Abstract
Gene expression analyses may play useful roles in determining the prognosis of cancer patients and in selecting antitumor drugs. This retrospective study examined potential prognostic factors in patients with pancreatic cancer who received adjuvant chemotherapy after surgery. The study group consisted of 79 patients who had received gemcitabine or S-1 as adjuvant chemotherapy for advanced pancreatic cancer. Using laser-captured microdissection and real-time RT-PCR assay, we quantitatively evaluated the mRNA levels of 10 genes associated with patient prognosis and sensitivity to chemotherapy using paraffin-embedded specimens of the primary tumors resected before the start of adjuvant chemotherapy. In univariate analyses, a low gene expression level of γ-glutamyl hydrolase (GGH) and a high gene expression level of folylpolyglutamate synthase correlated with a favorable outcome. In a multivariate analysis, a low gene expression level of dihydropyrimidine dehydrogenase (DPD) and GGH significantly correlated with outcome (hazard ratio of the high DPD group to the low DPD group: 5.55; 95% confidence interval (CI) 1.27–24.05; P=0.022; the high GGH group to the low GGH group: 3.77; 95% CI 1.04–13.79, P=0.043). For adjuvant chemotherapy of patients with pancreatic cancer, the mRNA level of DPD and GGH may affect the prognosis of these patients.
Keywords: pancreatic cancer, prognostic factors, adjuvant chemotherapy, gene expression analysis, retrospective study
Introduction
Pancreatic cancer, the fourth most common cause of cancer-related death, has a 5-year survival rate of 5% or less (1). Surgical removal of the tumor may improve survival, but survival remains poor even in optimally resected patients. The optimum adjuvant therapy for resected cases of pancreatic cancer is unclear. Surgical resection followed by maintenance chemotherapy [using gemcitabine, as reported in the CONKO-001 (2,3), or 5-FU with leucovorin (LV), also known as folinic acid, as reported in the ESPAC-1 trial (4)] has been considered the most beneficial strategy and is regarded as the standard of care for improving survival in North America and Europe. Furthermore, the ESPAC-3 (v2) trial showed that no significant survival differences were observed between adjuvant 5-FU/LV and adjuvant gemcitabine (5).
Chemotherapy is generally administered to patients without knowledge of the genetic background of their disease, which may affect drug efficacy. Considerable evidence has suggested that the intratumor gene expression of drug-metabolizing enzymes or angiogenic enzymes are useful predictors of treatment outcomes, such as survival and the response to anticancer drugs. However, the clinical significance of these biomarkers remains unclear.
Gemcitabine has a complex metabolic pathway, and several mechanisms are thought to contribute to gemcitabine cytotoxicity and/or chemoresistance. In a recent study, human equilibrative nucleoside transporter-1 (hENT-1) was found to be the major transporter of gemcitabine (6). In addition to being incorporated into DNA, gemcitabine exerts its cytotoxicity by inhibiting ribonucleotide reductase regulatory subunits M1 (RRM1) and M2 (RRM2) (7). Although the genes for gemcitabine transport and metabolism are thought to be involved in the mechanism of cellular resistance to gemcitabine, it is not fully understood how gemcitabine influences its own transport and metabolism during the process of acquired resistance.
S-1 is an oral fluoropyrimidine derivative consisting of tegafur (FT) and two modulators, 5-chloro-2,4-dihydoxypyrimidine (CDHP) and potassium oxonate (Oxo), in a molar ratio of 1:0.4:1. The antitumor effect is provided by the 5-FU prodrug FT. CDHP competitively inhibits the 5-FU degradative enzyme dihydropyrimidine dehydrogenase (DPD), resulting in the retention of a prolonged concentration of 5-FU in the blood. At present, S-1 is widely used to treat multiple types of cancer, including gastric, colorectal, breast and head and neck cancers, mainly in Japan. Very recently, the GEST study, a randomized phase III study of gemcitabine plus S-1 vs. S-1 vs. gemcitabine in unresectable advanced pancreatic cancer, demonstrated that S-1 was confirmed to be non-inferior to gemcitabine with respect to overall survival (8). As a predictive marker for the treatment of S-1, certain reports indicate that the status of thymidylate synthase (TS) gene expression is negatively correlated with response to tumor and survival of patients with gastric cancer (9,10).
In this study, we retrospectively examined intratumoral mRNA levels of several genes, including several potential prognostic factors (hENT-1, RRM1, RRM2, TS and DPD), regulating factors of intracellular folate level [dihydrofolate reductase (DHFR), folylpolyglutamate synthase (FPGS) and γ-glutamyl hydrolase (GGH)] and growth factors [epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF)] in patients with advanced pancreatic cancer who had received gemcitabine or S-1 as an adjuvant chemotherapy.
Materials and methods
Patient population
We studied data on 79 cases of pancreatic cancer, almost all of whom (96%) had T3 or T4 disease. All patients had undergone curative surgical resection between June 2003 and June 2008 at the Department of Gastroenterology of our institution. Sixty-three of the 79 patients had been treated with gemcitabine (1,000 mg/m2/day on Days 1, 8 and 15 every 28 days), while the remaining 16 patients had been treated with S-1 (twice daily for 28 days, followed by a 2-week period of no treatment every 6 weeks). The dose of S-1 was based on each patient's body surface area (BSA) as follows: BSA <1.25 m2, 40 mg; BSA 1.25–1.5 m2, 50 mg; and BSA >1.5 m2, 60 mg. None of the patients had received pre-operative neoadjuvant chemotherapy, while 38 patients (48.1%) had received second-line chemotherapy after relapse. All patients were Japanese; written informed consent was obtained from each patient according to institutional regulations.
Microdissection
Formalin-fixed, paraffin-embedded (FFPE) tumor specimens were cut into serial sections with a thickness of 10 μm. For the pathological diagnosis, one slide was stained with H&E and evaluated by a pathologist. Other sections were stained using Nuclear Fast Red (American MasterTech Scientific Inc., Lodi, CA, USA) to facilitate the visualization of the histological features. All of the tumor samples were then subjected to laser-captured microdissection (P.A.L.M. Microlaser Technologies AG, Munich, Germany) to ensure that primarily the tumor cells were dissected.
RNA isolation and cDNA synthesis
RNA was extracted and cDNA was prepared from each of the samples as described previously (11).
Reverse transcription-PCR
The quantification of 10 genes plus an internal reference gene (β-actin) was performed using a fluorescence-based real-time detection method [ABI PRISM 7900 Sequence Detection System (TaqMan); Applied Biosystems, Foster City, CA, USA], as described previously (12). The primers and probe sequences that were used are listed in Table I. The PCR reaction mixture consisted of 1,200 nM of each primer, 200 nM of probe, 0.4 units of AmpliTaq Gold Polymerase, 200 nM each of dATP, dCTP, dGTP and dTTP, 3.5 mM of MgCl2 and 1X TaqMan buffer A containing a reference dye, for a final volume of 20 μl (all reagents from Applied Biosystems). The cycling conditions were 50°C for 2 min and 95°C for 10 min, followed by 46 cycles at 95°C for 15 sec and 60°C for 1 min. The gene expression values (relative mRNA levels) were expressed as ratios (differences between the Ct values) between the gene of interest (target gene) and the internal reference gene (β-actin), enabling the data to be normalized according to the amount of RNA isolated from each specimen.
Table I.
Primer and probe sequences for quantitative RT-PCR.
| Gene | Gene symbol (HUGO) | GenBank no. | Forward primer (5′-3′) | Reverse primer (3′-5′) | TaqMan probe (5′-3′) |
|---|---|---|---|---|---|
| TS | TYMS | NM_001071.2 | GCCTCGGTGTGCCTTTCA | CCCGTGATGTGCGCAAT | TCGCCAGCTACGCCCTGCTCA |
| DPD | DPYD | NM_000110.3 | AGGACGCAAGGAGGGTTTG | GTCCGCCGAGTCCTTACTGA | CAGTGCCTACAGTCTCGAGTCTGCCAGTG |
| hENT1 | SLC29A1 | NM_004955.2 | CCAAGTTGGACCTCATTAGCA | TGGGCTGAGAGTTGGAGACT | TGCCTGCTCTTGGCTCCTCTCC |
| RRM1 | RRM1 | NM_111033.3 | ACTAAGCACCCTGACTATGCTATCC | CTTCCATCACATCACTGAACACTTT | CAGCCAGGATCGCTGTCTCTAACTTGCA |
| RRM2 | RRM2 | NM_001034.2 | ACCGCGAGGAGGATCT | TCAGCAGCGGCTCATC | TTTCGGCTCCGTGGGCTCCT |
| DHFR | DHFR | NM_000791.3 | GTCCTCCCGCTGCTGTCA | GCCGATGCCCATGTTCTG | TTCGCTAAACTGCATCGTCGCTGTGTC |
| FPGS | FPGS | M98045 | GGCTGGAGGAGACCAAGGAT | CATGAGTGTCAGGAAGCGGA | CAGCTGTGTCTCCATGCCCCCCTAC |
| GGH | GGH | NM_003878.2 | GTGGCAATGCCGCTGAA | CAACTCAGTAGGAAAATTCTGGAACA | TTCACTGGAGGTCAATTGCACAGCAGA |
| EGFR | EGFR | NM_005228.3 | TGCGTCTCTTGCCGGAAT | GGCTCACCCTCCAGAAGGTT | ACGCATTCCCTGCCTCGGCTG |
| VEGF | VEGFA | NM_003376.4 | AGTGGTCCCAGGCTGCAC | TCCATGAACTTCACCACTTCGT | TGATTCTGCCCTCCTCCTTCTGCCAT |
| β-actin | ACTB | NM_001101.3 | GAGCGCGGCTACAGCTT | TCCTTAATGTCACGCACGATTT | ACCACCACGGCCGAGCGG |
Statistical analysis
Overall survival (OS) was calculated as the period from the surgical resection until death. To assess the associations of the gene expression levels with OS, the expression level of each gene was categorized into high and low values based on median values. The clinical laboratory data were treated as continuous variables. The hazard ratio (HR) and 95% confidence interval (CI) were estimated using the Cox proportional hazards model to provide a quantitative summary of the gene expression data.
All reported P-values were two-sided, and the level of statistical significance was set at P<0.05. The variables that were included in the multivariate analysis were selected using the stepwise method model, with a significance level of <0.05 for entering into or remaining in the model. All analyses were performed using the SAS statistical package, version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Quantifiable mRNA levels were obtained in 57 of the 79 patients (72.2%). The demographic characteristics of the patient population in which the mRNA levels could be quantified are shown in Table II. All of the patients had received adjuvant chemotherapy using either gemcitabine (73.3%) or S-1 (26.7%) after surgical resection, and no significant difference was identified in the patient characteristics between these adjuvant chemotherapies (data not shown).
Table II.
Patient characteristics.
| Characteristic | n (%) |
|---|---|
| mRNA analysis population | 57 (100) |
| Gender | |
| Male | 39 (68) |
| Female | 18 (32) |
| Age (years) | |
| Median | 65 |
| Range | 39–80 |
| <65 | 25 (44) |
| ≥65 | 32 (56) |
| UICC T | |
| T1/2 | 2 (4) |
| T3/4 | 55 (96) |
| UICC N | |
| N(−) | 15 (26) |
| N(+) | 42 (74) |
| UICC M | |
| M(−) | 55 (96) |
| M(+) | 2 (4) |
| UICC stage | |
| I–II | 55 (96) |
| III–IV | 2 (4) |
| Histology | |
| Poor | 4 (7) |
| Moderate | 44 (77) |
| Well | 3 (5) |
| Other | 6 (11) |
| Adjuvant chemotherapy regimen | |
| Gemcitabine | 42 (74) |
| S-1 | 15 (26) |
mRNA and cut-off rates of gene expression levels in quantifiable cases
When the Ct value for a target gene was >39 and that for β-actin was <30, the mRNA expression level was designated as 0.00 (Table III). The gene expression cut-off values for the OS analyses were defined using the median value, and the number of measurable samples for each single gene is shown in Table IV.
Table III.
Gene expression levels in the mRNA analysis population (57 patients).
| Gene | mRNA expression levels relative to β-actin (x10−3)
|
No. of patients (%) below measurable limitsa | |
|---|---|---|---|
| Median | Range | ||
| ENT1 | 4.64 | 0.00–17.97 | 3 (5.26) |
| RRM1 | 0.00 | 0.00–2.78 | 35 (61.40) |
| RRM2 | 1.34 | 0.00–12.72 | 20 (35.09) |
| TS | 1.34 | 0.00–5.54 | 3 (5.26) |
| DPD | 0.53 | 0.00–2.61 | 7 (12.28) |
| DHFR | 1.14 | 0.00–8.44 | 12 (21.05) |
| FPGS | 1.21 | 0.00–5.17 | 0 (0.00) |
| GGH | 0.00 | 0.00–19.34 | 35 (61.40) |
| EGFR | 1.52 | 0.00–6.28 | 5 (8.77) |
| VEGF | 6.35 | 0.00–63.19 | 1 (1.75) |
When the Ct value for a target gene was >39 and that for β-actin was <30, the mRNA expression level was designated as 0.00.
Table IV.
Univariate and multivariate Cox regression analysis of overall survival in patients with adjuvant chemotherapy.
| Factor | Cut-off point | No. of patients | Median time (months) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Hazard ratio (95% CI) | P-value log-rank | P-value Wilcoxon | Hazard ratio (95% CI) | P-value | ||||
| Gender | ||||||||
| Male | 39 | 24.1 | 1.00 | 0.667 | 0.865 | - | ||
| Female | 18 | 25.1 | 1.19 (0.51–2.63) | |||||
| Age (years) | ||||||||
| <65 | 25 | 30.6 | 1.00 | 0.052 | 0.045a | - | ||
| ≥65 | 32 | 24.1 | 2.16 (0.99–4.96) | |||||
| UICC pTNM | ||||||||
| pT | 1/2 | 2 | Not reached | - | 0.653 | 0.656 | - | |
| 3/4 | 55 | 25.1 | - | |||||
| pN | − | 15 | 30.6 | 1.00 | 0.137 | 0.066 | - | |
| + | 42 | 23.3 | 2.22 (0.84–7.65) | |||||
| pM | − | 55 | 25.1 | 1.00 | 0.169 | 0.447 | - | |
| + | 2 | 18.4 | 2.70 (0.43–9.52) | |||||
| I–II | 55 | 25.1 | - | 0.169 | 0.447 | - | ||
| III–IV | 2 | 18.4 | - | |||||
| Histology | ||||||||
| Poor | 4 | 23.8 | 1.00 | 0.689 | 0.853 | - | ||
| Moderate | 44 | 24.1 | 0.78 (0.23–4.87) | 0.798 | 0.528 | |||
| Well | 3 | 25.4 | 0.68 (0.03–7.20) | 0.910 | 0.531 | |||
| Other | 6 | Not reached | 0.41 (0.02–4.29) | 0.512 | 0.807 | |||
| Genea | ||||||||
| ENT1 | <4.64 | 28 | 25.1 | 1.00 | 0.148 | 0.322 | - | |
| ≥4.64 | 27 | 25.1 | 1.79 (0.80–4.04) | |||||
| RRM1 | − | 35 | 25.4 | 1.00 | 0.264 | 0.211 | - | |
| + | 22 | 23.3 | 1.62 (0.65–3.70) | |||||
| RRM2 | <1.34 | 27 | 25.1 | 1.00 | 0.586 | 0.823 | - | |
| ≥1.34 | 26 | 23.3 | 1.24 (0.58–2.70) | |||||
| TS | <1.34 | 26 | 30.6 | 1.00 | 0.431 | 0.831 | - | |
| ≥1.34 | 26 | 24.1 | 1.37 (0.63–3.04) | |||||
| DPD | <0.53 | 28 | 27.2 | 1.00 | 0.166 | 0.333 | 5.55 (1.27–24.05) | 0.022a |
| ≥0.53 | 28 | 23.3 | 1.77 (0.77–4.04) | |||||
| DHFR | <1.14 | 18 | 27.3 | 1.00 | 0.203 | 0.271 | - | |
| ≥1.14 | 18 | 21.8 | 1.83 (0.70–4.80) | |||||
| FPGS | <1.21 | 26 | 16.9 | 1.00 | 0.129 | 0.023a | - | |
| ≥1.21 | 26 | 25.4 | 0.50 (0.19–1.20) | |||||
| GGH | − | 35 | 25.4 | 1.00 | 0.085 | 0.033a | 3.77 (1.04–13.79) | 0.043a |
| + | 15 | 15.4 | 2.23 (0.83–5.54) | |||||
| EGFR | <1.52 | 25 | 23.3 | 1.00 | 0.665 | 0.497 | - | |
| ≥1.52 | 26 | 24.1 | 0.84 (0.36–1.87) | |||||
| VEGF | <6.35 | 28 | 27.2 | 1.00 | 0.226 | 0.424 | - | |
| ≥6.35 | 28 | 23.3 | 1.63 (0.72–3.66) | |||||
With respect to nine genes other than RRM1, part of the data was excluded from the analysis because of high DNA contamination or high coefficient of variance.
Clinical outcomes of treatment with either gemcitabine or S-1
The OS curves for patients who received gemcitabine compared to those who received S-1 are shown in Fig. 1. The OS of patients treated with S-1 as an adjuvant chemotherapy was not significantly different from that of patients who received gemcitabine.
Figure 1.
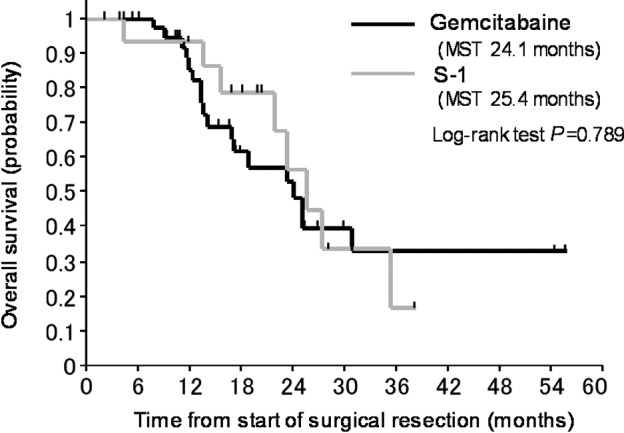
Kaplan-Meier plot for overall survival of the pancreatic cancer patients according to adjuvant chemotherapy.
Correlation between gene expression levels and overall survival
In subsequent analyses, we pooled the patients who received gemcitabine with those who received S-1. In univariate analyses, an age of <65 years, a high gene expression level of FPGS and a low gene expression level of GGH significantly correlated with a favorable prognosis in all 57 patients (Table IV, Fig. 2B and C). The expression levels of the other genes (TS, DHFR, hENT-1, RRM1, RRM2, EGFR and VEGF) did not significantly correlate with the prognosis. In a multivariate analysis including all of the significant factors obtained in the univariate analyses, low gene expression levels of DPD and GGH were identified as predictors of a favorable prognosis (Table IV, Fig. 2A and B).
Figure 2.
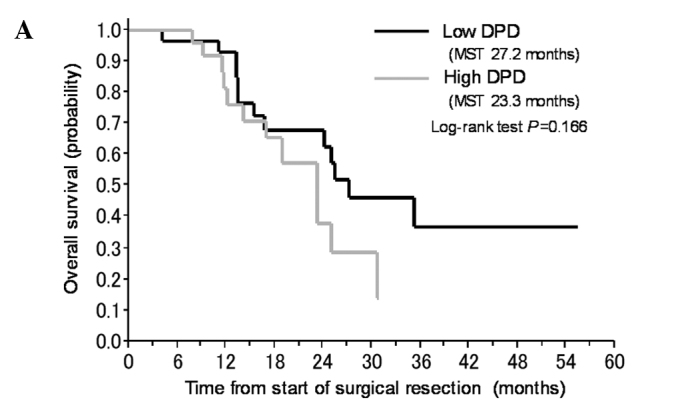
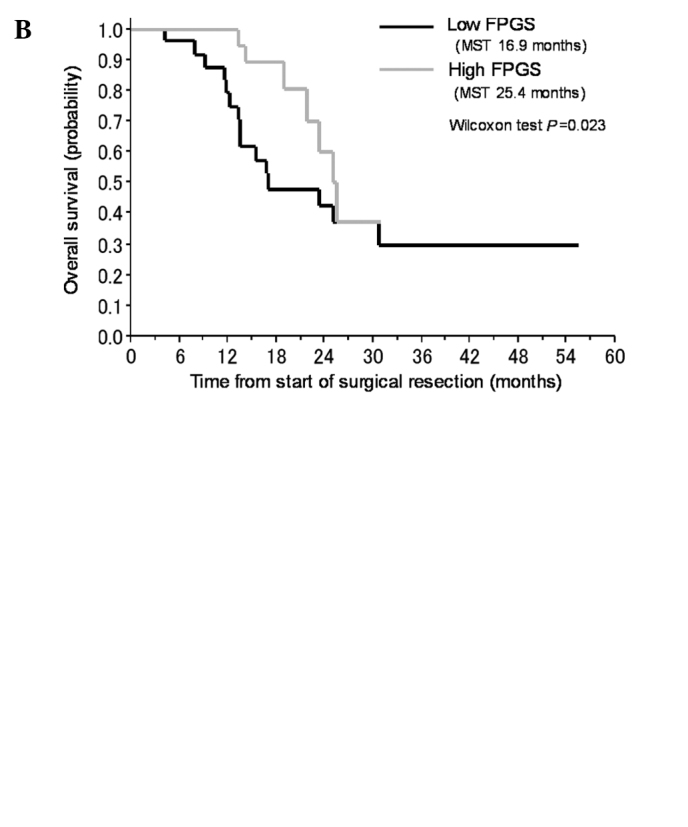
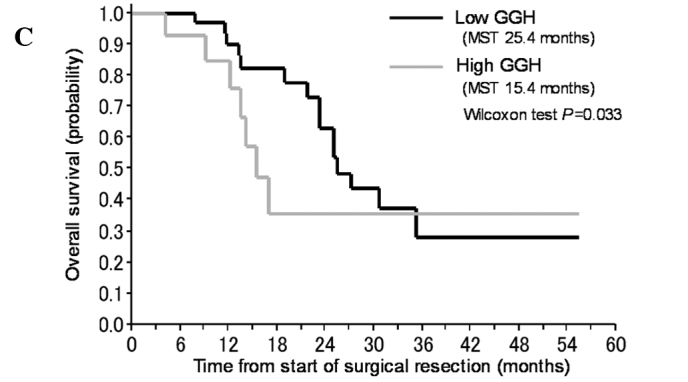
Kaplan-Meier plot for the overall survival of the patients with pancreatic cancer who received adjuvant chemotherapy according to (A) DPD, (B) FPGS or (C) GGH.
We also analyzed OS separately among the patients who received gemcitabine or S-1. Among the patients who received gemcitabine, a low gene expression level of hENT1 significantly correlated with a longer OS period compared to those who had a high expression level of hENT1 (Table V). A low gene expression level of GGH significantly correlated with a longer OS period among patients who received S-1, compared to those who had a high expression level of GGH (Table V).
Table V.
Univariate Cox regression analysis of the overall survival in patients who received adjuvant chemotherapy (gemcitabine or S-1).
| Factor | Cut-off point | Gemcitabine | S-1 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. of patients | Median time (months) | P-value log-rank | No. of patients | Median time (months) | P-value log-rank | ||
| ENT1 | <4.64 | 24 | 25.1 | 0.044a | 4 | 25.2 | 0.944 |
| ≥4.64 | 17 | 16.7 | 10 | 35.1 | |||
| RRM1 | − | 27 | 24.1 | 0.454 | 8 | 27.2 | 0.126 |
| + | 15 | 14.0 | 7 | 23.3 | |||
| RRM2 | <1.34 | 22 | 24.1 | 0.917 | 5 | 35.1 | 0.172 |
| ≥1.34 | 17 | 18.8 | 9 | 23.3 | |||
| TS | <1.34 | 19 | 30.60 | 0.397 | 7 | 23.3 | 0.807 |
| ≥1.34 | 20 | 23.30 | 6 | 25.4 | |||
| DPD | <0.53 | 17 | 25.1 | 0.193 | 11 | 27.2 | 0.908 |
| ≥0.53 | 25 | 18.8 | 3 | 23.3 | |||
| DHFR | <1.14 | 13 | Not reached | 0.329 | 5 | 27.2 | 0.141 |
| ≥1.14 | 15 | 18.8 | 3 | 21.8 | |||
| FPGS | <1.21 | 18 | 16.8 | 0.165 | 8 | 23.3 | 0.358 |
| ≥1.21 | 21 | 25.1 | 5 | 25.4 | |||
| GGH | − | 24 | 25.1 | 0.592 | 11 | 27.2 | 0.001a |
| + | 11 | 16.9 | 4 | 14.4 | |||
| EGFR | <1.52 | 19 | 23.3 | 0.716 | 6 | 23.6 | 0.763 |
| ≥1.52 | 20 | 24.1 | 5 | 35.1 | |||
| VEGF | <6.35 | 19 | Not reached | 0.150 | 9 | 27.2 | 0.737 |
| ≥6.35 | 22 | 18.8 | 6 | 22.6 | |||
Discussion
In this study, we examined whether the expression of genes in FFPE tumor specimens obtained from primary pancreatic cancer was related to prognosis and whether these expression levels could be used to determine whether gemcitabine or S-1 chemotherapy would be optimal in individual patients. We explored biomarkers that strongly correlated with the clinical outcomes of all of the patients and found that a high gene expression level of FPGS and low gene expression level of GGH correlated with a favorable OS in univariate analyses; furthermore, low expression level of DPD and GGH significantly correlated with a favorable outcome in multivariate analyses (Table IV).
To the best of our knowledge, this is the first report of single genes, DPD and GGH, correlating with OS in patients with pancreatic cancer. Miyake et al reported that 7 patients with pancreatic cancer and a high TP/DPD ratio showed a significantly poorer outcome compared to 14 patients with a low TP/DPD ratio (13). However, their report analyzed a small patient population (21 patients) and did not show a correlation between DPD alone and prognosis.
Our previous study suggested that the median DPD mRNA level in 33 patients with recurrent pancreatic cancer who had undergone resection was significantly lower among responders than among non-responders (P=0.02; median level 1.25 vs. 2.20), determining response to chemotherapy by measuring the serum CA19-9 tumor marker levels (11). This study indicated that the gene expression level of DPD in patients with pancreatic cancer who received adjuvant chemotherapy after undergoing a curative resection correlated with the patient outcome. Consequently, our studies support a correlation between the gene expression level of DPD and tumor response to chemotherapy or a survival advantage in patients with pancreatic cancer.
We previously reported that the intratumor expression level of DPD was significantly higher in patients with pancreatic cancer than in those with gastric or colorectal cancer [median level, 1.38 (n=33), 0.82 (n=20) and 0.44 (n=44), respectively] (11). Furthermore, Mori et al reported that the median expression level of DPD in patients with pancreatic cancer was 1.5–3 times higher than that in patients with gastric or colorectal cancer by an ELISA assay (14). Thus, the expression level of DPD in pancreatic cancer is higher than in gastrointestinal cancer. Several reports have shown an inverse correlation between the expression level of DPD and 5-FU sensitivity in patients with gastrointestinal cancer (15,16). Basically, Takechi et al demonstrated that CDHP, included in S-1, inhibits 5-FU degradation through the inhibition of intratumor DPD activity and enhances 5-FU cytotoxicity in pancreatic cancer cells (17). Collectively, these results suggest that treatment with S-1 may be useful for patients with pancreatic cancer, which has relatively high DPD.
A meta-analysis to estimate the effectiveness of adjuvant 5-FU/LV vs. resection alone for patients with pancreatic cancer showed that adjuvant chemotherapy using 5-FU/LV was more effective than resection alone (18). In addition, Larsson et al reported that an increased intake of folate may be associated with a reduced risk of pancreatic cancer (19). These results suggest a correlation between prognosis of patients with pancreatic cancer and folate metabolism in pancreatic tumors.
In this study, a low expression level of GGH was correlated with a longer OS period, compared to a high expression level of GGH, in all patients as well as in the subgroup of patients treated with S-1 (Tables IV and V). Collectively, the results suggest that the gene expression of DPD and folate metabolism may also affect the prognosis of patients with pancreatic cancer, and the gene expression of GGH may be useful for deciding whether gemcitabine or S-1 should be administered as an adjuvant chemotherapy to patients with advanced pancreatic cancer.
In colorectal cancer cells, intracellular folate levels were regulated by the expression of GGH and FPGS, and these genes may be responsible for the correlation between combined 5-FU and LV treatment and the antitumor effect (20). In colorectal tumor xenografts in nude mice fed a low-folate diet, the formation of much higher levels of a ternary complex with TS and 5-fluoro-2′-deoxyuridine 5′-monophosphate (FdUMP) derived from 5-FU was observed after LV treatment combined with S-1, leading to a prolonged inhibition of TS activity (21). These reports suggest that patients with activated folate metabolism in pancreatic tumor are likely to have a favorable outcome, and activated folate metabolism in tumor may be responsible for the improvement in therapeutic efficacy enabled by the combination of 5-FU and LV for the treatment of pancreatic cancer. Building on these data, we are now carrying out basic research on the effectiveness of LV used in combination with 5-FU and S-1 against pancreatic cancer.
In this study, the mRNA levels in the FFPE tumor tissue samples were quantifiable only in 57 of 79 patients (72.2%), whereas quantifiable mRNA levels in 88% of non-small cell lung cancer biopsy specimens have been previously reported (22). These results indicate that the RNA extracted from tumor tissue in the present study was relatively insufficient in quantity. Since FFPE samples of the pancreatic tumor tissue contained many interstitial normal cells, it was considered that the amount of tumor volume was comparatively small. The optimization and standardization of procedures for sampling and fixation of pancreatic tumor tissue are required in future studies.
In conclusion, our study provided evidence that the expression of DPD and GGH is correlated with the outcomes of patients with pancreatic cancer, suggesting that treatment with S-1 or S-1 combined with LV may be useful as adjuvant chemotherapy in patients with pancreatic cancer. However, this exploratory study was conducted retrospectively in a relatively small patient cohort. As basic research on the molecular markers identified in the present study advances in the future, the results obtained here should be validated in another large and well-defined population of patients treated with gemcitabine, S-1 or 5-FU combined with LV, as well as in prospective studies.
Acknowledgments
Statistical advice and fruitful support was provided by Dr Satoru Shimizu (Tokyo Women's Medial University, Tokyo, Japan). The authors thank Professor J. Patrick Barron of the Department of International Medical Communications of Tokyo Medical University (Tokyo, Japan), a remunerated consultant of Taiho Pharmaceutical, for his review of this report. They also thank Dr Masakazu Fukushima for the helpful advice and the many discussions. This study was funded by the Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan) and the Nakayama Cancer Research Institute (Tokyo, Japan).
Abbreviations:
- LV,
leucovorin;
- TS,
thymidylate synthase;
- DPD,
dihydropyrimidine dehydrogenase;
- DHFR,
dihydrofolate reductase;
- FPGS,
folylpolyglutamate synthase;
- GGH,
γ-glutamyl hydrolase;
- hENT-1,
human equilibrative nucleoside transporter-1;
- RRM1,
ribonucleotide reductase regulatory subunit M1;
- RRM2,
ribonucleotide reductase regulatory subunit M2;
- EGFR,
epidermal growth factor receptor;
- VEGF,
vascular endothelial growth factor;
- FFPE,
formalin-fixed, paraffin embedded;
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Oettle H, Neuhaus P. Adjuvant therapy in pancreatic cancer: a critical appraisal. Drugs. 2007;67:2293–2310. doi: 10.2165/00003495-200767160-00001. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos J, Büchler M, Stocken DD, et al. ESPAC-3(v2): a multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folinic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(suppl s18) abs LBA4505, (ASCO Annual Meeting). [Google Scholar]
- 6.Andersson R, Aho U, Nilsson BI, et al. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 7.Giovannetti E, del Tacca M, Mey V, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928–3935. doi: 10.1158/0008-5472.CAN-05-4203. [DOI] [PubMed] [Google Scholar]
- 8.Ioka T, Ohkawa S, Yanagimoto H, et al. Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol. 2011;29 doi: 10.1200/JCO.2012.43.3680. (ASCO Annual Meeting). [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa W, Takahashi T, Suto K, et al. Thymidylate synthase predictive power is overcome by irinotecan combination therapy with S-1 for gastric cancer. Br J Cancer. 2004;91:1245–1250. doi: 10.1038/sj.bjc.6602139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichikawa W, Sasaki Y. Challenges in predicting the clinical outcome in S-1-based chemotherapy for gastric cancer patients. Int J Clin Oncol. 2008;13:206–211. doi: 10.1007/s10147-008-0786-y. [DOI] [PubMed] [Google Scholar]
- 11.Kuramochi H, Hayashi K, Uchida K, et al. High intratumoral dihydropyrimidine dehydrogenase mRNA levels in pancreatic cancer associated with a high rate of response to S-1. Cancer Chemother Pharmacol. 2008;63:85–89. doi: 10.1007/s00280-008-0714-x. [DOI] [PubMed] [Google Scholar]
- 12.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 13.Miyake K, Imura S, Yoshizumi T, Ikemoto T, Morine Y, Shimada M. Role of thymidine phosphorylase and orotate phosphoribosyltransferase mRNA expression and its ratio to dihydropyrimidine dehydrogenase in the prognosis and clinicopathological features of patients with pancreatic cancer. Int J Clin Oncol. 2007;12:111–119. doi: 10.1007/s10147-006-0634-x. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Hasegawa M, Nishida M, et al. Expression levels of thymidine phosphorylase and dihydropyrimidine dehydrogenase in various human tumor tissues. Int J Oncol. 2000;17:33–38. doi: 10.3892/ijo.17.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa W. Prediction of clinical outcome of fluoropyrimidine-based chemotherapy for gastric cancer patients, in terms of the 5-fluorouracil metabolic pathway. Gastric Cancer. 2006;9:145–155. doi: 10.1007/s10120-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 16.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 17.Takechi T, Fujioka A, Matsushima E, Fukushima M. Enhancement of the antitumour activity of 5-fluorouracil (5-FU) by inhibiting dihydropyrimidine dehydrogenase activity (DPD) using 5-chloro-2,4-dihydroxypyridine (CDHP) in human tumour cells. Eur J Cancer. 2002;38:1271–1277. doi: 10.1016/s0959-8049(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 18.Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson SC, Hakansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006;98:407–413. doi: 10.1093/jnci/djj094. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto E, Tsukioka S, Oie S, et al. Folylpolyglutamate synthase and gamma-glutamyl hydrolase regulate leucovorin-enhanced 5-fluorouracil anticancer activity. Biochem Biophys Res Commun. 2008;365:801–807. doi: 10.1016/j.bbrc.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Tsukioka S, Uchida J, Tsujimoto H, et al. Oral fluoropyrimidine S-1 combined with leucovorin is a promising therapy for colorectal cancer: Evidence from a xenograft model of folate-depleted mice. Mol Med Reports. 2009;2:393–398. doi: 10.3892/mmr_00000111. [DOI] [PubMed] [Google Scholar]
- 22.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]


