Abstract
Background
Monocytes/macrophages from filaria-infected animals exhibit an alternatively activated phenotype; however, very little is known about the alternative activation phenotype of monocytes in human filarial infection.
Methods
To elucidate the activation and cytokine profile of monocytes in human filarial infection, we examined the expression patterns of genes encoding arginase, nitric oxide synthase 2, alternative activation markers, and cytokines in monocytes from individuals with asymptomatic filarial infection and individuals without filarial infection, ex vivo and in response to filarial antigen (Brugia malayi antigen [BmA]).
Results
Monocytes from patients with asymptomatic filarial infection exhibited significantly diminished expression of NOS2 and significantly enhanced expression of ARG1. These changes were associated with significantly increased expression of the genes encoding resistin, mannose receptor C type 1 (MRC1), macrophage galactose type C lectin (MGL), and chemokine ligand 18 (CCL18). In response to BmA, purified monocytes from infected individuals also expressed significantly lower levels of interleukin (IL)–12 and IL-18 but, in contrast, expressed significantly higher levels of transforming growth factor β, IL-10, and suppressor of cytokine signaling 1 mRNA. Inhibition of arginase-1 resulted in significantly diminished expression of the genes encoding resistin, MRC1, MGL, and CCL18, as well as significantly enhanced expression of NOS2 and the genes encoding IL-12 and IL-18.
Conclusion
Patent human filarial infection is associated with the presence of monocytes characterized by an alternatively activated immunoregulatory phenotype.
Filarial infection–induced monocyte dysfunction is an important feature of patent lymphatic filariasis. First, monocytes from individuals with filarial infection exhibit a diminished ability to respond to pathogen-associated molecular patterns because of their decreased activation through Toll-like receptor [1]. Second, monocytes from individuals with filarial infection exhibit diminished expression of the proinflammatory cytokines interleukin (IL)–1α, IL-1β, macrophage inflammatory protein (MIP)–1α, MIP-1β, and IL-8 and increased expression of the immunoregulatory cytokine IL-10 [2, 3]. Third, monocytes from patients with filiarial infection exhibit diminished expression of genes related to the antigen processing and presentation pathway, which is presumably a direct consequence of filarial antigen internalization [3]. Fourth, several life-cycle stages of the filarial parasite are known to induce down-regulation of activation markers, costimulatory molecules, cytokines, and effector activity of monocyte-derived dendritic cells and macrophages in vitro [4]. Fifth, classical activation of monocytes and macrophages (hereafter, “monocytes/macrophages”) has been suggested to be detrimental to filarial parasite survival, because the gene encoding nitric oxide synthase 2, NOS2, has, in several murine models of infection, been shown to be an important mediator of resistance to filarial parasites [5–8]. Finally, filarial parasites are potent inducers of IL-4 and IL-13, cytokines known to cause differentiation of macrophages to the alternatively activated phenotype [9], a process that could contribute to the establishment of chronic infections.
Macrophages activated by the T helper (Th) 1 cytokines interferon-γ (IFN-γ) and tumor necrosis factor–α (TNF-α) play a critical role in destroying intracellular pathogens through the production of proinflammatory mediators, such as nitric oxide [9–11]; however, macrophages can also be activated by Th2-type cytokines (particularly IL-4 and IL-13) that cause the development of a phenotype, termed “alternative activation,” that is distinct from the classical phenotype [9–11]. The alternative activation pathway is not simply defined by the down-regulation of IFN-γ–mediated processes but rather is a distinct lineage characterized by expression of specific cell surface and intracellular markers and by the ability to perform discrete functions [9 –11]. The most important distinction between the 2 categories of macrophages is the use of different metabolic pathways for catalysis of L-arginine. Under classical conditions, inflammatory macrophages produce nitric oxide as a result of their up-regulation of NOS2, which catalyzes the L-arginine substrate. Under alternatively activated conditions, ARG1 (which encodes liver arginase) is up-regulated, and induction of ARG1 leads to catalysis of L-arginine to L-ornithine and urea. The up-regulation of NOS2 is characteristically associated with suppression of ARG1, and up-regulation of ARG1 is associated with suppression of NOS2, indicating a competitive relationship between these differing states of macrophage metabolism [9].
In vitro studies with INF-γ– or lipopolysaccharide-activated macrophages suggest that classical activation of macrophages is a major mechanism of resistance to filarial parasites [7, 8]. This finding differs from observations of the macrophage response to gastrointestinal nematodes, in which the alternative activation pathway plays a more important role in resistance [12]. The mechanism by which classically activated macrophages damage filarial parasites is through the release of reactive oxygen species and nitric oxide derivatives [7, 8]. Studies involving animal models suggest that filarial parasites have evolved mechanisms to down-regulate the classical macrophage activation pathway and stimulate induction of alternatively activated macrophages [13]. These macrophages predominantly express ARG1 and 2 characteristic marker genes, YM1 and RELMa, and are profoundly down-regulatory, as indicated by their ability to suppress proliferation of a wide variety of cells [14, 15]. Whether a similar dichotomy exists for human monocytes/macrophages and whether filarial infections induce alternative activation of human monocytes/macrophages are not known. Moreover, the lack of specific markers of alternative activation in human monocytes/macrophages (and the failure to find distinct homologs of YM1 and RELMa in the human genome) has made it difficult to study this lineage of cells in human infections.
We have used a wide array of markers known to be associated with alternative activation of monocytes/macrophages in mice to identify and study the presence and function of alternatively activated monocytes in the peripheral blood of patients with filarial infection. Our study reveals the presence of a population of monocytes that predominantly express alternative activation protein and gene markers in individuals with filarial infection. These monocytes also have an immunoregulatory phenotype, owing to their enhanced expression of the cytokines transforming growth factor β (TGFβ) and IL-10, expression of diminished levels of the proinflammatory cytokines IL-12 and IL-18, and augmented induction of SOCS-1, which encodes suppressor of cytokine signaling–1. In addition, the alternatively activated and immunoregulatory phenotype of filaria-infected monocytes is dependent on the activity of the L-arginine–metabolizing enzyme arginase.
SUBJECTS, MATERIALS, AND METHODS
Study population
We studied a cohort of 20 individuals from an area in the Tamil Nadu state of southern India, where lymphatic filariasis is endemic. This included 10 individuals with asymptomatic filarial infection (AFI) and 10 uninfected individuals. The individuals in the AFI group had positive results of the ICT filarial antigen test (Binax) and the Trop Bio Og4C3 enzyme-linked immunosorbent assay (ELISA; Trop Bio) test for circulating filarial antigen. All uninfected individuals tested negative for filarial antigen and had no history, signs, or symptoms of filarial infection. Levels of Brugia malayi antigen (BmA)–specific immunoglobulin (Ig) G4 in individuals with AFI ranged from 1389 to 7830 pg/mL (geometric mean [GM], 4176.7 pg/ mL); BmA-specific IgG4 was not detected in uninfected individuals. Levels of BmA-specific total IgG ranged from 97.4 to 643.8 ng/mL (GM, 280.2 ng/mL) among individuals with AFI and from 4.3 to 350.5 ng/mL (GM, 40.1 ng/mL) among uninfected individuals. ELISAs for detection of BmA-specific IgG4 and IgG were performed exactly as described elsewhere [16]. All individuals provided informed consent and were examined as part of a clinical protocol approved by institutional review boards of the National Institute of Allergy and Infectious Diseases and the Tuberculosis Research Center.
Isolation of peripheral blood mononuclear cells (PBMCs)
Heparinized blood was collected, and peripheral blood lymphocytes were isolated by Ficoll diatrizoate gradient centrifugation (LSM; ICN Biomedicals). Erythrocytes were lysed using ACK lysis buffer (BioSource International). Cells were then washed and cultured in RPMI-1640 (BioWhittaker) supplemented with 20 mmol/L of glutamine (BioWhittaker), 10% heat-inactivated fetal calf serum (Harlan Bioproducts for Science), and 50 μg/mL of gentamicin (Mediatech).
Parasite and control antigen
Saline extracts of BmA were used as the parasite antigens, and mycobacterial purified protein derivative (PPD; Statens Serum Institute) was used as the control antigen. Final concentrations were 5 μg/mL for BmA and 10 μg/mL for PPD. The endotoxin level in final soluble BmA was determined to be <0.1 EU/mL, using the QCL-1000 Chromogenic LAL test kit (BioWhittaker).
In vitro culture and cell purification
PBMCs were cultured with BmA or PPD in 24-well tissue culture plates (Corning Life Sciences) at a concentration of 5 × 106 cells/well. After 24 h, CD14+ monocytes (confirmed as such on the basis of positive selection with anti-CD14 beads) were isolated by magnetic column purification (Miltenyi Biotec). The cells were >90% pure in all our experiments, as estimated by flow cytometry. A representative dot plot showing the purity of CD14+ monocytes after positive selection is shown in figure 1, which appears only in the electronic edition of the Journal. For arginase-1 inhibition, PBMCs were cultured with BmA in the presence of Nω-hydroxy-nor-L-arginine (LOHA; CalBiochem) at a concentration of 500 μmol/L per well for 72 h before monocyte isolation [17].
Figure 1.
Representative dot plot showing the purity of CD14+ monocytes after positive selection. FITC, fluorescein isothiocyanate; SSC, side scatter.
RNA preparation
Monocytes were lysed using the reagents of a commercial kit (QIAshredder; Qiagen). Total RNA was extracted according to the manufacturer’s protocol (RNeasy mini kit; Qiagen), and RNA was dissolved in 50 μL of RNase-free water.
Complementary DNA (cDNA) synthesis
RNA (1 μg) was used to generate cDNA by means of TaqMan reverse transcription reagents, according to the manufacturer’s protocol (Applied Biosystems). In brief, random hexamers were used to prime RNA samples for reverse transcription using MultiScribe reverse transcriptase.
Real-time reverse transcription–polymerase chain reaction (RT-PCR)
Real-time quantitative RT-PCR was performed in an ABI 7700 sequence detection system (Applied Bio-systems), using Applied Biosystems TaqMan Assays-on-Demand reagents, to quantify messenger RNA (mRNA) levels of NOS2; ARG1; genes encoding resistin, mannose receptor C type-1 (MRC1), macrophage galactose type C lectin (MGL), chemokine ligand 18 (CCL18), IL-12p40, IL-18, TNF-α, IL-8, TGFβ, IL-10, and suppressor of cytokine signaling (SOCS) family proteins (i.e., cytokine inducible SH2–containing protein [CIS], SOCS1–5, and SOCS7); and an endogenous 18S ribosomal RNA control. Relative transcript numbers were determined by the formula 0.5CT of target − CT of control, where CT is the threshold cycle during the exponential phase of amplification.
Statistical analysis
Comparisons were done using the nonparametric Mann-Whitney U test (for unpaired data) and the Wilcoxon signed rank test (for paired data). Correlations were performed using the Spearman rank correlation test. All statistic analyses were performed with GraphPad Prism, version 5.0 (GraphPad Software).
RESULTS
Filarial antigen induces down-regulation of NOS2 and up-regulation of ARG1 in monocytes from patients with AFI
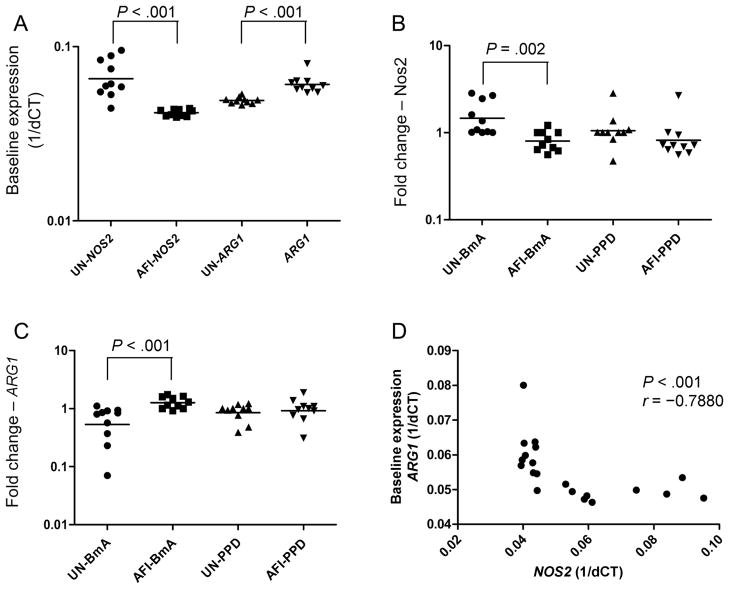
Because induction of ARG1 and inhibition of NOS2 are essential for establishment of alternatively activated monocytes, we stimulated PBMCs from uninfected persons and persons with AFI with BmA or PPD for 24 h and measured the mRNA levels of NOS2 and ARG1 by RT-PCR. As shown in figure 2A, monocytes from patients with AFI expressed significantly less NOS2 mRNA and significantly more ARG1 mRNA, compared with monocytes from uninfected patients (GM relative transcript levels, 0.04174 vs. 0.06551 for NOS2 and 0.06078 vs. 0.04913 for ARG1). The parasite antigen BmA induced significant additional down-regulation of NOS2 and significant up-regulation of ARG1 in the AFI group, compared with the uninfected group (GM fold changes, 0.8016 vs. 1.465 for NOS2 [figure 2B] and 1.265 vs. 0.5329 for ARG1 [figure 2C]). Of interest, no significant alterations in the expression of NOS2 or ARG1 were observed between the groups in response to the nonparasite antigen PPD. Furthermore, as shown in figure 2D, baseline data for all 20 subjects revealed an inverse relationship between NOS2 expression and ARG1 expression (r = −.7880; P < .001, by the Spearman rank correlation test), suggesting that the presence of a patent filarial infection is associated with induction of the alternative pathway of monocyte activation.
Figure 2.
Filarial infection is associated with elevated ARG1 and decreased NOS2 expression. Peripheral blood mononuclear cells from 10 patients with asymptomatic filarial infection (AFI) and 10 uninfected patients (UN) were examined at baseline (A) or were stimulated with Brugia malayi antigen (BmA; 10 μg/mL; B ) or purified protein derivative (PPD; 10 μg/mL; C ) for 24 h, after which monocytes were isolated. NOS2 and ARG1 messenger RNA (mRNA) levels were measured by real-time reverse transcription–polymerase chain reaction and normalized to the levels of the 18S ribosomal RNA control gene in the isolated monocytes. Results are shown as fold change versus media control. D, Correlation between baseline levels of ARG1 and NOS2 mRNA is shown as an x-y scatterplot. P values were calculated using the Mann-Whitney U test or the Spearman rank correlation test. 1/dCT, 1/[change in cycle threshold for the target gene minus that for the control gene].
Filarial antigen induces increased expression of alternative activation genes in monocytes from patients with AFI
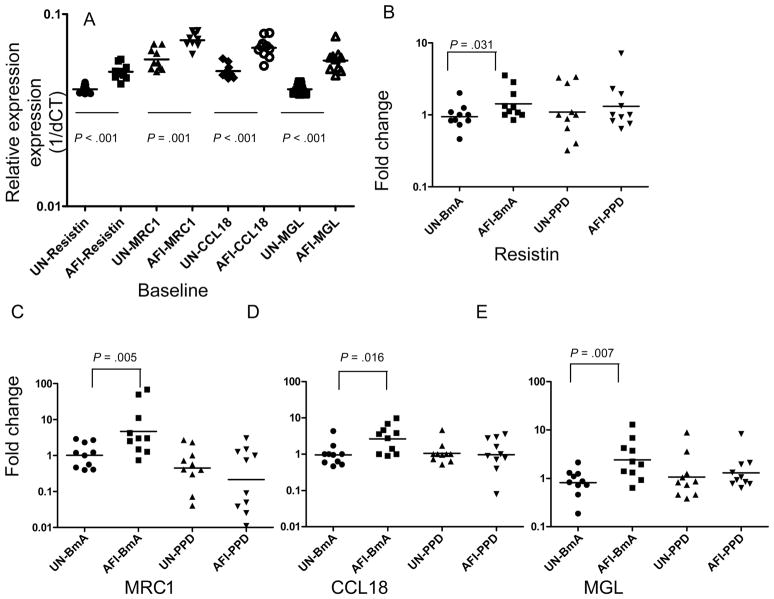
To characterize the alternative activation pathway in human monocytes further, we examined the patterns of mRNA expression for resistin, MRC1, CCL18, and MGL, each of which is known to be associated with the alternative activation pathway in macrophages [18, 19]. As shown in figure 3A, baseline expression in the AFI group was significantly greater than that in the uninfected group for resistin (GM relative transcript level, 0.05013 vs. 0.04064), MRC1 (0.07306 vs. 0.05810), CCL18 (0.06673 vs. 0.05045), and MGL (0.05729 vs. 0.04064). As with ARG1, expression induced by BmA was significantly greater in the AFI group than in the uninfected group for resistin (GM fold change, 1.425 vs. 0.939), MRC1 (4.686 vs. 1.011), CCL18 (2.654 vs. 0.954), and MGL (4.014 vs. 0.8392) (figure 3B–3E). In contrast, PPD induced no significant differences between the 2 groups in the expression of these proteins. Thus, induction of the markers for alternative monocyte activation (i.e., resistin, MRC1, CCL18, and MGL) is filaria-antigen specific.
Figure 3.
Filarial infection is associated with enhanced expression of alternative activation genes. Peripheral blood mononuclear cells from 10 patients with asymptomatic filarial infection (AFI) and 10 uninfected patients (UN) were examined at baseline (A) or were stimulated with Brugia malayi antigen (BmA; 10 μg/mL) or purified protein derivative (PPD; 10 μg/mL) for 24 h, after which monocytes were isolated. Resistin (B), mannose receptor C type 1 (MRC1; C ), chemokine ligand 18 (CCL18; D ), and macrophage galactose type C lectin (MGL; E ) messenger RNA levels were measured by real-time reverse transcription–polymerase chain reaction and normalized to the levels of the 18S ribosomal RNA control gene in the isolated monocytes. Results are shown as relative transcript levels or fold change versus media control. P values were calculated using the Mann-Whitney U test. 1/dCT, 1/[change in cycle threshold for the target gene minus that for the control gene].
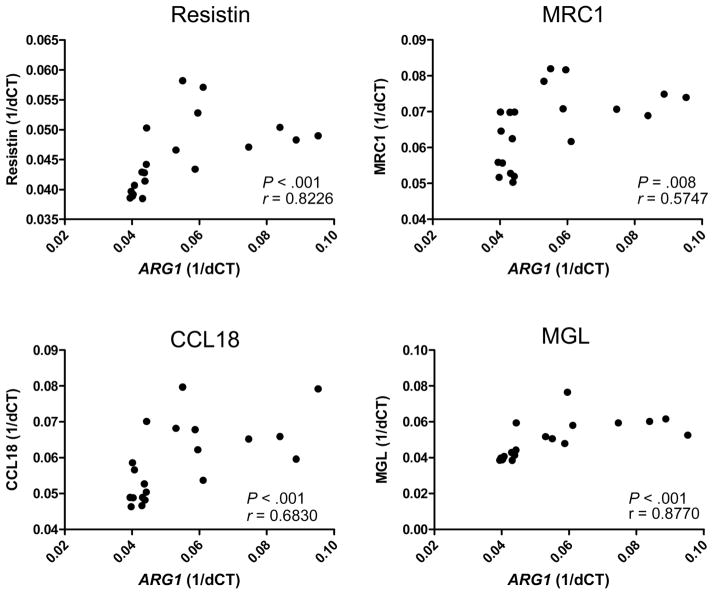
To determine whether there is an association between the expression of ARG1 and the expression of other genes implicated in the alternative activation pathway, we examined the relationship between ARG1 expression and the expression of the genes encoding resistin, MRC1, CCL18, and MGL. As shown in figure 4, we found a statistically significant association between the baseline expression of ARG1 and that of the genes encoding resistin (r = 0.8226; P < .001), MRC1 (r = 0.5747; P = .008), CCL18 (r = 0.6830; P < .001), and MGL (r = 0.8770; P < .001).
Figure 4.
Baseline levels of ARG1 correlate with alternative activation genes. Baseline correlation between messenger RNA levels of ARG1 and alternative activation genes encoding resistin, mannose receptor C type 1 (MRC1), chemokine ligand 18 (CCL18), and macrophage galactose type C lectin (MGL), as measured by real-time reverse transcription–polymerase chain reaction. Results are shown as x-y scatterplots for the correlation studies and as fold change versus media control for the inhibition studies. P values were calculated using the Spearman rank correlation test. 1/dCT, 1/[change in cycle threshold for the target gene minus that for the control gene].
Filarial antigen induces down-regulation of IL-12 and IL-18 and up-regulation of TGFβ and IL-10 by monocytes from patients with AFI
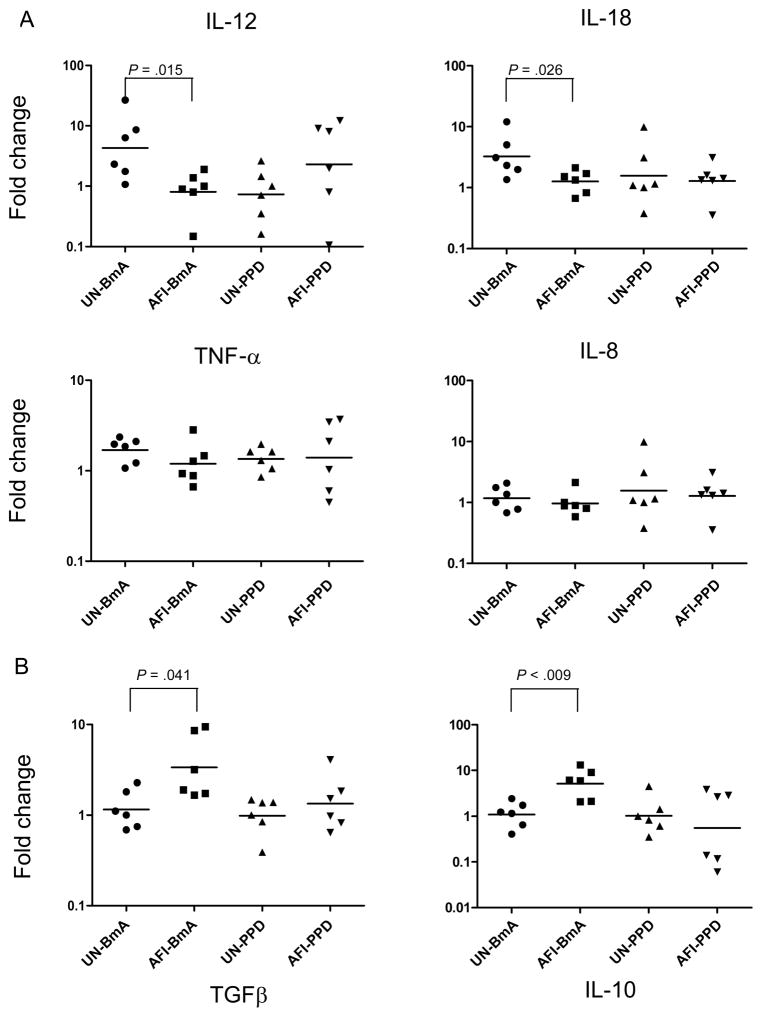
Because classically activated monocytes are predominantly associated with secretion of proinflammatory cytokines [20], we stimulated PBMCs from individuals from both study groups with BmA or PPD for 24 h, purified the monocytes, and measured the IL-12, IL-18, TNF-α, and IL-8 mRNA levels by RT-PCR. As shown in figure 5A, BmA caused significantly down-regulated expression of IL-12 and IL-18 mRNA in monocytes from patients with AFI, compared with those from uninfected patients (GM fold changes, 0.8079 vs. 4.292 for IL-12 and 1.262 vs. 3.241 for IL-18), but not of TNF-α mRNA (1.195 vs. 1.696) or IL-8 (0.9624 vs. 1.172). Of note, no significant alteration in the expression of IL-12 or IL-18 mRNA was observed in response to the nonparasite antigen PPD or at baseline.
Figure 5.
Filarial infection is associated with decreased expression of proinflammatory cytokines and increased expression of down-regulatory cytokines. Peripheral blood mononuclear cells from 6 patients with asymptomatic filarial infection (AFI) and 6 uninfected patients (UN) were stimulated with Brugia malayi antigen (BmA; 10 μg/mL) or purified protein derivative (PPD; 10 μg/mL) for 24 h, after which monocytes were isolated. Interleukin (IL)–12, IL-18, tumor necrosis factor–α (TNF-α), IL-8 messenger RNA levels (A) and transforming growth factor β (TGFβ) and IL-10 messenger RNA levels (B) were measured by real-time reverse transcription–polymerase chain reaction and normalized to the levels of the 18S ribosomal RNA control gene in the isolated monocytes. Results are shown as fold change versus media control. P values were calculated using the Mann-Whitney U test.
Because alternatively activated monocytes are predominantly associated with secretion of regulatory cytokines [20], we stimulated PBMCs from individuals in the uninfected group and patients in the AFI group with BmA or PPD for 24 h, purified the monocytes, and measured the levels of TGFβ and IL-10 mRNA by RT-PCR. Although baseline expression was not significantly different between the 2 groups, BmA induced significant up-regulation of the genes encoding TGFβ and IL-10 in patients from the AFI group, compared with uninfected persons (GM fold changes, 3.353 vs. 1.153 for TGFβ and 5.0845 vs. 1.081 for IL-10; figure 5B). Of interest, no significant alteration in the expression of TGFβ or IL-10 mRNA was observed in response to the nonparasite antigen PPD. Thus, augmented induction of the genes encoding regulatory cytokines IL-10 and TGFβ in monocytes is restricted to filarial antigen stimulation.
Filarial antigen induces increased expression of SOCS-1 mRNA in patients with AFI
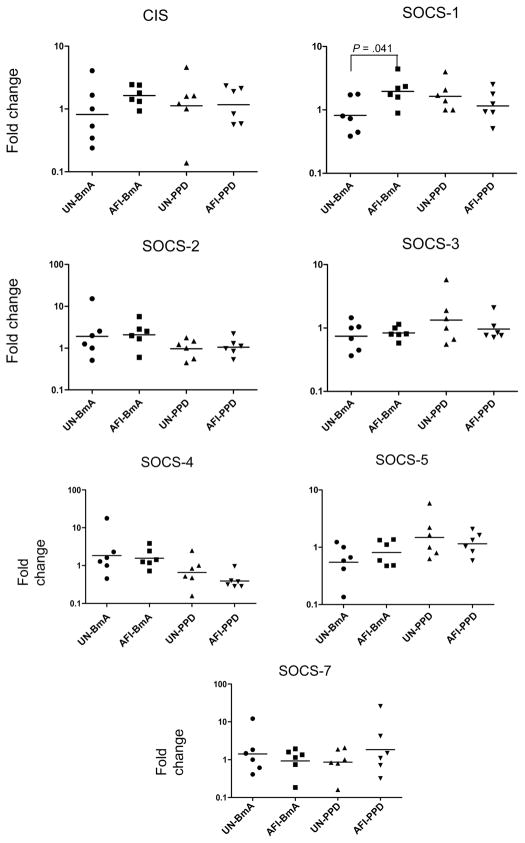
Because the SOCS family of genes is involved in the regulation of cytokine secretion in monocytes [21], we stimulated PBMCs from uninfected individuals and patients with AFI with BmA or PPD for 24 h and examined the patterns of CIS, SOCS-1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, and SOCS-7 mRNA expression in the purified monocytes. Although baseline expression of this protein family in patients with AFI was not significantly different from that in uninfected persons, we found that BmA induced significantly increased expression of SOCS-1 (GM fold change, 1.963 vs. 0.8207) but not of the other SOCS family genes (figure 6). PPD had no significant effect on induction of the SOCS family genes in either group.
Figure 6.
Filarial infection is associated with increased expression of SOCS-1. Peripheral blood mononuclear cells from 6 patients with asymptomatic filarial infection (AFI) and 6 uninfected patients (UN) were stimulated with Brugia malayi antigen (BmA; 10 μg/mL) or purified protein derivative (PPD; 10 μg/mL) for 24 h, after which monocytes were isolated. Cytokine inducible SH2– containing protein (CIS), suppressor of cytokine signaling (SOCS)–1, SOCS-2, SOCS-3, SOCS-4, SOCS-5, and SOCS-7 messenger RNA levels were measured by real-time reverse transcription–polymerase chain reaction and normalized to the levels of the 18S ribosomal RNA control gene in the isolated monocytes. Results are shown as fold change over media control. P values were calculated using the Mann-Whitney U test.
Arginase-1 inhibition negates the increased expression of alternative activation markers in patients with AFI
To examine whether arginase-1 is involved in the establishment of the alternative activation differentiation pathway in human monocytes, we stimulated PBMCs from patients in the AFI group with BmA for 72 h in the presence or absence of the arginase-1 inhibitor LOHA and examined the patterns of resistin, MRC1, CCL18, and MGL mRNA expression in purified monocytes. As shown in figure 7, a comparison of LOHA-exposed PBMCs with non–LOHA exposed PBMCs revealed that BmA induced significantly decreased PBMC expression of genes encoding resistin (GM fold change, 0.6276 vs. 1.384), MRC1 (0.6118 vs. 1.389), CCL18 (0.6915 vs. 3.245), and MGL (0.7761 vs. 1.920), thereby establishing a link between induction of ARG1 and expression of these known markers of the alternative activation pathway in human filarial infection.
Figure 7.
Inhibition of ARG1 leads to down-regulation of alternative activation genes and up-regulation of NOS2 and the genes encoding interleukin (IL)–12 and IL-18. Peripheral blood mononuclear cells from 6 patients with asymptomatic filarial infection were stimulated with Brugia malayi antigen (10 μg/mL) for 24 h in the presence or absence of ARG1 inhibitor Nω-hydroxy-nor-L-arginine, after which monocytes were isolated. Resistin, mannose receptor C type 1 (MRC1), chemokine ligand 18 (CCL18), and macrophage galactose type C lectin (MGL) messenger RNA levels were measured by real-time reverse transcription–polymerase chain reaction and normalized to the levels of the 18S ribosomal RNA control gene in the isolated monocytes. Results are shown as fold change versus media control in the presence or absence of inhibitor. P values were calculated using the Wilcoxon signed rank test.
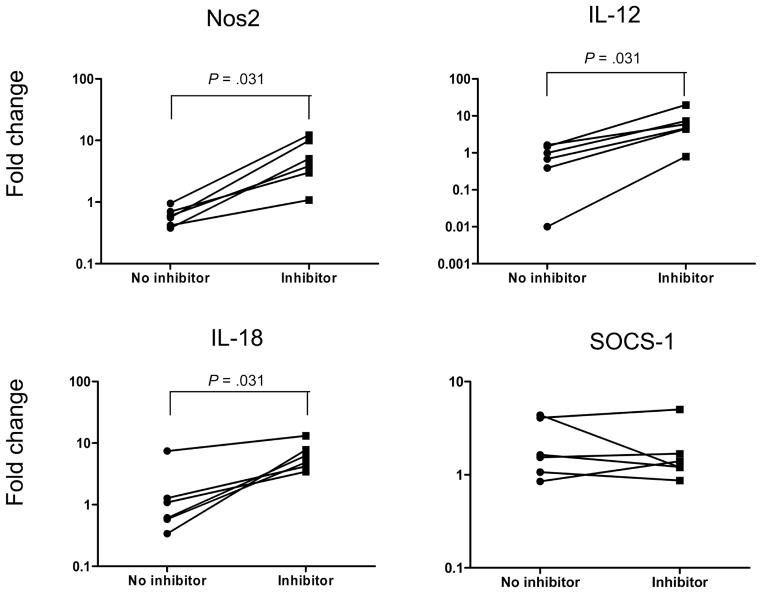
Arginase-1 inhibition up-regulates the expression of IL-12 and IL-18 mRNA in patients with AFI
To examine the functional importance of the impairment in the induction of alternative activation genes by arginase-1 inhibition, we examined mRNA expression for NOS2 and the genes encoding IL-12, IL-18, and SOCS-1. As shown in figure 8, expression of NOS2 in the presence of arginase-1 inhibition was significantly greater than that in the absence of arginase-1 inhibition (GM fold change, 4.4446 vs. 0.5737). Of note, arginase-1 inhibition also significantly increased the expression of IL-12 (GM fold change, 4.888 vs. 0.4292) and IL-18 (GM fold change, 5.959 vs. 1.038) mRNA, suggesting that the arginase-1 pathway in monocytes is mediating down-regulated proinflammatory cytokine expression in filarial infections. Arginase-1 inhibition, however, had no significant effect on expression of SOCS-1 mRNA (GM fold change, 1.570 vs. 1.857). Thus, the induction of arginase-1 activity is critical for the function of alternatively activated monocytes in human filarial infections.
Figure 8.
Inhibition of ARG1 leads to up-regulation of NOS2 and the genes encoding interleukin (IL)–12 and IL-18. Monocytes from 6 patients with asymptomatic filarial infection were stimulated with Brugia malayi antigen (10 μg/mL) for 24 h in the presence or absence of ARG1 inhibitor Nω-hydroxy-nor-L-arginine. NOS2, interleukin (IL)–12, IL-18, and suppressor of cytokine signaling–1 (SOCS-1) messenger RNA levels were measured by real-time reverse transcription–polymerase chain reaction and normalized to the levels of the 18S ribosomal RNA control gene. Results are shown as fold change versus media control in the presence or absence of inhibitor. P values were calculated using the Wilcoxon signed rank test.
DISCUSSION
The classical pathway of IFN-γ– dependent activation of macrophages by Th1 responses is an important feature of cellular immunity to infection with intracellular pathogens such as Mycobacterium tuberculosis and human immunodeficiency virus [22]. The concept of an alternative pathway of macrophage activation induced by IL-4 and IL-13 (Th2-type cytokines) has been postulated to account for a distinctive macrophage phenotype that is consistent with a role in humoral immune responses and wound healing in murine models [9]. The main defining characteristic of the alternative activation pathway is the induction of the enzyme arginase. This pathway in macrophages has been seen commonly in several models of infection caused by helminths, most notably Schistosoma mansoni, Heligmosomoides polygyrus, Nippostrongylus brasiliensis, Taenia crassiceps, Trichinella spiralis, Fasciola hepatica, Ascaris suum, and a number of filarial parasites [23, 24].
Helminth-induced alternatively activated macrophages have been shown to be important in 3 different processes: control of Th1-type inflammation, wound healing, and worm expulsion [23]. These cells have been characterized by the expression of a number of markers (apart from ARG1): IL-4Rα, CD206 (MRC1), resistin-like molecule a (RELMa; also known as FIZZ1), YM1, acidic mammalian chitinase (AMCase), MGL (mMGL1 and mMGL2), hemoglobin scavenger receptor (CD163), IL-27Rα, CCL17, CCL18, CCL22, matrix metalloproteinases, and the extracellular matrix protein βIG-H3 [18, 19, 23, 25]; however, studies of alternatively activated human monocytes/ macrophages are sparse because of the lack of specific markers associated with alternative activation in human myeloid cells and because of relatively poor homology between human and mouse with respect to some of the prototypical molecules associated with alternative macrophage activation (e.g., YM1 and RELMa in mice) [19, 26, 27]. Thus, although ARG1 is a classical marker for alternative activation in mouse macrophages, its expression in human monocytes/macrophages is not well-defined [19, 28–30]. We therefore examined the induction of NOS2 and ARG1 in purified monocytes from patients with AFI and individuals without filarial infection to elucidate the role of these enzymes in monocyte differentiation. Our results suggest that ARG1 expression is moderately induced in filarial infections, whereas NOS2 expression is suppressed, and that this differentiation occurs at baseline and is amplified by filarial-antigen stimulation. Hence, ARG1 induction can be used to delineate distinct monocyte differentiation patterns in filarial infections.
This is corroborated by the examination of known markers of alternative activation in human monocytes/macrophages. Human MRC1 (CD206), CCL18, and MGL are known markers of human alternatively activated macrophages [18, 25, 31]. RELMa is encoded by a prominent gene that, in murine models, was induced after exposure to a wide variety of helminths [30]. Although no RELMa ortholog has been identified in the human genome, the human gene encoding resistin shows the greatest similarity in sequence and expression pattern to murine RELMa [32]. Our examination of gene expression in filarial antigen–exposed monocytes revealed up-regulation of all these molecules in patients in the AFI group but not in uninfected individuals. Although resistin is commonly associated with metabolic pathways related to adipose tissue [33], our results suggest a potentially new role for resistin as a marker for alternatively activated monocytes under Th2 conditions. The results from this study also reveal a novel link between induction of ARG1 in monocytes and expression of genes encoding the alternative activation markers (i.e., resistin, MRC1, CCL18, and MGL).
To elucidate the functional phenotype of the filaria-infected monocytes, we examined the expression of both proinflammatory and immunoregulatory cytokines in response to filarial and control antigens. In agreement with previous reports, monocytes from individuals with filarial infection expressed increased levels of the immunoregulatory cytokine IL-10 [2, 3, 34]. In addition, expression of monocyte-derived TGFβ is also up-regulated. Thus, the alternatively activated monocytes in filarial infections appear to have a function that is predominantly regulatory. This is further reinforced by the finding that filaria-infected monocytes also express diminished levels of the proinflammatory cytokines IL-12 and IL-18. Because IL-12 and IL-18 are important in the establishment of Th1 responses [35], monocytes in individuals with filarial infection might potentially be responsible for the down-regulated Th1 responses in filarial infections. Regulation of cytokine expression is modulated by the SOCS family in a variety of settings, including filarial infections [35]. Our examination of the expression patterns of SOCS family members in monocytes reveals that transcriptional regulation of SOCS-1 is a feature of filarial infections. SOCS-1 is a known down-modulator of Th1 responses [21], and SOCS-1 up-regulation identifies another pathway for modulating Th1 responses to filarial antigen.
Inhibition of arginase-1 induction by the competitive inhibitor LOHA significantly decreased the expression of resistin, MRC1, CCL18, and MGL mRNA in filaria-infected patients. Thus, the differentiation of monocytes during filarial infection to the alternative activation pathway, with expression of characteristic markers, is dependent on arginase. To elucidate the mechanism by which arginase-1 inhibition suppresses development of alternatively activated monocytes, we examined the induction of the competing enzyme encoded by NOS2. Indeed, NOS2 expression was significantly enhanced in the presence of LOHA, as was the expression of genes encoding IL-12 and IL-18. This cytokine expression pattern was not associated with altered expression of SOCS-1. Taken together, these data identify an important role for arginase-1 in establishing or maintaining the alternative activation phenotype and function in human monocytes. Although we have begun elucidating the role of arginase-1 in alternative activation of monocytes, further studies will be needed to look at the influence of arginase-1 on T and B cell responses. The mechanism by which arginase-1 influences the establishment and/or maintenance of the alternatively activated phenotype in monocytes also needs further dissection.
Thus, we have identified a pathway of differentiation for monocytes in human lymphatic filariasis that involves up-regulation of genes associated with alternative activation and a cytokine profile that is profoundly inhibitory in nature. To our knowledge, this is the first description of infection-elicited alternative activation of monocytes in humans. This phenotype is influenced by arginase-1 and suggests that alternatively activated monocytes have an important role in antigen-specific immune tolerance in human lymphatic filariasis.
Acknowledgments
We thank Colin O’Shea and B. Sangeetha for technical assistance and Brenda Rae Marshall (intramural editor, National Institute of Allergy and Infectious Diseases) for editorial assistance.
Financial support: Intramural Research Program, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J Immunol. 2005;175:1170– 6. doi: 10.4049/jimmunol.175.2.1170. [DOI] [PubMed] [Google Scholar]
- 2.Sasisekhar B, Aparna M, Augustin DJ, et al. Diminished monocyte function in microfilaremic patients with lymphatic filariasis and its relationship to altered lymphoproliferative responses. Infect Immun. 2005;73:3385–93. doi: 10.1128/IAI.73.6.3385-3393.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semnani RT, Keiser PB, Coulibaly YI, et al. Filaria-induced monocyte dysfunction and its reversal following treatment. Infect Immun. 2006;74:4409–17. doi: 10.1128/IAI.01106-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semnani RT, Nutman TB. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol Rev. 2004;201:127–38. doi: 10.1111/j.0105-2896.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 5.Pfaff AW, Schulz-Key H, Soboslay PT, Geiger SM, Hoffmann WH. The role of nitric oxide in the innate resistance to microfilariae of Lito-mosoides sigmodontis in mice. Parasite Immunol. 2000;22:397– 405. doi: 10.1046/j.1365-3024.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajan TV, Porte P, Yates JA, Keefer L, Shultz LD. Role of nitric oxide in host defense against an extracellular, metazoan parasite, Brugia malayi. Infect Immun. 1996;64:3351–3. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor MJ, Cross HF, Mohammed AA, Trees AJ, Bianco AE. Susceptibility of Brugia malayi and Onchocerca lienalis microfilariae to nitric oxide and hydrogen peroxide in cell-free culture and from IFN gamma-activated macrophages. Parasitology. 1996;112:315–22. doi: 10.1017/s0031182000065835. [DOI] [PubMed] [Google Scholar]
- 8.Thomas GR, McCrossan M, Selkirk ME. Cytostatic and cytotoxic effects of activated macrophages and nitric oxide donors on Brugia malayi. Infect Immun. 1997;65:2732–9. doi: 10.1128/iai.65.7.2732-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 10.Goerdt S, Politz O, Schledzewski K, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222– 6. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 12.Anthony RM, Urban JF, Jr, Alem F, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955– 60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JE, Loke P. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 2001;23:345–52. doi: 10.1046/j.1365-3024.2001.00394.x. [DOI] [PubMed] [Google Scholar]
- 14.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald AS, Loke P, Allen JE. Suppressive antigen-presenting cells in helminth infection. Pathobiology. 1999;67:265– 8. doi: 10.1159/000028107. [DOI] [PubMed] [Google Scholar]
- 16.Lal RB, Ottesen EA. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J Infect Dis. 1988;158:1034–7. doi: 10.1093/infdis/158.5.1034. [DOI] [PubMed] [Google Scholar]
- 17.Iniesta V, Gomez-Nieto LC, Corraliza I. The inhibition of arginase by Nω-hydroxy-L-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777– 84. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raes G, Brys L, Dahal BK, et al. Macrophage galactose-type C-type lec-tins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321–7. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 19.Raes G, Van den Bergh R, De Baetselier P, et al. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174:6561. doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]
- 20.Bouhlel MA, Derudas B, Rigamonti E, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137– 43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477– 87. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953– 64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 23.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–53. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes JL, Terrazas LI. The divergent roles of alternatively activated macrophages in helminthic infections. Parasite Immunol. 2007;29:609–19. doi: 10.1111/j.1365-3024.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 25.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boot RG, Bussink AP, Aerts JM. Human acidic mammalian chitinase erroneously known as eosinophil chemotactic cytokine is not the ortho-log of mouse YM1. J Immunol. 2005;175:2041–2. doi: 10.4049/jimmunol.175.4.2041-a. [DOI] [PubMed] [Google Scholar]
- 27.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflam-mation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–9. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munder M, Mollinedo F, Calafat J, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–56. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa JB, Bernard AC, O’Brien WE, et al. Arginase I expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393–9. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouzaut A, Subira ML, de Miguel C, et al. Co-expression of inducible nitric oxide synthase and arginases in different human monocyte subsets. Apoptosis regulated by endogenous. NO Biochim Biophys Acta. 1999;1451:319–33. doi: 10.1016/s0167-4889(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional pro-filing of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 32.Yang RZ, Huang Q, Xu A, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–35. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 33.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Mahanty S, Mollis SN, Ravichandran M, et al. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic fil-ariasis. J Infect Dis. 1996;173:769–73. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 35.Murphy KM, Ouyang W, Farrar JD, et al. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–94. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]