Abstract
Until recently, the treatment of frostbite injuries has been limited to supportive care only, with mediocre outcomes. The use of thrombolytic therapy has been presented in a limited fashion in the literature since 2005. This case study describes the work-up and treatment of a patient with severe frostbite injury who received tPA. We then discuss thrombolytic therapy in more detail, with particular attention to the two studies outlining different treatment regimens.
Keywords: Frostbite, thrombolytic, cold injury, hypothermia
INTRODUCTION
Frostbite represents a spectrum of tissue injury, ranging from superficial insult to deep tissue freezing. Although it is not usually associated with mortality, there is a high level of morbidity that can occur with severe frostbite with potential loss of digits or limbs.
Historically, frostbite has affected military personnel; however, the incidence of frostbite in the military population is declining, as shown in a 2010 study by Hall et al., who found only 19 reported cold injuries, two of which were frostbite, in a retrospective review of over 18,000 patients identified by trauma registry, compared to 6,300 cases of cold weather injury seen previously in the Korean war.[1]
The epidemiology of this condition has changed over the past twenty years with greater involvement of the civilian population.[2] There is no formal reporting system for frostbite cases and the incidence of frostbite in the general population has not been widely studied. The prevalence and incidence of frostbite varies widely depending on the study population. A 2009 cross-sectional study performed in Finland sent out a cold questionnaire to the general population. Subjects were randomly chosen from a pre-existing nationwide health survey system from all over Finland in the years 1997 and 2002. Of 13,713 participants ranging from ages 25-74 years, 697, or 5.1% sustained some type of frostbite.[3] In another cross-sectional Finnish study of male military recruits aged 17-30, the prevalence of frostbite was 2555/5839, or 44% with an annual incidence of 2.2%. The questionnaire addressed frostbite sustained prior to military service.[4] The incidence of frostbite was also examined in a high risk population, mountaineers, and found to be 366/1000 mountaineers per year.[5]
Frostbite or damage to tissues from exposure to temperatures below their freezing point, can occur at -2°C (28°F). Tissue freezes faster at lower temperatures and in the presence of wind. The severity of frostbite is proportional to the duration of the exposure.[2,6,7] Risk factors for frostbite injury in the civilian population include prolonged exposure, decreased ambient temperature, inadequate clothing, smoking, peripheral vascular disease, diabetes, Raynaud's disease, sepsis, previous cold injury, concomitant use of alcohol or drugs, psychiatric illness, dementia, trauma, vehicular breakdown, and homelessness.[6–8]
Until the early 1990s the goal of frostbite treatment was to preserve viable tissue and prevent infection, with rapid thawing, daily wound care, antibiotics as indicated, and watchful waiting being the mainstays of therapy. The treatment of frostbite had not progressed until the introduction of tPA for limb and digit salvage in the 1990s. The following case report outlines a case of frostbite treated with tPA at our institution.
CASE REPORT
A 59-year-old woman was found outside her home by a neighbor. The average temperature that day was -7°F. The patient was confused initially, and gave various stories to different providers about how she came to be outside and exposed for a prolonged period of time. She was outside for 2 hours according to her initial report. She was unable to provide a complete medical history; however, she was able to state she had a history of depression, anxiety, and migraines, and medications included alprazolam and sumatriptan as needed. She denied drug or alcohol use. Complaints on arrival included bilateral hand, arm, knee, and ankle pain.
Examination revealed mild hypothermia (temp 34.2°C) and cold, mottling and woody induration of all ten digits of the hands. Passive re-warming techniques were used to correct her hypothermia. Her hands were placed in a water bath at 40°C. After re-warming, her hands became purple and swollen [Figures 1 and 2].
Figure 1.
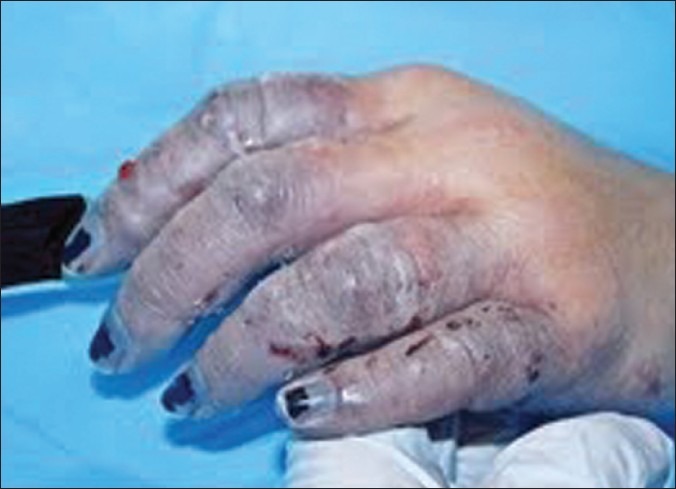
Left hand after rewarming
Figure 2.
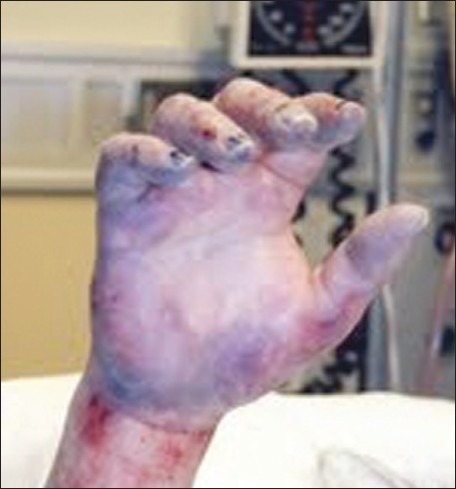
Right hand after rewarming
Multiple imaging and laboratory studies were obtained in the ED. Pertinent studies included a normal head CT, a WBC count of 17.5k, and a CK of 441. Her mental status improved, and although unable to recall details of her exposure, she thought she had been outside longer than 2 hours. The surgery team was consulted regarding the patient's frostbite and she was admitted to the burn service. A bone scan was urgently obtained which revealed: (1) No perfusion identified within the left third through 5th digits and right 4th and 5th digits, and (2) Small amount of uptake at the base of the right first 3 digits and left first and second digits [Figure 3]. The patient was given aspirin. Intravenous (IV) tPA was given as a 0.15 mg/kg bolus followed by an infusion of 100mg at 0.15 mg/kg/hr. After tPA, a heparin drip at 12 units/kg/hr was administered for 36 hours. A repeat bone scan was performed two days after admission, showing increased perfusion in the first through 4th digits bilaterally to at least the proximal interphalangeal joints, and in the 5th digits bilaterally, more modestly improved flow to the mid proximal phalanx level [Figures 4a and b].
Figure 3.
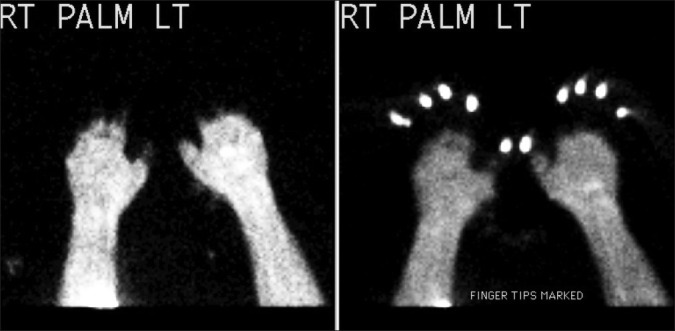
Initial bone scan prior to thrombolytic therapy. There is no perfusion identified within the left 3rd through 5th digits and right 4th and 5th digits, and a small amount of uptake at the base of the right first 3 digits and left 1st and 2nd digits
Figure 4.
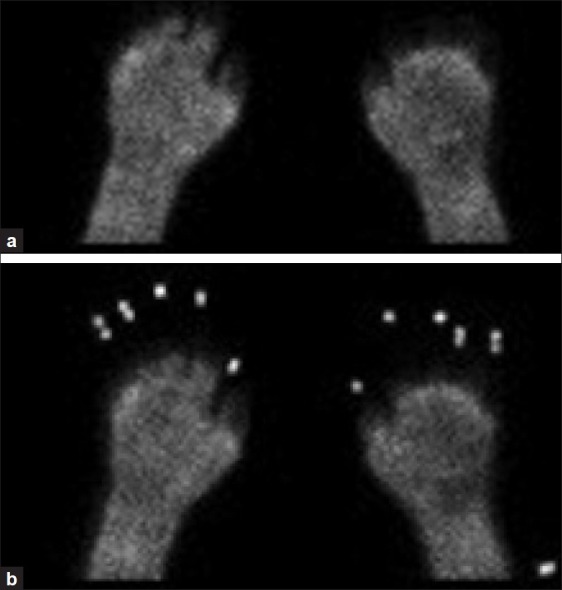
(a-b): Bone scan after thrombolytic therapy showing increased perfusion in the first through 4th digits bilaterally to at least the proximal interphalangeal joints, and in the 5th digits bilaterally, more modestly improved flow to the mid proximal phalanx level
After a twelve day hospital stay, the patient was discharged to a nursing facility to allow demarcation of her injuries. The patient returned to the hospital approximately one month after her initial presentation for amputation of mummified tissue. All ten fingers had amputation: bilaterally, her index, middle and ring fingers were amputated to the level of the proximal phalanx. Thumbs were amputated to level of the proximal phalanx. Pinky fingers were amputated at the level of metacarpo-phalangeal joint. She spent 1.5 months in a nursing facility. Upon discharge, she was able to write her name and perform activities of daily living.
DISCUSSION
The treatment of frostbite injury had been stagnant for many years prior to the introduction of thrombolytic therapy. The mainstays of treatment had been re-warming, local wound care, watchful waiting, and then progression to either recovery or amputation. Other investigations into anti-thrombotics, dextran, and hyperbaric oxygen therapy have not been fruitful.[9–11]
In frostbite, two elements, tissue freezing and reperfusion, contribute to the damage seen in frostbite patients. Parts of the mechanism of injury are not clearly understood.First, tissue freezing leads to crystallization of the extracellular space with increase of extracellular oncotic pressure, causing fluid shifts out of the cells. This causes cellular dehydration and disruption of intracellular metabolism and instability of the cell membrane. The cell itself is also prone to mechanical damage by extracellular ice crystals. Vasoconstriction, ice crystal formation in the plasma, and increased blood viscosity with subsequent microvascular damage leads to an inflammatory response.[6,7,12] This begins a cascade of events that ends with thrombosis, tissue ischemia, edema, and endothelial injury.[13]
Reperfusion injury then occurs when blood flow is restored. Early thrombolytic therapy could limit microvascular thrombosis and help prevent reperfusion injury. With thawing, further endothelial damage and lysis of cells occur, with release of inflammatory cytokines and further development of microvascular clot and edema.[6,7] Early thrombolytic therapy can theoretically limit microvascular thrombosis and help prevent reperfusion injury.
The initial examination of the frostbitten extremity may reveal a range of severity of injury from superficial (erythematous area around a white center) to deep (hemorrhagic blisters or full thickness tissue necrosis). The final demarcation of viable tissue is hard to determine at this stage. At this time, some supportive measures can be started, including addressing hypothermia, pain management, IV hydration, and consideration of transfer to a higher-level facility. The decision to re-warm the affected extremity should not be taken lightly. If there is another period of freezing, the prognosis is worse. Rapid transport to a facility capable of giving tPA is of utmost importance; it may be the re-warming needs to be done there.
The decision to proceed with thrombolysis of frostbitten extremities is typically based on results of technetium-99m triple-phase bone scanning or angiography. Bone scanning has been found to predict tissue viability.[14] Findings on physical exam that should prompt further evaluation by one of these two methods include absent capillary refill, absent Doppler pulses, purple discoloration and hemorrhagic blisters. Ultimately, a decrease in perfusion should lead to treatment with thrombolytic therapy.
There are few studies describing thombolytic therapy in humans. Two methods have been described – catheter directed intra-arterial (IA) and systemic IV therapy. There have not been any prospective ranndomized studies comparing the two methods.
Bruen, et al. published a 2007 retrospective review of patients at their institution who received IA therapy for severe frostbite.[15] They compared patients treated with IA tPA starting in 2001, (when they implemented their protocol), to historical controls from 1995-2001 as well as more recent patients presenting greater than 24 hours after injury. Patients were given IA tPA if they had evidence of significant perfusion defect on 99Tc-scintigraphy or distal angiography and had no contraindications for tPA. A total of 32 patients were identified. They reported a 10% amputation rate among those treated with IA tPA within 24 hours of injury (vs. 41% in those not receiving tPA). The authors reported one complication in the patients who received IA tPA, with one patient developing a retroperitoneal hematoma that was managed non operatively and resolved without further issue.
Bruen recommends dosing as follows: (1) IA catheter(s) are placed, (2) an initial bolus of 2-4 mg is given, (3) a maximum infusion of 1 mg/hr is started; this infusion dose is divided amongst the catheters (e.g., two extremities involved = 0.5 mg/hr per extremity, etc.). Heparin is given at 500 mg/hr concurrently through the access sheath. tPA is continued until there is evidence of tissue reperfusion, 48 hours has passed, or the attending surgeon and interventional radiologist feel there is no further therapeutic gain by continuing the infusion. Heparin is continued for 72-96 hours. More recently, adding a vasodilator to IA tPA has been suggested.[16]
In 2005, Twomey et al. published a non-randomized prospective trial with historical controls to assess the safety and efficacy of tPA for severe frostbite.[17] The study was funded in part by the manufacturer of r-TPA. Trial enrollment began in 1989 and stopped in 2003. Patients received either IA tPA (6 patients) or IV tPA (13 patients), and were included in the study if they had no improvement with re-warming, absent Doppler pulses in limbs and/or digits, and no perfusion on Technetium 99m three-phase bone scan, and no contraindications to tPA. Patients also received heparin acutely and warfarin for 4 weeks after tPA treatment. The authors reported of 174 digits at risk in 19 patients treated with tPA; part or all of 33 digits were amputated (10 fingers and bilateral BKAs in one patient with 60 hours of cold exposure with repeat freeze-thaw cycles). Response was seen in 16 of the 19 patients. This compares to 12 of 16 patients requiring amputation in the control group. No complications were reported in the IV tPA group. In the IA group, two patients had reported complications, where one patient had bleeding at arterial puncture sites requiring premature cessation of therapy and the other developed hematuria that required cessation of therapy.
Overall, this study showed safety and efficacy of tPA in a small number of patients, with equivalent results in IV and IA patients. Two patients who received IA tPA patients had complications. Ultimately the authors recommend tPA at dosing of 0.15 mg/kg bolus with a 0.15 mg/kg/hr infusion over 6 hours up to a maximum of 100 mg and heparin therapy (defined as PTT twice control values) for 3-5 days, and aspirin therapy for several weeks after discharge.[17]
Contraindications to use of tPA in the Bruen study included superficial frostbite, involvement of the tips of the distal phalanges, concurrent trauma, neurological impairment, recent surgery or hemorrhage, or bleeding diasthesis. Contraindications to the use of tPA in the Twomey study included: Severe hypertension, recent trauma, stroke, or bleeding disorder, pregnancy, mental incapacity, drug or alcohol intoxication, repeated freeze-thaw cycles, or more than 48 hours of cold exposure.
The optimal timing of tPA administration is as fast as possible after exposure. However, the outer range of tPA administration after exposure is not clear. In the Bruen study, one patient received tPA treatment approximately 48 hours after exposure with no improvement in blood flow. The remainder of their patients treated with tPA were treated within 24 hours after exposure.[15] In the Twomey study, the authors found that patients that did not respond to tPA were those with greater than warm ischemia time greater than 6 hours, more than 24 hours of cold exposure, or those with multiple freeze-thaw cycles.[17] Based on these two studies, most of the literature now recommends tPA be given within 24 hours of exposure.
CONCLUSIONS
Although our patient's bone scan did show improvement after tPA and heparin infusion, her digits were ultimately unable to be salvaged. Further discussion with family revealed that she had likely been exposed for a much longer period of time, possibly 7 or 8 hours.
This case outlines the current evaluation and management of frostbite. IV tPA shows promise for a condition that has had no advances in treatment for decades. Evidence is limited but suggests IV tPA is an effective treatment, and possibly the only opportunity for digit and limb salvage.
In the ED, re-warming the affected digits in a 40°C water bath along with concomitant treatment of hypothermia or co-morbid trauma and medical conditions is the first priority. Access to a facility with bone scanning or angiography and the ability to monitor a tPA infusion is important and should be considered early in the disposition decision. The risk/benefit ratio must be considered before starting tPA empirically prior to any bone scan or angiography. Transferring the patient may be appropriate to obtain imaging and treatment. Consultation with a plastic/burn or general surgeon is encouraged prior to transfer in questionable cases. Our facility recommends transfer to a burn center for new severe frostbite, danger of skin or limb loss, or if the transferring facility feels uncomfortable caring for the patient.
Based on limited existing literature, patients who are eligible for tPA should have less than 24 hours of cold exposure, no evidence of multiple freeze/thaw cycles, show no improvement with rapid re-warming in tepid water, absent doppler pulses in limbs and/or digits, and absence of perfusion on angiography or technetium-99m triple-phase bone scanning, with no indications to tPA treatment. Patients should be able to consent for treatment or have a representative able to consent for them. Traditional contraindications for tPA should be employed. IV tPA appears to be equally as effective as IA tPA with a lower complication rate.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hall A, Evans K, Pribyl S. Cold injury in the United States military population: Current trends and comparison with past conflicts. J Surg Educ. 2010;67:1350–5. doi: 10.1016/j.jsurg.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: Pathogenesis and treatment. J Trauma. 2000;48:171–8. doi: 10.1097/00005373-200001000-00036. [DOI] [PubMed] [Google Scholar]
- 3.Mäkinen TM, Jokelainen J, Näyhä S, Laatikainen T, Jousilahti P, Hassi J. Occurence of frost bite in the general population- work related and individual factors. Scan J Work Environ Health. 2009;35:384–93. doi: 10.5271/sjweh.1349. [DOI] [PubMed] [Google Scholar]
- 4.Ervasti O, Juopperi K, Kettunen P, Remes J, Rintamäki H, Latvala J, et al. The occurrence of frostbite and its risk factors in young men. Int J Circumpolar Health. 2004;63:71–80. doi: 10.3402/ijch.v63i1.17650. [DOI] [PubMed] [Google Scholar]
- 5.Harirchi I, Arvin A, Vash JH, Zafarmand V. Frostbite: Incidence and predisposing factors in mountaineers. Br J Sports Med. 2005;39:898–901. doi: 10.1136/bjsm.2004.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohr WJ, Jenabzadeh K, Ahrenholz DH. Cold Injury. Hand Clin. 2009;25:481–96. doi: 10.1016/j.hcl.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Hallam MJ, Cubison T, Dheansa B, Imray C. Managing frostbite. BMJ. 2010;341:1151–6. doi: 10.1136/bmj.c5864. [DOI] [PubMed] [Google Scholar]
- 8.Koljonen V, Andersson K, Mikkonen K, Vuola J. Frostbite injuries treated in the Helsinki area from 1995-2002. J Trauma. 2004;57:1315–20. doi: 10.1097/01.ta.0000151258.06910.83. [DOI] [PubMed] [Google Scholar]
- 9.Mundeth ED, Long DM, Brown RB. Treatment of experimental frostbite with low molecular weight dextran. J Trauma. 1964;128:246–57. doi: 10.1097/00005373-196403000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Theis FV, O’connor WR, Wahl FJ. Anticoagulants in acute frostbite. JAMA. 1951;146:992–5. doi: 10.1001/jama.1951.03670110012004. [DOI] [PubMed] [Google Scholar]
- 11.Finderle Z, Cankar K. Delayed treatment of frostbite injury with hyperbaric oxygen therapy: A case report. Aviat Space Environ Med. 2002;73:392–4. [PubMed] [Google Scholar]
- 12.Heggers JP, Robson MC, Manavalen K, Weingarten MD, Carethers JM, Boertman JA, et al. Experimental and clinical observations on frostbite. Ann Emerg Med. 1987;16:1056–62. doi: 10.1016/s0196-0644(87)80758-8. [DOI] [PubMed] [Google Scholar]
- 13.McCauley RL, Hing DN, Robson MC, Heggers JP. Frostbite injuries: A rational approach based on the pathophysiology. J Trauma. 1983;23:143–7. [PubMed] [Google Scholar]
- 14.Mehta RC, Wilson MA. Frostbite injury: Prediction of tissue viability of with triple-phase bone scanning. Radiology. 1989;170:511–4. doi: 10.1148/radiology.170.2.2911677. [DOI] [PubMed] [Google Scholar]
- 15.Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546–51. doi: 10.1001/archsurg.142.6.546. [DOI] [PubMed] [Google Scholar]
- 16.Saemi AM, Johnson JM. Treatment of bilateral hand frostbite using transcatheter arterial thrombolysis after paparavine infusion. Cardiovasc Intervent Radiol. 2009;32:1280–3. doi: 10.1007/s00270-009-9584-9. [DOI] [PubMed] [Google Scholar]
- 17.Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safetyand efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350–5. doi: 10.1097/01.ta.0000195517.50778.2e. [DOI] [PubMed] [Google Scholar]


