTORC1/2 and PI3K efficiently inhibits AKT S473 phosphorylation. A, MCF7 and BT474 cells were treated for 4 and 24 h with vehicle control (DMSO), 20 nmol/L RAD001, and/or 50 nmol/L NVP-BEZ235. NVP-BEZ235 inhibits mTORC1 signaling (S6 and 4E-BP1 phosphorylation). Combination treatment inhibits basal and RAD001-induced AKT S473 phosphorylation. B, MCF7 and BT474 cells transfected with siRNA (20 nmol/L) for 48 h were treated for another 24 h with DMSO, 20 nmol/L RAD001, and/or 50 nmol/L NVP-BEZ235. Reduced rictor expression or NVP-BEZ235 treatment attenuates AKT S473 phosphorylation induced after treatment with RAD001 or raptor siRNA. C, TSC2+/+ and TSC2−/− MEFs were treated with DMSO, 20 nmol/ L RAD001, and/or 50 nmol/L NVP-BEZ235 for 4 h. NVP-BEZ235 inhibits basal and RAD001-induced AKT S473 phosphorylation.
Consistent with the observation that elevated AKT S473 phosphorylation after RAD001 treatment was attenuated after loss of rictor expression (Fig. 3B), increased phosphorylation after raptor down-regulation was also rictor dependent, as shown by combining raptor with rictor siRNAs (Fig. 4B, bottom). NVP-BEZ235 also attenuated the increased AKT S473 phosphorylation associated with raptor down-regulation, to an extent similar to that observed with RAD001 and NVP-BEZ235 in combination (Fig. 4B, compare last lanes of top and bottom blots). These data support the conclusion that induction of AKT S473 phosphorylation related to chemical (RAD001 treatment) or biological (raptor siRNA) mTORC1 inhibition is modulated in a rictor-dependent manner and can be blocked using a compound active against PI3K and mTORC2.
Sensitivity to RAD001- nor RAD001-Induced AKT S473 Phosphorylation Predicts Tumor Cell Response to the PI3K/mTOR Inhibitor NVP-BEZ235
In the previous section we showed that NVP-BEZ235 prevents rictor-dependent induction of AKT S473 phosphorylation, presumably through direct inhibition of mTORC2, although inhibition of class I PI3Ks may also contribute. To evaluate the cellular consequences of dual PI3K/mTOR inhibition, a genetically defined model of mTORC1 deregulation was used. As expected (21, 22), MEFs lacking expression of the mTORC1 negative regulator TSC2 (TSC2−/−) exhibited reduced basal AKT S473 phosphorylation when compared with isogenic controls (TSC2+/+; Fig. 4C). Strikingly, RAD001 caused a similar induction of AKT S473 phosphorylation versus basal in both lines. However, the TSC2+/+ line was found to be ~25-fold less sensitive to RAD001 than the TSC2−/− line as determined by YO-PRO proliferation assay (TSC2+/+ IC50 = 7.2 nmol/L; TSC2−/− IC50 = 0.26 nmol/L). In both lines, treatment with an optimal concentration of NVP-BEZ235 (50 nmol/L; see Fig. 5A and B) reduced basal and RAD001-associated AKT S473 phosphorylation levels (Fig. 4C) and a significant concentration-dependent effect on cell proliferation was observed (P < 0.001, two-way ANOVA on Tukey test), with the TSC2−/− MEFs being twice as sensitive to NVP-BEZ235 treatment (TSC2+/+ IC50 = 7.2 nmol/L; TSC2−/− IC50 = 3.0 nmol/L; see Fig. 5A and B). Interestingly, combining NVP-BEZ235 with optimal RAD001 (20 nmol/L) significantly increased antiproliferative effects in both lines (P < 0.001), albeit reaching the same end point as optimal NVP-BEZ235 alone (Fig. 5A and B, right), an observation consistent with the fact that NVP-BEZ235 also targets the mTORC1 kinase. In TSC2+/+ MEFs, NVP-BEZ235 alone had a minor but significant effect on cell survival only at high concentrations (P < 0.001), with the addition of optimal RAD001 having little effect (Fig. 5A, left). Strikingly, in TSC2−/− MEFs, NVP-BEZ235 alone or in combination with RAD001 induced a statistically significant increase in cell death (P < 0.01; Fig. 5B, left), with a dramatic interaction between RAD001 and NVP-BEZ235, particularly at low NVP-BEZ235 concentrations (<25 nmol/L). Hence, MEFs with a genetically defined mTORC1 pathway deregulation exhibit a more profound cellular response to both RAD001 and NVP-BEZ235.
To evaluate the same question in the heterogeneous genetic background of tumor cells, the same analysis was done in MCF7 and BT474 cells, which have a similar anti-proliferative response to mTORC1 inhibition (see Table 1), associated with differential effects on AKT S473 phosphorylation. The former line shows minimal induction of AKT S473 phosphorylation even after 72 hours RAD001 treatment, as compared with a robust and sustained induction in BT474 cells (Figs. 1B, 3B, and 4A and B). In both cell lines, NVP-BEZ235 efficiently reduced basal and RAD001-induced AKT S473 phosphorylation (see Fig. 4A and B), exerting a significant concentration-dependent effect on cell proliferation (P < 0.001), with the MCF7 cells being slightly more sensitive (Fig. 5C and D, right). Combining NVP-BEZ235 with optimal RAD001 (20 nmol/L) significantly increased the antiproliferative effect in both lines (P < 0.001), again reaching the same end point as optimal NVP-BEZ235 alone (Fig. 5C and D, right). In MCF7 cells, significant cell death was observed with NVP-BEZ235 (P < 0.001) and a significant positive interaction occurred with the combination (P < 0.001; Fig. 5C, left). Again, cell death induced by optimal NVP-BEZ235 (>50 nmol/L) was similar to the combination at lower NVP-BEZ235 concentrations. Strikingly, in BT474, which showed a more rapid and dramatic increase in AKT S473 phosphorylation after RAD001 treatment, neither NVP-BEZ235 nor the combination induced a marked increase in cell death (Fig. 5D, left).
From these data, derived from both a genetically defined model of mTORC1 deregulation and genetically heterogeneous tumor cells, we have shown that concomitant targeting of the PI3K and mTOR kinase elicits more profound cellular responses (including loss of cell viability). However, cellular outcome in tumor cells cannot be predicted from antiproliferative or AKT S473 phosphorylation responses induced by mTORC1 inhibition alone.
Discussion
The prototypic pathway that promotes cellular survival is the PI3K/AKT/mTOR pathway, which is constitutively activated in many cancers (20, 22). Many tumor cell lines and animal models of cancer are sensitive to the mTORC1 inhibitor RAD001, whereas some are intrinsically insensitive (2). Previous work has shown that, in serum-deprived conditions (39–41) or in combination with DNA-damaging agents (5), p53 status is indicative of the degree of cellular response to mTORC1 inhibition. In contrast, in nutrient-replete conditions, we were unable to show an association between p53 status and RAD001 single-agent activity, both in the large panel of tumor lines analyzed here (see Table 1) and in a panel of 15 non–small-cell lung cancer lines (data not shown), consistent with the observations of others (5, 42). However, we have shown that activation of the PI3K/ AKT pathway (high phospho-AKT S473) is indicative of acertain dependency on mTORC1 activity, an observation consistent with mTOR being downstream of the PI3K pathway and with the enhanced activity of mTORC1 inhibitors in cells expressing constitutively activated AKT (2, 23). Because AKT S473 phosphorylation levels are suggested to dictate substrate utilization (43), a quantitative analysis of AKT substrate phosphorylation was done. There were differential correlations with RAD001 sensitivity, with some apparently being predictive (phospho-GSK3β S9, phospho-TSC2 T1462) and others not [phospho-FoxO1(T24)/3a (T32), phospho-PRAS40 T246]. In this context, it is interesting to note that enhanced TSC2 T1462 phosphorylation is indicative of inactivation of the TSC1-TSC2 mTORC1 inhibitory complex (44). Moreover, loss of TSC2 function has been associated with elevated GSK3β phosphorylation (45). Hence, the association between elevated GSK3β and TSC2 phosphorylation, together with the enhanced sensitivity of TSC2−/− MEFs to RAD001, supports the conclusion that these epitopes may be useful as predictive biomarkers of RAD001 sensitivity.
It has been suggested that increased AKT signaling following mTORC1 inhibition could attenuate anticancer efficacy, confer resistance, or over time, contribute to the development of resistance (8, 14). Our data indicate that changes in AKT S473 phosphorylation upon treatment with RAD001 cannot be used as a surrogate biomarker predictive of acute antiproliferative responses. This may seem at odds with the observation that PI3K/AKT pathway activation is associated with sensitivity to RAD001 treatment. In this regard, however, there are clear differences in the biochemical end points depending on how AKT activation occurs (e.g., AKT substrate phosphorylation at baseline versus after mTORC1 inhibition versus after raptor depletion). The most striking observation was that FoxO1 phosphorylation increased after RAD001 treatment of sensitive lines, a phenomenon not observed after raptor down-regulation. Additionally, TSC2 phosphorylation increased in all lines after RAD001 treatment, with raptor siRNA having little effect. Based on this limited analysis, it is difficult to make solid conclusions on the significance of these observations to the cell. However, the fact remains that changes in AKT S473 phosphorylation can be associated with pleiotropic effects on downstream signaling molecules. Hence, we suggest that mTORC1-induced changes in AKT S473 phosphorylation levels per se may not be so informative with regard to relevance to cellular outcome.
Recent data have suggested that mTORC2 is directly involved in AKT activation (3, 43). A limited analysis in human cancer lines indicated that silencing raptor, but not mTOR or rictor, induced AKT S473 phosphorylation (3), whereas silencing rictor decreased it (3, 46). We have extended this analysis to a large panel of tumor lines, and our data strongly support the role of rictor as a modulator of the increased AKT S473 phosphorylation associated with mTORC1 inhibition resulting from RAD001 treatment or raptor depletion. During revision of this article, Wang et al. (47) suggested no association between rictor and increased phospho-AKT S473 levels after rapamycin treatment of tumor cells. The reason for this contrasting observation is presently unclear, but could be related to treatment times. In five tumor lines, we have observed that attenuation of RAD001-induced AKT S473 phosphorylation in the absence of rictor occurred most strikingly after prolonged treatment times (24–48 hours; see Fig. 3B), an observation confirmed in raptor siRNA–transfected cells (Fig. 4B). In comparison, Wang et al. (47) analyzed cell extracts only after acute rapamycin treatment (1 hour).
Highlighting the importance of rictor in development, rictor mutant mice are characterized by embryonic lethality, associated with inhibition of AKT S473 phosphorylation (3). Moreover, analysis of rictor−/− MEFs showed a requirement for insulin signaling to FoxO3, but not to TSC2 or GSK3β (48). Hence, rictor may be a necessary component of the AKT-FoxO pathway, a hypothesis supported by work in Drosophila (49). Here, siRNA-mediated down-regulation of rictor in five tumor lines did not lead to any significant modulation of FoxO, TSC2, or GSK3β phosphorylation. This could indicate differential regulation of AKT signaling during development as compared to cancer. Alternatively, different experimental approaches may explain these discrepancies. For example, we performed our experiments in full medium, whereas Guertin and coworkers (48) performed insulin treatment after serum starvation.
Attenuation of mTORC1 inhibitor–induced AKT activation using logical drug combinations may avoid any potentially negative effects on tumor response. Indeed, concomitant inhibition of mTORC1 and PI3K has induced increased antiproliferative effects in some tumor lines (34, 37, 47). The phenotype of rictor-deficient embryos is similar to that of mice deleted for PI3K (p110α; ref. 48), suggesting that mTORC2 lies downstream of PI3K. Furthermore, direct mTORC2 inhibition may prove to be a promising strategy for cancer therapy because mTORC2 activity permits high-level PI3K/AKT signaling in vivo (50). Using NVP-BEZ235 (30, 38), an inhibitor of PI3K and mTORC1 and mTORC2 kinases, together with siRNA approaches, cotargeting these kinases efficiently inhibited RAD001- and raptor siRNA–induced AKT S473 phosphorylation. Moreover, this was associated with more profound effects on cell proliferation and viability as compared to mTORC1 inhibition alone. Notably, although specific deregulation of mTORC1 signaling seemed to sensitize cells to the action of NVP-BEZ235, treatment of tumor lines that exhibit similar antiproliferative responses to mTORC1 inhibition resulted in quite different effects on proliferation and viability. Strikingly, loss of cell viability was observed in MCF7 cells but not in BT474 breast carcinoma cells, despite the latter having the more dramatic and prolonged increase in AKT S473 phosphorylation after RAD001 treatment. Together with data from TSC2+/+ and TSC2−/− MEFs, this suggests that increases in AKT S473 phosphorylation after mTORC1 inhibition will not predict cellular outcome to a dual PI3K/ mTOR inhibitor.
It is possible that the rictor-dependent AKT S473 phosphorylation we have observed could be due to modulation of rictor-dependent ILK activity rather than mTORC2 complex regulation (6). Indeed, this is a question worthy of further investigation. However, despite extensive tests against a panel of protein kinases, NVP-BEZ235 has only been found to efficiently inhibit the class I PI3K and mTOR kinase, presumably because of the high homology between the kinase domains of mTOR and class IA PI3Ks (30). Hence, based on the data with NVP-BEZ235, we can conclude that increased AKT signaling associated with mTORC1 inhibition is modulated by mTORC2 and potentially PI3Ks, inhibition of which leads to more profound effects on tumor biology.
Taken together, the data in this article stimulate the question of just how much AKT responses following mTORC1 inhibition actually contribute to the attenuation of antitumor responses. One could speculate that over long treatment periods AKT activation may overcome the antitumor activity of mTORC1 inhibitors, a hypothesis recently supported through long-term in vitro culturing of A549 cells in the presence of rapamycin (47). However, in the latter study, a lack of inhibition of the p70S6K1 pathway after mTORC1 inhibition was also shown in the resistant cells, suggesting that other parameters may also play a role. These points, coupled with our demonstration that there is a high level of complexity associated with the biochemical effect of AKT phosphorylation events induced by mTORC1 inhibition, indicate that there is as yet no simple explanation with regard to the consequences of this phenomenon for cancer patients. The data with NVP-BEZ235 suggest that targeting PI3K and mTORC2 together with mTORC1 can bring benefit in terms of enhanced tumor cell responses. However, the definition of tumors more likely to respond to NVP-BEZ235 cannot be simply based on biochemical or cellular responses to mTORC1 inhibition, a conclusion that could have implications for patient selection.
Supplementary Material
Acknowledgments
We thank S. Zumstein-Mecker [Novartis Institutes for Biomedical Research (NIBR) Oncology, Basel, Switzerland] for technical assistance; Dr. F. Natt (NIBR Biologics) for providing siRNA; Dr. M. Hegi (Department of Neurosurgery, University Hospital Lausanne, Switzerland) for providing the glioblastoma lines; Prof. N. Hynes (FMI, Basel, Switzerland) for providing BT474 and T47D cells; Dr. R.M. Baker (RPMI, Buffalo, NY) for providing KB31 cells; and Dr. I.J. Fidler (M. D. Anderson Cancer Center, Houston, TX) for providing PC3M cells. We also thank Dr. C. Garcia-Echeverria for proofreading the manuscript, Dr. T. O’Reilly for statistical analyses, and Dr. R. McCabe for editorial assistance.
Footnotes
Disclosure of Potential Conflicts of Interest H.A. Lane, C. Stephan, and S-M. Maira: own shares in Novartis Pharma AG. D. Kwiatkowski: research grant from Novartis Pharma AG. No other potential conflicts of interest were disclosed.
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Boulay A, Lane HA. The mammalian target of rapamycin kinase and tumor growth inhibition. Recent Results Cancer Res. 2007;172:99–124. doi: 10.1007/978-3-540-31209-3_7. [DOI] [PubMed] [Google Scholar]
- 3.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Chiang GC, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–42. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 6.McDonald PC, Oloumi A, Mills J, et al. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–24. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 7.Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–6. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 8.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell A, Faivre S, Burris HA, III, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun SY, Rosenberg LM, Wang XR, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 12.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–10. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 14.Rosen N, She QB. AKT and cancer—is it all mTOR? Cancer Cell. 2006;10:254–6. doi: 10.1016/j.ccr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–81. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 16.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 17.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2006;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 20.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/ mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–8. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Cicchetti G, Onda H, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–33. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milton DT, Riely GJ, Azzoli CG, et al. Phase I trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer. 2007;110:599–605. doi: 10.1002/cncr.22816. [DOI] [PubMed] [Google Scholar]
- 25.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–73. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 26.Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91. doi: 10.1016/j.ejca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Yao JC. Neuroendocrine tumors. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab. 2007;21:163–72. doi: 10.1016/j.beem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–61. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka C, O’Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;6:1596–602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 30.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo anti-tumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 31.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;127:5–19. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Sarbassov DD, Ali SM, et al. MTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 33.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 34.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–9. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han EKH, Leverson JD, McGonigal T, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–61. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi H, Kondo Y, Fujiwara K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 37.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–82. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 38.Stauffer F, Maira S-M, Furet P, Garcia-Echeverria C. Imidazo(4,5-c)quinolines as inhibitors of the PI3K/PKB-pathway. Bioorg Med Chem Lett. 2008;18:1027–30. doi: 10.1016/j.bmcl.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Huang S, Shu L, Dilling MB, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21 (Cip1) Mol Cell. 2003;11:1491–501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 40.Huang S, Shu L, Easton J, et al. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–6.4. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 41.Teachey DT, Obzut DA, Cooperman J, et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107:1149–55. doi: 10.1182/blood-2005-05-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 43.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Huang J, Manning BD. The TSC1–2 complex: a molecular switch-board controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185–97. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frias MA, Thoreen CC, Jaffe JD, et al. mSin1 is necessary for Akt/ PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–18. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Lee G, Chung J. Discrete functions of rictor and raptor in cell growth regulation in Drosophila. Biochemical Biophys Res Commun. 2007;357:1154–9. doi: 10.1016/j.bbrc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 50.Hietakangas V, Cohen SM. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21:632–7. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.