Abstract
The type I insulin-like growth factor receptor (IGF-1R) signaling pathway is an important growth-regulatory pathway that is prevalent in a variety of cancer types, including human non-small cell lung cancer (NSCLC). To observe the combined effects of gefitinib and AG1024-induced IGF-1R inhibition on the growth of NSCLC, PC9/G cells, a NSCLC cell line with acquired resistance to gefitinib, were treated with AG1024 and gefitinib, alone or in combination. The proliferative activity of PC9/G cells upon different treatments was assessed by CCK-8, and the median-effects principle was used to assess the effect of the combined treatment. Apoptotic rates of the PC9/G cells for the different treatment groups were analyzed by flow cytometry. The expression of phosphorylated epidermal growth factor receptor (p-EGFR), phosphorylated-Akt (p-Akt), and phosphorylated extracellular signal-regulated kinase (p-ERK) in PC9/G cells was examined by Western blotting. PC9/G cells exhibited apoptotic features after treatment with AG1024 and gefitinib alone, and their proliferation rates were inhibited to different degrees. The treatment of AG1024 combined with gefitinib resulted in a synergistic effect in inducing apoptosis, inhibiting cell proliferation and decreasing the expression of p-EGFR, p-Akt and p-ERK. In conclusion, combined inhibition of IGF-1R signaling enhances the anti-proliferative and pro-apoptotic effects of gefitinib in NSCLC gefinitib-resistant cells. Moreover, the addition of an anti-IGF-1R strategy to gefitinib treatment may be more effective than a single-agent approach.
Keywords: insulin-like growth factor-1 receptor, gefitinib, non-small cell lung cancer, apoptosis
Introduction
Lung cancer is the leading cause of cancer-related mortality in both men and women (1). Non-small cell lung cancer (NSCLC) represents 80% of lung cancers, and most patients are diagnosed with stage IIIB and IV disease. Five-year survival rates for these patients remain below 10%. Current treatments, including chemotherapy, radiotherapy and surgery, have provided only limited improvement in the natural history of the disease (2). This dismal clinical and epidemiological picture underscores the need for novel treatment strategies to target this aggressive disease.
Over the last decade, the epidermal growth factor receptor (EGFR) has emerged as one of the most important signaling components involved in cell growth and survival. EGFR and its family members, ErbB2, ErbB3 and ErbB4, are receptor tyrosine kinases which send signals into the cell to regulate many critical processes including development, tissue homeostasis, and tumorigenesis (3). The binding of a ligand (e.g. EGF) to the extracellular region of the EGFR induces receptor dimerization and activation of the intracellular region. The intracellular tyrosine kinase domain then phosphorylates several tyrosine residues of the receptor and relays the signal to downstream signaling pathways (4). Extracellular signal-regulated kinase (ERK)1/2, which is one of the three major groups of mitogen-activated protein kinases (MAPKs) in mammals, is activated by the EGFR tyrosine kinase and plays an essential role in cell proliferation. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway which is activated by the EGFR has been implicated in both cell proliferation and survival (5). Furthermore, recent large-scale retrospective analyses have reported that EGFR is overexpressed in 62% of NSCLCs (6). EGFR is implicated in the development and progression of the majority of common human epithelial cancers; therefore, various agents have been developed to block EGFR activation in cancer cells. Small molecule kinase inhibitors targeting the ErbB family have been the subject of intensive drug development and clinical trials in cancer therapy (7). Gefitinib (Iressa®) and erlotinib (Tarceva®) are members of a class of quinazolium-derived agents that inhibit the EGFR pathway by binding in a reversible fashion to the EGFR ATP pocket domain (8). Both agents are approved for treatment of patients with advanced NSCLC and have provided hope for better survival. However, their efficacy is still limited, predominantly due to drug resistance (9). Despite initial and sometimes dramatic responses of specific NSCLC cases to EGFR TKIs, nearly all patients develop resistance and relapse.
Recently, the T790M mutation (10,11) and amplification of MET (12) were identified as two main mechanism of acquired resistance. Although together they account for approximately 50% of cases with acquired resistance, the mechanism involved in the remaining 50% of cases remains unknown (13).
The type I insulin-like growth factor receptor (IGF-1R) signaling pathway is another important growth-regulatory pathway that is prevalent in a variety of cancer types, including NSCLC (14). IGF-1R is a heterotetrameric receptor (two extracellular 125-kDa α chains and two transmembrane 95-kDa β chains) that auto-phosphorylates after ligand binding and activates several downstream signaling routes, including the PI3K and MAPK pathways. Signaling through IGF-1R stimulates proliferation, promotes angiogenesis and metastasis, and inhibits apoptosis (15,16). Elevated IGF-1R expression and activity have been associated with multiple aspects of cancer progression including enhanced carcinogenesis, tumorigenesis, metastasis, resistance to chemotherapeutics and other molecularly targeted drugs and to transformation (17). Major signaling pathways activated by the IGF-1R and EGFR include the PI3K and MAPK pathways. Because these receptors appear to be so similar in their signaling mechanisms, it raises the possibility that IGF-1R signaling may be involved in tumor resistance to EGFR-TKIs. Investigations to date have also demonstrated that persistent activation of PI3K/Akt signaling is involved in acquired resistance to EGFR TKIs. In this study, we evaluated the effects of a combined treatment of the IGF-1R inhibitor AG1024 with gefitinib on the human non-small cell lung cancer PC9/G cell line with acquired resistance to gefitinib, and investigated the possible mechanisms through inhibition of IGF-1R.
Materials and methods
Cell lines and culture
Human non-small lung cancer cell lines PC9 and PC9/G were kindly provided by Dr Caicun Zhou, Department of Oncology, Shanghai Pulmonary Disease Hospital affiliated with Tongji University, Shanghai, China. Both were cultured in RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS), 1% penicillin -streptomycin (GIBCO, Invitrogen) at 37°C in a humidified atmosphere with 5% CO2 and 95% air. Subcultures were produced by trypsinization and were reseeded for experiments.
Cell proliferation assay and combination index (CI)
The proliferative activity of PC9 and PC9/G cells for the different treatments was assessed using the Cell Counting Kit-8 (CCK-8) (Dojindo, Japan). Cells were plated in 96-well plates at 2,000 cells/well in complete medium and cultured for 24 h. The media were then replaced with RPMI-1640, 1% FBS with or without inhibitors. Each condition in each experiment was studied in five replicate wells. After incubation for 72 h, 10 μl of CCK-8 was added to each well, and the plates were further incubated for 4 h in an incubator. The absorbance at 450 nm was read spectrophotometrically using a microplate reader. The combination effect was evaluated by the CCK-8 assay. CI values <1, 1 and >1 indicated synergism, additive effect and antagonism, respectively.
Apoptosis assay
Cell apoptosis was determined by the Annexin V fluorescein isothiocyanate (FITC) Apoptosis Kit I (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol. Briefly, after treatment with different inhibitors for 24 h, cells were harvested, washed with PBS and resuspended in binding buffer. Cells were stained with Annexin V-FITC and 5 μl propidium iodide (PI), and incubated for 15 min at room temperature in the dark. Binding buffer 1X (400 μl) was added, and the cells were analyzed by flow cytometry (Beckman Coulter).
Western blot analysis
After treatment with the different inhibitors for 24 h, cells were harvested and washed with ice-cold PBS, lysed in lysis buffer at 0°C for 10 min, and centrifuged. Briefly, the lysates containing 30 μg protein were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) polyacrylamide gels and transferred to PVDF membranes. Antibodies were obtained from the following sources: EGFR phospho-antibody (py1068) was purchased from Epitomics, Inc. (Burlingame, CA, USA), EGF receptor rabbit antibody, Akt rabbit antibody, phospho-Akt (Ser473) mouse mAb, p44/42 MAPK (Erk1/2) rabbit antibody and P-p44/42MAPK (Thr202/Tyr204) rabbit antibody were purchased from Cell Signaling Technology (Boston, MA, USA). All target proteins were immunoblotted with appropriate primary and horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized by the Enhanced Chemiluminescence system (CWBIO Biotechnology, China). One Step Western Kit HRP (rabbit) and One Step Western Kit HRP (mouse) were purchased from CWBIO Biotechnology. β-actin was used as an internal control.
Statistical analysis
The experiments were repeated three times. Data are expressed as means ± SD. For comparison of two groups, the two-tailed, paired t-test was used. A value of P<0.05 was considered statistically significant. Statistical tests were performed using SAS 13.0 (SAS Institute, Cary, NC, USA).
Results
Acquired resistance of PC9/G cells to gefitinib
The PC9 cells were induced to mutate by the mutagen N-methyl-N’-nitro-N-nitroso-guanidine (MNNG) and then selected. The resistant cell line to gefitinib, PC9/G, was obtained by limited dilution. The sensitivity and IC50 for gefitinib and AG1024 were determined by the CCK-8 assay in the PC9 and PC9/G cells. Cells were treated with various concentrations of gefitinib and AG1024 for 72 h. IC50 values for gefitinib in the PC9 and PC9/G cells were 0.04±0.02 and 9.66±3.14 μmol/l, respectively. PC9/G cells showed a more than 100-fold higher IC50 for gefitinib than the parental cells. In addition, IC50 values for AG1024 in the PC9 and PC9/G cells were 5.10±0.60 and 7.56±0.63 μmol/l, respectively, indicating that the PC9/G cells were also more resistant to AG1024 than the PC9 cells.
AG1024 enhanced the growth inhibitory effects of gefitinib in the PC9/G cells
Both agents inhibited cell proliferation to different degrees (Fig. 1). To evaluate the cell growth inhibitory effect of the combination of AG1024 and gefitinib, we treated both cells with gefitinib and AG1024 for 72 h at a concentration ratio of 1:2. The combined treatment revealed that this co-targeting approach achieved a greater growth inhibition (Fig. 2). The combination index (CI) values were determined using the Chou and Talalay method (18), a well-established mathematical analysis to determine the pharmacologic interaction between two drugs. We evaluated the interactions between inhibitors as additive (CI=1), antagonistic (CI>1) or synergistic (CI<1). CI values significantly <1 in PC9 (CI=0.092) and PC9/G (CI=0.022) cells at the 50% inhibition level were observed, indicative of a synergistic effect. A similar synergestic effect was observed over the entire range of tested concentrations (Fig. 3).
Figure 1.
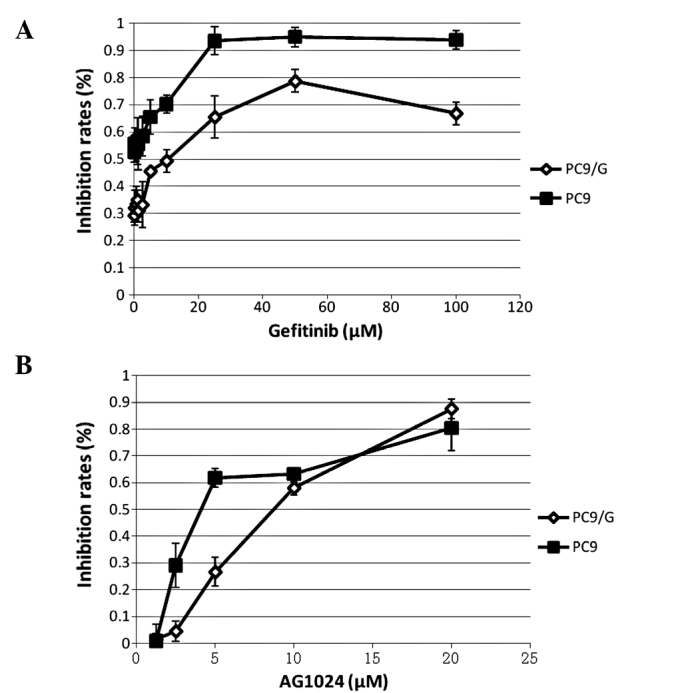
Comparative growth inhibition rates of PC9 and PC9/G cells exposed to gefitinib or AG1024. Cells (2×103 cells/well) were plated in 96-well plates and treated with various concentrations of (A) gefitinib and (B) AG1024 for 72 h. The ability of the drugs to inhibit cell proliferation was determined by the CCK-8 assay. The results represent the means ± SD of at least three independent experiments.
Figure 2.
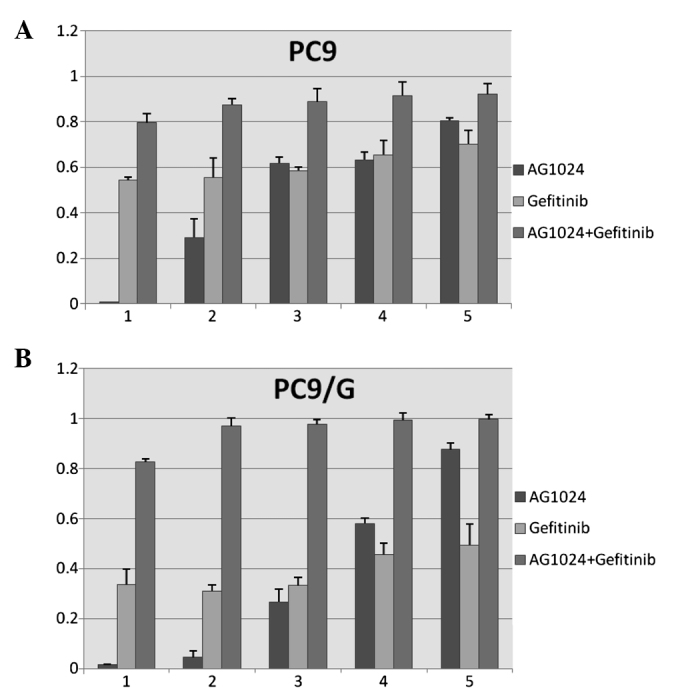
AG1024 enhances the inhibitory effect of gefitinib on cell proliferation in (A) PC9 and (B) PC9/G cells. Cells (2×103 cells/well) were plated in 96-well plates and treated with gefitinib and AG1024 alone or their combination for 72 h at a concentration ratio of 1:2. The ability of the drugs to inhibit cell proliferation was determined by the CCK-8 assay. The results represent the means ± SD of at least three independent experiments.
Figure 3.
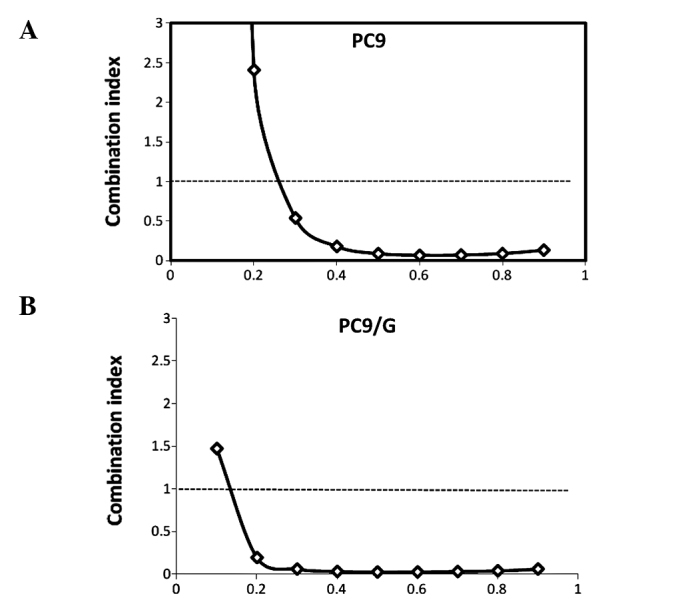
Combination index plots for (A) PC9 and (B) PC9/G cells treated with gefitinib and AG1024.
Adding an anti-IGF-1R strategy to gefitinib treatment increases the levels of apoptosis
The rates of apoptosis of the PC9/G cells in the different treatment groups were analyzed by flow cytometry. PC9/G cells were treated with AG1024 (10 μmol/l) and gefitinib (5 μmol/l) for 24 h, alone or in combination. Consistent with the results of the CCK-8 assay, the PC9/G cells displayed apoptotic features after treatment with AG1024 and gefitinib alone. Addition of AG1024 to gefitinib significantly increased the levels of apoptosis in the PC9/G cells (Fig. 4).
Figure 4.
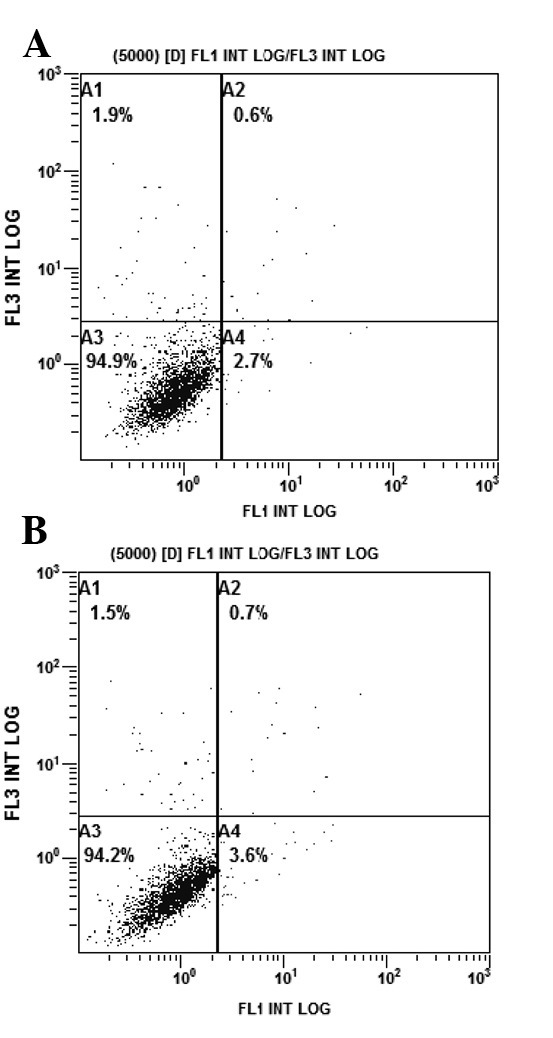
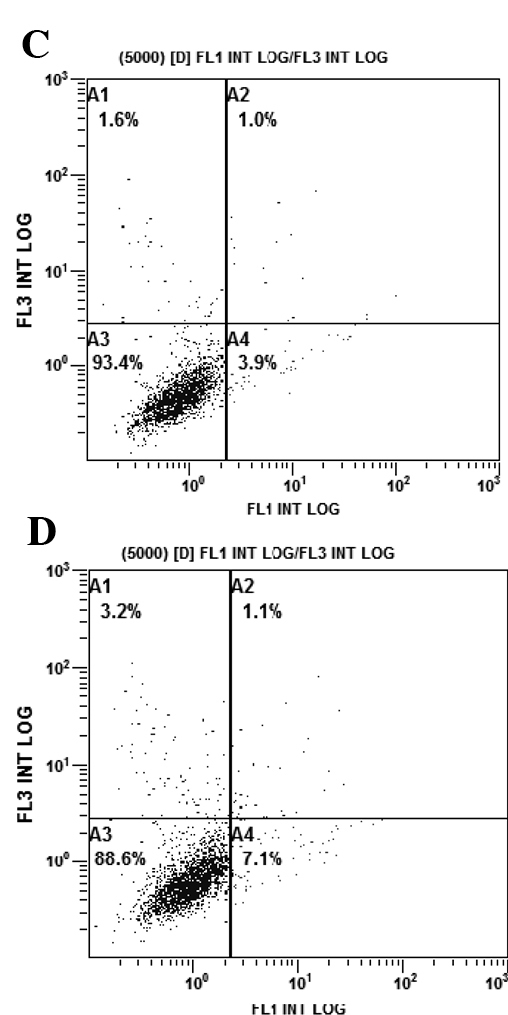
AG1024 increases the apoptotic activity of gefitinib. After treatment with the different inhibitors for 24 h, cells were harvested and stained with 5 μl Annexin V-FITC and 5 μl propidium iodide (PI). Cells were then analyzed by flow cytometry. (A) Untreated group as the control; (B) AG1024 (5 μmol/l, 24 h) treatment group; (C) gefitinib (2.5 μmol/l, 24 h) treatment group; (D) gefitinib (2.5 μmol/l) combined with AG1024 (5 μmol/l, 24 h) treatment group.
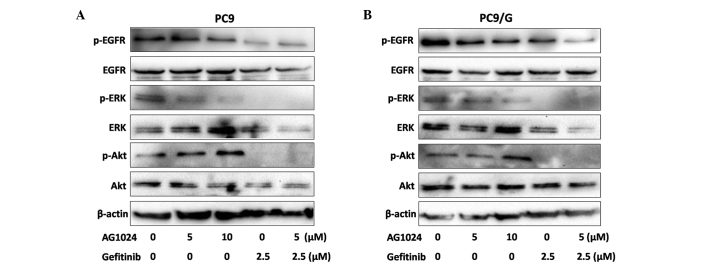
Blockade of IGF-1R in combination with gefitinib inhibits the expression of p-Akt and p-ERK in PC9/G cells
To examine the effect of AG1024 and gefitinib on the EGFR downsteam signaling pathway, we measured the level of phosphorylation and expression of signaling molecules by Western blotting (Fig. 5). After treatments for 24 h, phosphorylation levels of EGFR were decreased by gefitinib in the parental cells, while only moderately affected in the resistant cells. Both agents reduced the expression of p-ERK, but AG1024 was unable to reduce the expression of p-Akt. When treated with AG1024 and gefitinib in combination, the phosphorylation of Akt and ERK was completely abolished.
Figure 5.
Expression of p-EGFR, p-Akt and p-ERK in (A) PC9 and (B) PC9/G cells treated with gefitinib (2.5 μmol/l) and AG1024 (5 μmol/l) alone or in combination as detected by Western blotting.
Discussion
Despite remarkable advances in oncology medicine, the prognosis of lung cancer patients has not greatly improved over the past few decades. One of the most significant developments in cancer research in recent years has been the clinical validation of molecularly targeted drugs that inhibit the action of pathogenic tyrosine kinases. Treatment of appropriately selected patients with these drugs can alter the natural history of their disease and improve survival (7). Beneficial responsiveness to these EGFR tyrosine kinase inhibitors in patients with NSCLC was found to be closely associated with EGFR mutations such as del746–750 and L858R in the kinase domain (19,20). In the present study, we established gefitinib-resistant NSCLC cells from the PC9 cell line, which harbors the delE746-A750 mutation in EGFR exon 19 and is highly sensitive to gefitinib, by inducing mutation with the mutagen MNNG. The gefitinib-resistant cells were subsequently selected for the experiments. A subclone of the gefitinib-resistant cell line was obtained by limited dilution, and its sensitivity for gefitinib was determined by the CCK-8 assay. We found that PC9/G cells showed more than a 100-fold higher IC50 for gefitinib than the parental cells. A previous study showed that the gene expression profile was also significantly different between PC9/G cells and the parental cells, as determined by a DNA microarray (21). Gene function annotation and grouping showed that fatty acid metabolism and oxidative phosphorylation-related genes were down-regulated, while glycolysis genes were up-regulated in this cell line. Moreover, the insulin-like receptor and positive regulative factors for the NF-κB cascade were up-regulated. These result suggest that the resistance to gefitinib may be due to activation of an alternative signaling pathway and the downstream molecules of the signaling pathway.
The IGF-1R and its associated signaling system has provoked considerable interest over recent years as a novel therapeutic target in cancer (22). Two classes of IGF-1R inhibitors are currently under clinical development for cancer therapy: mAbs and small molecule tyrosine kinase inhibitors, such as AG1024. In our study, gefitinib and AG1024 used as single agents showed antiproliferative activity in PC9 and PC9/G cells, and their combination resulted in a synergistic enhancement of growth inhibition. The mechanism of this action is associated with reversible G1 arrest induced by gefitinib (23). The antiproliferative effect was mainly found to be cytostatic, but high doses of the drug are needed to induce apoptosis in normal mammary epithelial cells and primary cultures of mammary carcinoma cells (24). Inhibiting the IGF-1R pathway by the addition of AG1024 improves the induction of apoptosis in PC9/G cells at a level higher than that upon treatment with gefitinib alone.
In addition, Western blot analysis showed that both gefitinib and AG1024 affect phosphorylation levels of ERK to different degrees, but that AG1024 is unable to reduce phosphorylation levels of Akt. Combination treatment induces a further reduction in the activation of the Akt and ERK signaling pathways downstream of EGFR. Notably, PC9 cells expressed high levels of phosphorylated and total EGFR, which were inhibited by gefitinib. In contrast, the phosphorylation levels of EGFR were slightly affected by the treatment in PC9/G cells. Therefore, we suggest that the maintenance of this survival pathway may be related to acquired resistance to gefitinib. IGF-1R signaling can activate downstream signaling pathways such as ERK/MAPK and PI3K/Akt, which are also modulated by EGFR (25). A previous study has suggested that crosstalk and interaction between the IGF-1R and EGFR is important, and clear evidence exists for the involvement of constitutive activation of the PI3K/Akt signaling pathway in lung carcinogenesis and resisitance to tyrosine kinase inhibitors (26). For example, EGFR-amplified cells with loss of PTEN exhibit resistance to EGFR inhibitors, even though inhibiting EGFR led to down-regulation of ERK signaling (27).
Some studies, for example in NSCLC and breast cancer, have suggested that IGF-1R expression is associated with improved survival, suggesting that the relationship of the IGF-1R system to outcome is more complex than initially thought (28). The mechanism of the IGF-1R pathway involvement in cellular resistance to gefitinib remains unclear. Further development of rational clinical strategies require greater clarification of the key signaling factors which are potential targets for cancer therapies (29). The data presented here support further research into NSCLC therapeutic strategies combining gefitinib with anti-IGF-1R agents.
In conclusion, the present study suggests that the IGF-1R pathway contributes to the acquired resistance of gefitinib in NSCLC. Combination therapy with AG1024 and gefitinib markedly inhibited the growth of PC9/G cells and showed a synergistic effect in inducing apoptosis. Combined blockade of EGFR and IGF-1R signaling may be considered as a new effective therapeutic approach for NSCLC patients to overcome gefitinib resistance.
Abbreviations:
- NSCLC,
non-small cell lung cancer;
- IGF-1R,
insulin-like growth factor-1 receptor;
- EGFR,
epidermal growth factor receptor;
- ERK,
extracellular signal-regulated kinase;
- PI3K,
phosphatidylinositol 3-kinase
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Carney DN. Lung cancer - time to move on from chemotherapy. N Engl J Med. 2002;346:126–128. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 3.Bose R, Zhang X. The ErbB kinase domain: structural perspectives into kinase activation and inhibition. Exp Cell Res. 2009;315:649–658. doi: 10.1016/j.yexcr.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010;277:316–326. doi: 10.1111/j.1742-4658.2009.07450.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 8.Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- 9.Van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009;9:748–760. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 12.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 13.Yao Z, Fenoglio S, Gao DC, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA. 2010;107:15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachdev D, Yee D. The IGF system and breast cancer. Endocr Relat Cancer. 2001;8:197–209. doi: 10.1677/erc.0.0080197. [DOI] [PubMed] [Google Scholar]
- 15.Camirand A, Zakikhani M, Young F, Pollak M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005;7:R570–R579. doi: 10.1186/bcr1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 17.Knowlden JM, Jones HE, Barrow D, Gee JM, Nicholson RI, Hutcheson IR. Insulin receptor substrate-1 involvement in epidermal growth factor receptor and insulin-like growth factor receptor signalling: implication for gefitinib (‘Iressa’) response and resistance. Breast Cancer Res Treat. 2008;111:79–91. doi: 10.1007/s10549-007-9763-9. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 20.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 21.Su B, Su CX, Zhang HP, Dun QF, Zhao YM, Zhou CC. Selection and establishment of gefitinib-resistant PC9 cell line and its gene expression profile. Tumor. 2008;28:552–557. [Google Scholar]
- 22.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 23.Busse D, Doughty RS, Ramsey TT, et al. Reversible G(1) arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27(KIP1) independent of MAPK activity. J Biol Chem. 2000;275:6987–6995. doi: 10.1074/jbc.275.10.6987. [DOI] [PubMed] [Google Scholar]
- 24.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drug activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]
- 25.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 26.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 27.Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 28.Cappuzzo F, Toschi L, Tallini G, et al. Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2006;17:1120–1127. doi: 10.1093/annonc/mdl077. [DOI] [PubMed] [Google Scholar]
- 29.Dobashi Y, Koyama S, Kanai Y, Tetsuka K. Kinase-driven pathways of EGFR in lung carcinomas: perspectives on targeting therapy. Front Biosci. 2011;16:1714–1732. doi: 10.2741/3815. [DOI] [PubMed] [Google Scholar]