Abstract
DBC1/KIAA1967 (deleted in breast cancer 1) is a putative tumor-suppressor gene cloned from breast cancer specimens and is reported to regulate p53-dependent apoptosis through its specific inhibition of SIRT1 deacetylase. Although SIRT1 plays a pivotal role in carcinogenesis by regulating cellular proliferation, survival and death, its role in breast cancer remains controversial. Therefore, we aimed to investigate the expression status and clinicopathological significance of DBC1 and SIRT1 in breast cancer tissues. We evaluated the expression of DBC1 and SIRT1 in breast core-needle biopsy specimens from 48 primary breast cancer patients between 2005 and 2008. These patients were treated with primary systemic chemotherapy and subsequent surgical resection of the lesions. Immunohistochemical expression scores of DBC1 and SIRT1 were evaluated, and the relationship between their expression levels and clinicopathological features of breast cancer was analyzed. The expression was observed exclusively in the nuclei of normal and neoplastic ductal cells. In breast biopsy specimens, positive expression of DBC1 and SIRT1 was noted in 85 and 98% of patients, respectively. Expression of DBC1 was significantly associated with the tumor nuclear grade (P=0.019). DBC1 and SIRT1 expression was inversely correlated with HER2 expression (P=0.026 and 0.003, respectively). Lower expression of DBC1 and SIRT1 indicated a tendency for a favorable pathological response to chemotherapy, although this was not statistically significant. Our results reveal that the expression of DBC1 and SIRT1 in breast tissues is associated with tumor characteristics.
Keywords: DBC1, SIRT1, breast cancer, nuclear grade, HER2
Introduction
Although the gene encoding DBC1 was identified as a candidate breast tumor-suppressor gene (1), the expression of DBC1 is postulated to be a poor prognostic factor in gastric (2) and breast cancer (3,4). Currently, the molecular and cellular functions of DBC1 are being extensively investigated to reveal its precise physiological role (5–9). The endogenous DBC1 is a nuclear protein and the amino-terminus of DBC1 has been shown to be a protein-interaction surface and DBC1 serves as a transcriptional factor to repress transcriptional activation function, such as BRCA1 (8) and estrogen receptor β (9). During TNF-α induced apoptosis, DBC1 is translocated to the cytoplasm with loss of the nuclear localization signal by caspase-dependent cleavage, and this cleavage promotes apoptosis due to the death-promoting activity of its carboxyl-terminal coiled-coil domain (7). Previous studies have demonstrated that DBC1 promotes p53-mediated apoptosis through specific inhibition of SIRT1 (5,6). However, the functions of DBC1 in living cells remain largely unknown and it should be determined whether DBC1 plays a pivotal role in tumor suppression or promotion.
SIRT1, the mammalian homologue of yeast silent information regulator 2 (Sir2), functions as an NAD+-dependent class III histone deacetylase (10). SIRT1 deacetylates multiple targets in mammalian cells. By regulating these molecules, SIRT1 functions as a master regulator of energy homeostasis, gene silencing, metabolism, genomic stability and cell survival. Recent reports have revealed that SIRT1 may be involved in both tumorigenesis and anti-tumorigenesis. The expression of SIRT1 has been shown to be increased in human prostate (11), gastric (2), colon (12), ovarian (13) and breast cancer (3,4,14), and SIRT1 was found to promote cellular survival by deacetylating key cell cycle molecules and apoptosis regulatory proteins (15,16). SIRT1 inactivates p53 by deacetylation and then allows cells to proliferate in the presence of damaged DNA and subsequently promotes tumor progression (15). In contrast to these tumorigenic activities, SIRT1 inactivates β-catenin by deacetylation and protects colonic tissue from tumor formation (17). Collectively, these studies implicate that the DBC1 and SIRT1 expression axis may play an important role in the development of malignant tumors.
Therefore, we aimed to identify the role of DBC1 and SIRT1 expression in breast cancer specimens obtained as core biopsy specimens prior to surgery. We revealed that DBC1-positive cells may constitute an unfavorable environment for breast cancer. These results suggest the pivotal role of the DBC1 and SIRT1 expression axis in patients with breast cancer.
Patients and methods
Patients and tissue sampling
A total of 52 patients who underwent primary systemic chemotherapy followed by definitive surgery during December 2005 and April 2008 at the Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital (Tokyo, Japan) were consecutively enrolled in this study. Informed consent was obtained from all patients and approval of the Institutional Review Board of Komagome Hospital was also obtained. All patients were diagnosed with invasive breast carcinoma by core needle biopsy and 4 patients were excluded since they were treated with primary hormonal therapy. Thus, 48 of the originally enrolled patients were evaluated. Four cycles of FEC (fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cyclophosphamide 500 mg/m2) administered intravenously (i.v.) on day 1 every 21 days were followed by four cycles of docetaxel i.v. (75 mg/m2) every 21 days, prior to surgery. None of the patients were administered with trastsuzumab prior to surgery.
Pathological response evaluation
The Japanese Pathological Response Criteria were applied, defined as follows: grade 0, no chemotherapeutic change in remnant cancer cells; grade 1a, 0–1/3 of remnant cancer cells in degeneration or necrosis; grade 1b, 1/3–2/3; grade 2, >2/3; grade 3, no viable cancer cells in duct and stroma (18,19).
Immunohistochemical staining of DBC1- and SIRT1-positive cell
Immunohistochemistry was performed to visualize the signal. Paraffin sections (4 μm) mounted on organosilane-coated glass slides were dewaxed in xylene and rehydrated through a graded ethanol series. The tissue sections were treated with a microwave antigen retrieval procedure in sodium citrate buffer (pH 6.0) for 20 min. They were subsequently treated with 0.3% hydrogen peroxide in methanol for 15 min to quench endogenous peroxidase activity. The primary antibodies were anti-DBC1 rabbit polyclonal antibody (produced in our laboratory) (8,9) and anti-SIRT1 rabbit polyclonal antibodies (H-300; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). These primary antibodies were diluted (DBC1 1/100,000; SIRT1 1/200), and the tissue sections were incubated for 30 min at room temperature using reagents provided with the ChemMate EnVision™ Detection system (Dako, Carpinteria, CA, USA). Cells were visualized using the chromogen diaminobenzidine and counterstained with Mayer’s hematoxylin. Appropriate positive and negative controls were included.
Evaluation of DBC1 and SIRT1 expression
For semi-quantitative evaluation of DBC1/SIRT1 expression, immunohistochemical scoring was performed, and nuclear staining of DBC1 and SIRT1 was evaluated according to the Allred score (20). The immunostaining results were interpreted as positive when at least 5% of cells were stained. No expression or expression of <5% of tumor cells was considered negative. The semi-quantification for immunostaining intensity was scored on a scale of: 0, negative; 1, weak; 2, moderate and 3, intense. Average numbers of immunopositive cells within the neoplastic tissues were determined in at least five areas at x400 magnification. The semi-quantification of the percentage of immunopositive cells was scored on a scale of 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) and 4 (>75%). The percentage of the staining intensity and positive tumor cells were multiplied to produce a weighted score for each case. These scores were determined as the positive index. Immunohistochemical analysis was performed by three authors by consensus without knowledge of the clinicopathological information.
Breast cancer subtyping according to estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) status
For ER, PR and HER2 evaluation, immunohistochemical staining was performed using anti-ER mouse monoclonal antibody (clone 1D5; Dako), anti-PR mouse monoclonal antibody (clone PgR636; Dako) and a HercepTest kit (Dako), respectively. Hormone receptor status was evaluated as the percentage of positive nuclear staining among cancer cells, and the cut-off value was set to 10%. HER2 scoring was carried out according to the standard HercepTest guidelines. HER2 expression status was further confirmed by fluorescence in situ hybridization using Vysis Path Vision HER2/neu DNA probe kit (Abott, Chicago, IL, USA), when the tumor was evaluated as 2+ by the HercepTest. The breast tumor samples were classified into four subtypes, namely luminal A (ER+ and/or PR+, and HER2−), luminal B (ER+ and/or PR+, and HER2+), HER2+ (ER− and PR−, and HER2+) and triple-negative (ER−, PR− and HER2−), according to the system for the immunohistochemical subtyping of breast cancer (21).
Statistical analysis
The association between DBC1 and SIRT1 expression and clinicopathological features was examined by the Mann-Whitney U-test. The correlation test was used to analyze correlations between SIRT1 and DBC1 using Spearman’s rank correlation test. All tests were two-tailed, and a P-value <0.05 was considered statistically significant.
Results
Patient background
The clinical characteristics and pathological data of the 48 breast cancer patients are shown in Table I. All patients were female and the median age of the enrolled patients was 53 years (range 32–75). Lymph node involvement was found in 18 patients and metastasis occurred in 22 patients. The TNM staging of the tumors ranged from stage 0 to stage IV: stage 0 (n=1), stage I (n=5), stage II (n=31), stage III (n=6) and stage IV (n=5). All tumors were graded according to the modified Bloom-Richardson system (22): grade 1 (n=12), grade 2 (n=10) and grade 3 (n=26). ER, PR and HER2 were positive in 28, 16 and 35 patients, respectively. The breast cancer subtypes in the present study included luminal A (n=8, 16.7%), luminal B (n=20, 41.7%), HER2+ (n=5, 10.3%) and triple-negative (n=15, 31.3%) subtypes.
Table I.
Patient clinical and pathological characteristics.
| No. of patients (%) | |
|---|---|
| Age (years) | |
| Median | 53 |
| Range | 32–75 |
| Menopausal status | |
| Pre-menopause | 21 (43.8) |
| Postmenopause | 27 (56.3) |
| Tumor stage | |
| 0 | 1 (2.1) |
| 1 | 5 (10.4) |
| 2 | 31 (64.6) |
| 3 | 6 (12.5) |
| 4 | 5 (10.4) |
| Nodal stage | |
| N0 | 29 (60.5) |
| N1 | 17 (35.5) |
| N2 | 1 (2.0) |
| Unknown | 1 (2.0) |
| Nuclear grade | |
| 1 | 12 (25.0) |
| 2 | 10 (20.8) |
| 3 | 26 (54.2) |
| ER | |
| Positive | 28 (58.3) |
| Negative | 20 (41.7) |
| PR | |
| Positive | 16 (33.3) |
| Negative | 32 (66.7) |
| HER2 (IHC) | |
| 0 | 12 (25.0) |
| 1+ | 22 (45.8) |
| 2+ | 6 (12.5) |
| 3+ | 7 (14.6) |
| Unknown | 1 (2.1) |
| Subtypes of breast cancer | |
| Luminal A | 8 (16.7) |
| Luminal B | 20 (41.7) |
| HER2+ | 5 (10.3) |
| Triple negative | 15 (31.3) |
| Pathological response | |
| Grade 0 | 3 (6.3) |
| Grade 1a | 22 (45.8) |
| Grade 1b | 10 (20.8) |
| Grade 2 | 6 (12.5) |
| Grade 3 | 5 (10.4) |
| Unknown | 2 (4.2) |
Pathological response defined as grade 0, no chemotherapeutic change in remnant cancer cells; grade 1a, 0–1/3 of remnant cancer cells in degeneration or necrosis; grade 1b, 1/3–2/3; grade 2, >2/3; grade 3, no viable cancer cells in duct and stroma. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor-2; IHC, immunohistochemistry.
DBC1 and SIRT1 expression in core needle biopsy specimens
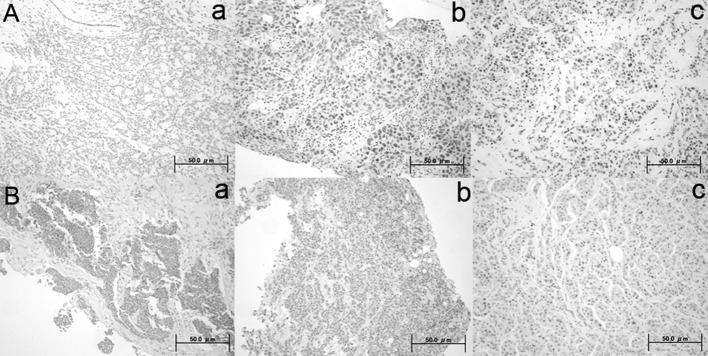
DBC1 and SIRT1 immunoreactivity was present in the nuclei of normal and tumor cells. Positive expression of DBC1 and SIRT1 was noted in 85% (41 of 48) and in 98% (47 of 48) of patients, respectively. Representative immunostained tissues are shown in Fig. 1. Positive indices of DBC1 and SIRT1 judged by immunohistochemistry were calculated, and the association between the positive indices and the clinicopathological characteristics of the patients was further investigated. Elevated DBC1 expression was significantly associated with nuclear grade as determined by the modified Bloom-Richardson system (P= 0.019). Expression of DBC1 was inversely correlated with the HER2 expression status (P=0.026), while other clinical factors exhibited no significant correlation with the DBC1-positive index. Expression of SIRT1 was also negatively correlated with the HER2 expression status (P=0.003). However, in contrast to other studies, SIRT1 expression showed no relation with hormone receptor status and luminal subtype. We also analyzed the correlation coefficient between DBC1 and SIRT1 in breast cancer tissue and a marginal correlation was detected between the expression of DBC1 and the expression of SIRT1 (P=0.047, r=0.34).
Figure 1.
Representative immunohistochemical staining with (A) DBC1 and (B) SIRT1 antibodies. (A) Positive indices of DBC1: (a) 0, (b) 3 and (c) 8. (B) Positive indices of SIRT1: (a) 0, (b) 3 and (c) 6.
Correlation between DBC1 and SIRT1 expression and pathological response
Three patients were diagnosed with a grade 0 pathological response, and 22, 10, 6 and 5 patients were diagnosed with grades 1a, 1b, 2 and 3, respectively. To investigate whether DBC1 and SIRT1 expression is associated with chemotherapeutic response of the patients, the correlation between immunohistochemical positive index and pathological response of the invasive component of the breast carcinoma was evaluated, as pathological response is proposed to provide accurate information for prognosis. Expression of DBC1 and SIRT1 judged by immunohistochemistry was compared to the pathological response of the patients. Patients who exhibited a favorable response to neoadjuvant chemotherapy showed lower positive indices for DBC1 and SIRT1 rather than those who exhibited an unfavorable response, but this inverse relationship was not statistically significant (Table II).
Table II.
Correlation between positive index (PI) score (DBC1 and SIRT1) and clinicopathological features of the 48 specimens.
| DBC1 PI score
|
SIRT1 PI score
|
|||
|---|---|---|---|---|
| (mean, 95% CI) | P-value | (mean, 95% CI) | P-value | |
| Menopausal status | NS | NS | ||
| Premenopause | 2.91 (2.06–3.77) | 3.65 (3.27–4.03) | ||
| Postmenopause | 3.32 (2.54–4.10) | 3.72 (3.16–4.29) | ||
| Tumor stage (T) | NS | NS | ||
| I–II (n=37) | 3.19 (2.55–3.83) | 3.68 (3.27–4.08) | ||
| III–IV (n=11) | 2.91 (1.55–4.27) | 3.73 (3.05–4.41) | ||
| Nodal status | NS | NS | ||
| Negative (n=29) | 3.00 (2.19–3.81) | 3.62 (3.16–4.08) | ||
| Positive (n=19) | 3.32 (2.06–4.07) | 3.79 (3.27–4.31) | ||
| Nuclear grade | 0.0189a | NS | ||
| 1 (n=12) | 2.08 (1.39–2.77) | 3.33 (2.42–4.25) | ||
| 2 and 3 (n=36) | 3.47 (2.79–4.16) | 3.81 (3.45–4.16) | ||
| ER status | NS | NS | ||
| Negative (n=20) | 3.11 (1.91–4.32) | 3.89 (3.47–4.30) | ||
| Positive (n=28) | 3.13 (2.54–3.73) | 3.57 (3.08–4.05) | ||
| PR status | NS | NS | ||
| Negative (n=32) | 3.31 (2.57–4.05) | 3.56 (3.11–4.01) | ||
| Positive (n=16) | 2.75 (1.89–3.61) | 3.94 (3.44–4.43) | ||
| HER2 status | 0.0264a | 0.0028b | ||
| 0 (n=12) | 4.08 (2.86–5.31) | 4.50 (4.17–4.83) | ||
| 1–3 (n=36) | 2.80 (2.19–3.43) | 3.42 (3.02–3.82) | ||
| Luminal subtype | NS | NS | ||
| Luminal (n=28) | 3.32 (2.66–3.98) | 3.64 (3.13–4.15) | ||
| Non-luminal (n=20) | 2.85 (1.82–3.88) | 3.75 (3.32–4.18) | ||
| Pathological response | NS | NS | ||
| 0-1a (n=25) | 3.32 (2.54–4.01) | 3.80 (3.37–4.23) | ||
| 1b-3 (n=21) | 2.91 (2.06–3.77) | 3.57 (3.02–4.12) | ||
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor-2.
P<0.05;
P<0.01.
Discussion
Recently, the physiological significance and tumor-promoting functions of DBC1 have been revealed. DBC1 has been found to associate with unliganded-ERα and to manipulate ligand-independent growth of breast cancer cells (23). Our previous data also indicated the possible tumorigenic role of DBC1 (8,9). However, given that DBC1 inhibits the deacetylase activity of SIRT1 and promotes p53-dependent apoptosis (5,6), DBC1 expression may not be directly associated with tumorigenesis. DBC1 expression has been shown to be elevated in breast cancer (14,24). In patients with breast cancer, expression of DBC1 and SIRT1 was significantly associated with distant metastatic relapse, shorter relapse-free survival and reduced overall survival (4). These findings suggest the possibility that the expression of DBC1 is a clinically significant prognostic indicator for breast carcinoma patients. However, another recent study found that overexpression of DBC1 in tumor tissue had no significant correlations with clinicopathological factors of breast cancer, but overexpression of SIRT1 was significantly correlated with luminal subtypes, ER and PR expression by immunohistochemistry (3). Further studies are required to define DBC1 as a tumor promoter, since DBC1 was originally identified during a genetic search for candidate breast tumor-suppressor genes (1).
In the present study, the expression of DBC1 was significantly associated with nuclear grade, which is considered an unfavorable prognostic factor. Histological and nuclear grades have almost the same prognostic relevance, and a high nuclear grade is a significant prognostic factor for the development of ipsilateral breast recurrences in numerous retrospective and prospective studies. Since histological grade has a strong correlation with HER2 status, inactivation of p53, hormone receptor negativity and accumulation of chromosomal alterations, we may expect that the elevated expression of DBC1 has certain clinical significance in breast cancer patients. The fact that DBC1 and SIRT1 expression correlates with ErbB2/HER2 status may simply be translated that DBC1 and SIRT1 expression affects cellular homeostasis because the overexpression of ErbB2/HER2 has been reported as an adverse prognostic factor in invasive breast cancer and is considered to be a marker of aggressive biology.
It remains questionable why the overall expression of SIRT1 and DBC1 simultaneously increases in breast tumor tissues. Since SIRT1 and DBC1 possess simultaneous roles both in tumor promotion and tumor suppression, their individual expression is not sufficient to determine the fate of tumorigenic cells. Therefore, it is not surprising that the correlation between SIRT1 and DBC1 was not lost in tumor tissue, in contrast to a previous study (3). However, beyond the balance between SIRT1 and DBC1, a more exquisite determinant of tumorigenesis may exist between these two proteins. Although our data were insufficient to show that lower expression of DBC1 and SIRT1 suggests favorable pathological response to chemotherapy, we expect that the expression of DBC1 and SIRT1 in breast tissues reflects baseline tumor characteristics. Due to the limited sample size of patients in our investigation, further studies are required to verify our findings and establish the role of DBC1 and SIRT1 as a reliable clinical predictor for the outcome of breast cancer patients.
In conclusion, our study demonstrated that the expression levels of DBC1 and SIRT1 may constitute important tumor characteristics for patients with breast cancer. Our study suggests that DBC1 may be a more useful prognostic factor in breast cancer rather than SIRT1. In clinical practice, considering that the activation of SIRT1 by small molecules have been extensively investigated for the treatment of diabetes, our study may implicate that these molecules have certain roles in the pathophysiology of breast cancer.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, the JMS Bayer Schering Pharma Grant, the Kowa Life Science Foundation and the Kanzawa Medical Research Foundation, Japan.
References
- 1.Hamaguchi M, Meth JL, von Klitzing C, et al. DBC2, a candidate for a tumor-suppressor gene involved in breast cancer. Proc Natl Acad Sci USA. 2002;99:13647–13652. doi: 10.1073/pnas.212516099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha EJ, Noh SJ, Kwon KS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453–4459. doi: 10.1158/1078-0432.CCR-08-3329. [DOI] [PubMed] [Google Scholar]
- 3.Sung JY, Kim R, Kim JE, Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101:1738–1744. doi: 10.1111/j.1349-7006.2010.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H, Kim KR, Noh SJ, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42:204–213. doi: 10.1016/j.humpath.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W, Kruse JP, Tang Y, et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundararajan R, Chen G, Mukherjee C, White E. Caspase-dependent processing activates the proapoptotic activity of deleted in breast cancer-1 during tumor necrosis factor-alpha-mediated death signaling. Oncogene. 2005;24:4908–4920. doi: 10.1038/sj.onc.1208681. [DOI] [PubMed] [Google Scholar]
- 8.Hiraike H, Wada-Hiraike O, Nakagawa S, et al. Identification of DBC1 as a transcriptional repressor for BRCA1. Br J Cancer. 2010;102:1061–1067. doi: 10.1038/sj.bjc.6605577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama S, Wada-Hiraike O, Nakagawa S, et al. Repression of estrogen receptor beta function by putative tumor suppressor DBC1. Biochem Biophys Res Commun. 2010;392:357–362. doi: 10.1016/j.bbrc.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 11.Huffman DM, Grizzle WE, Bamman MM, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 12.Kabra N, Li Z, Chen L, et al. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem. 2009;284:18210–18217. doi: 10.1074/jbc.M109.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang KY, Kim KS, Hwang SH, et al. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41:366–371. doi: 10.1080/00313020902884451. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhang M, Dong H, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 16.Wong S, Weber JD. Deacetylation of the retinoblastoma tumour-suppressor protein by SIRT1. Biochem J. 2007;407:451–460. doi: 10.1042/BJ20070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberdoerffer P, Michan S, McVay M, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japanese Pathological Response Criteria of Breast Cancer General Rules for Clinical and Pathological Recording of Breast Cancer. (15th edition) 2008:75–76. [Google Scholar]
- 19.Kurosumi M, Akashi-Tanaka S, Akiyama F, et al. Histopatho-logical criteria for assessment of therapeutic response in breast cancer (2007 version) Breast Cancer. 2008;15:5–7. doi: 10.1007/s12282-007-0016-x. [DOI] [PubMed] [Google Scholar]
- 20.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda H, Akiyama F, Kurosumi M, et al. Establishment of histological criteria for high-risk node-negative breast carcinoma for a multi-institutional randomized clinical trial of adjuvant therapy. Japan National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) Pathology Section. Jpn J Clin Oncol. 1998;28:486–491. doi: 10.1093/jjco/28.8.486. [DOI] [PubMed] [Google Scholar]
- 23.Trauernicht AM, Kim SJ, Kim NH, Boyer TG. Modulation of estrogen receptor alpha protein level and survival function by DBC-1. Mol Endocrinol. 2007;21:1526–1536. doi: 10.1210/me.2007-0064. [DOI] [PubMed] [Google Scholar]
- 24.Richardson AL, Wang ZC, de Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]