Abstract
Human harvests can select against phenotypes favoured by natural selection, and natural resource managers should evaluate possible artificial selection on wild populations. Because the required genetic data are extremely difficult to gather, however, managers typically rely on harvested animals to document temporal trends. It is usually unknown whether these data are unbiased. We explore our ability to detect a decline in horn size of bighorn sheep (Ovis canadensis) by comparing harvested males with all males in a population where evolutionary changes owing to trophy hunting were previously reported. Hunting records underestimated the temporal decline, partly because of an increasing proportion of rams that could not be harvested because their horns were smaller than the threshold set by hunting regulations. If harvests are selective, temporal trends measured from harvest records will underestimate the magnitude of changes in wild populations.
Keywords: artificial selection, sport hunting, time series, ungulates
1. Introduction
Recently, it has become evident that exploitation can lead to artificial selection [1–4]. As humans often prefer sex-age classes or morphological traits associated with high natural survival, harvest mortality differs from natural mortality [5]. Thus, harvests may lead to evolutionary responses in life histories and morphology [6], outpacing other selective agents [7,8]. There have been calls to consider potentially undesirable artificial selection in management and conservation [9,10].
A first step to avoid artificial selection is evaluating which practices have undesired consequences. As detailed, individual monitoring is rarely available, attempts to quantify artificial selection in wild populations usually rely on morphological, life history and demographic data collected from harvested animals [6,11]. Harvest data, however, will not reflect population values if harvest is selective and varies in intensity over time, as may occur with trophy hunting and size-selective fisheries. For example, the age distribution of shot red grouse (Lagopus lagopus scoticus) was biased by harvest intensity [12]. Similar biases may affect temporal trends in morphological traits estimated from harvest data. If harvest probability is affected by regulations for minimum size, gear selectivity [13] or by cultural preferences, the average size of harvested animals should be greater than the population average. These biases have been acknowledged [14], but it remains unknown how they affect our ability to detect temporal trends in phenotypic traits.
To test whether data from selective harvests can be used to detect temporal trends in phenotype, we analysed nearly four decades of detailed individual monitoring of bighorn sheep (Ovis canadensis) in a population where unlimited trophy hunting favoured rams with slow-growing horns [2,15]. We compared harvested animals with the whole population. In most of the province of Alberta, harvest of bighorn rams is based on minimum horn curl (figure 1). This management strategy protects sub-adults but leads to the harvest of rams with rapidly growing horns aged 4–6 years [5,16], before they obtain the high reproductive success associated with large horns at ages 7–11 [17,18]. Similar regulations for both bighorn and thinhorn (Ovis dalli) sheep, combining curl restrictions and unlimited resident permits, exist in most of Canada and in Alaska. The ‘legal’ definition of a harvestable ram is thus comparable to antler point restrictions often used for cervids [19].
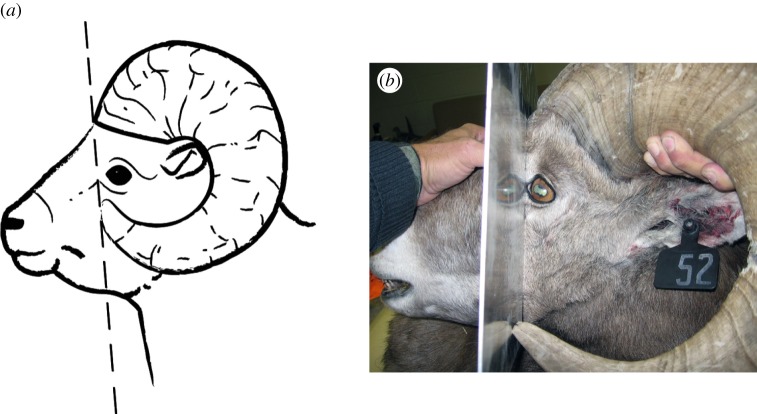
Figure 1.
(a) Legal definition of a ‘4/5-curl’ bighorn sheep ram in Alberta, Canada: a straight line drawn from the front of the base of the horn to the tip of the horn passes in front of the eye when viewed in profile. Image: Groupe PVP. (b) Harvested 4-year-old ram that just meets the definition. Photo: Alberta Fish and Wildlife.
2. Material and methods
From 1975, 95 per cent of resident bighorn sheep at Ram Mountain, Alberta, Canada, have been individually marked and repeatedly measured [20,21]. We used data on horn size for males aged 4–7 years from 1975 to 2004, when rams from elsewhere were introduced to rescue the population from demographic and genetic decline. Each year, we determined whether or not each ram fitted the legal definition of 4/5-curl, similarly to how a hunter would evaluate a potential target (figure 1). Rams in this population are never legal before age 4, and only nine of 59 (15%) harvested in 1975–1996 were aged 8 years or older. Because hunters selectively harvest rams with fast-growing horns at younger ages [22,23], horn size of surviving rams is increasingly biased by sport harvest as they age. This population was subject to unlimited harvest of 4/5-curl rams until 1995. From 1996, only ‘full-curl’ rams could be harvested. Average horn size, however, began to decline about 1983, so that only 13.7 per cent of rams aged 4 years and older were legal in 1996–2004 under the revised definition. To simulate a continuing hunting season under 4/5ths curl, we randomly assigned 37.5 per cent of ‘4/5-curl’ rams to the ‘harvested’ group from 1996 onward, based on harvest rate in 1975–1995. The simulation harvested only two rams aged 4–7 over these 11 years, as most were not ‘4/5-curl’.
Analyses involved two steps. We first explored temporal trends in the relationship between horn size and year using broken stick regression models (see Crawley et al. [24] and electronic supplementary material) without any covariates. Once the threshold year was identified, we compared temporal trends in horn length and basal circumference during the decline between harvested rams and all males aged 4–7 years. Horn size was adjusted on June 5 (see Festa-Bianchet et al. [22]). All models included age as a covariate. Linear-mixed effects models for all rams were fitted with restricted maximum likelihood including ram identity as a random effect to account for repeated measurements over time [25]. For harvest data, however, each ram contributed only one datum, in the year of death. All analyses were implemented in R v. 2.12.0 [26], using the nlme library.
3. Results
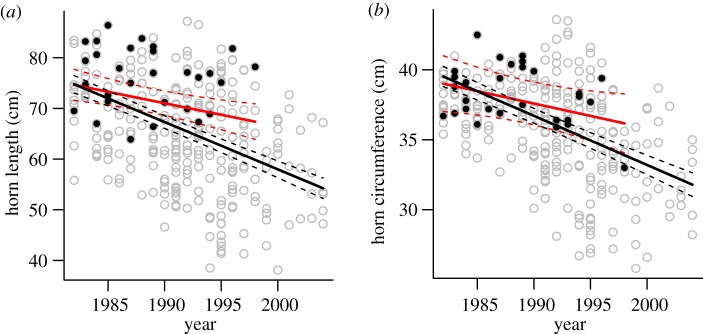
Horn size began to decline in the 1980s, but Akaike information criterion (AIC) values for successive years were similar. The decline in length began in the early 1980s (thresholds between 1980 and 1983 were equivalent), while that in base circumference was delayed by approximately 5 years (electronic supplementary material, table S1). We quantified the decline in horn size from 1982, when horn length began a linear decline (electronic supplementary material, table S1). All models indicate a decline in horn size through time (electronic supplementary material, table S2), but trends were underestimated by analyses of harvested rams (figure 2). Using different ‘threshold’ years provide similar results (results not shown). The complete dataset revealed a decrease in horn length of 0.82 cm yr−1, while data from harvested rams suggested a decline of 0.45 cm yr−1. Hunting data predicted a decline of 7.7 cm over 17 years, half the actual decline of almost 14 cm. Horn base circumference showed a similar trend, although confidence intervals of the slopes for all rams and harvested rams overlapped over most of the period (figure 2). The complete dataset suggested a decrease of 5.6 cm in circumference while the harvest dataset detected a decline of only 3 cm. As horn size declined, fewer rams were at risk of being harvested, because small horns cannot form 4/5 of a curl. Of 100 rams born in 1969–1985 that survived to age 4, only 29 died without their horns reaching 4/5 curl. In contrast, 68 per cent of 77 born in 1986–2000 died without reaching 4/5 curl (χ2 = 26.6, p < 0.001).
Figure 2.
Predicted temporal trends in horn size of 5-year-old bighorn rams at Ram Mountain from 1982 to 2004 for (a) horn length and (b) horn base circumference. Black circles and red line represent data from shot rams. This regression line stops in 1998 because no rams were harvested thereafter. Empty grey circles and black line are horn measurements of all rams in the population. Dotted lines are the 95% confidence intervals, whose divergence on (a) suggests that the slope estimate from shot rams differs significantly from the estimate for the entire population.
4. Discussion
Our results suggest that trophy harvest records underestimate temporal trends in horn size of bighorn rams. Average horn length declined by approximately 20 per cent, but harvest data suggested a decline of only 11 per cent. Despite the overlap in confidence interval of temporal trends in horn circumference, this trait showed a similar pattern of steeper decline (15%) for the population than for the subset of harvested rams (8%). In several years after horn size declined, there were no legal rams in the population, so it would have been impossible for harvest statistics to monitor horn size. These results are likely representative of those sport-hunted ungulates where hunters typically harvest the largest available animals [19]. The extent of hunter selectivity is rarely known [27]. Size-selective harvests are particularly likely for gregarious species such as wild sheep, where hunters can choose the largest individual in a group. In our study, this situation was exacerbated by regulations making it illegal to harvest small males. Similar preferences for harvesting larger individuals are well known in fisheries [6,13]. For example, size-selectivity of fisheries for cod (Gadus morhua) in the Gulf of St Lawrence resulted in fast-growing fish being more likely to be caught than slow-growing fish. Consequently, data from declining commercial fisheries might underestimate the decline in age-specific mass for the population [6].
Base circumference began to decline about 5 years later than horn length, and the difference in temporal decline between all rams and harvested rams was not as evident as for horn length. Hunting regulations specify a minimum degree of horn curl, and short horns cannot achieve that minimum degree of curl. Horn base circumference, on the other hand, is not a direct target of artificial selection. Therefore, selective hunting could lead to changes in horn shape, as reported in European mouflon (Ovis aries) [28].
A combination of legal requirements and hunter preferences makes information from harvested animals a biased estimator of changes in trait values. These legal and cultural factors produce an ‘invisible fraction’ of animals whose size may vary according to artificial selection [16], population density and environmental effects [22], all drivers of horn and antler size. Similar considerations would apply to other species, such as fish harvested with size-selective gear [13] or birds where harvest is age-biased [12]. Thus, evolutionary ecology studies of wild populations based on data from selectively harvested animals will erroneously estimate temporal changes in phenotypic traits unless they can account for the effects of non-random harvest and temporal changes in harvest intensity.
Acknowledgements
We thank all assistants and students who worked on Ram Mountain over decades. M.F.B. and F.P. are funded by NSERC. F.P. holds the Canada Research Chair in Evolutionary Demography and Conservation. Our research is also supported by the Government of Alberta and the Alberta Conservation Association.
References
- 1.Sinclair A. R. E., Fryxell J. M., Caughley G. 2006. Wildlife ecology, conservation and management, 2nd edn 469 p Oxford, UK: Blackwell Publishing [Google Scholar]
- 2.Coltman D. W., O'Donoghue P., Jorgenson J. T., Hogg J. T., Strobeck C., Festa-Bianchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 10.1038/nature02177 (doi:10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 3.Olsen E. M., Heino M., Lilly G. R., Morgan M. J., Brattey J., Ernande B., Dieckmann U. 2004. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935 10.1038/nature02430 (doi:10.1038/nature02430) [DOI] [PubMed] [Google Scholar]
- 4.Law W., Salick J. 2005. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae). Proc. Natl Acad. Sci. USA 102, 10 218–10 220 10.1073/pnas.0502931102 (doi:10.1073/pnas.0502931102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonenfant C., Pelletier F., Garel M., Bergeron P. 2009. Phenotypic consequences of hunting on the horn growth–longevity relationship in wild sheep. J. Anim. Ecol. 78, 161–171 10.1111/j.1365-2656.2008.01477.x (doi:10.1111/j.1365-2656.2008.01477.x) [DOI] [PubMed] [Google Scholar]
- 6.Swain D. P., Sinclair A. F., Hanson J. M. 2007. Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. Lond. B 274, 1015–1022 10.1098/rspb.2006.0275 (doi:10.1098/rspb.2006.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darimont C. T., Carlson S. M., Kinnison M. T., Paquet P. C., Reimchen T. E., Wilmers C. C. 2009. Human predators outpace other agents of trait change. Proc. Natl Acad. Sci. USA 106, 952–954 10.1073/pnas.0809235106 (doi:10.1073/pnas.0809235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendry A. P., Farrugia T. J., Kinnison M. T. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29 10.1111/j.1365-294X.2007.03428.x (doi:10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 9.Stockwell C. A., Hendry A. P., Kinnison M. T. 2003. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94–101 10.1016/S0169-5347(02)00044-7 (doi:10.1016/S0169-5347(02)00044-7) [DOI] [Google Scholar]
- 10.Mysterud A. 2011. Selective harvesting of large mammals: how often does it result in directional selection. J. Appl. Ecol. 48, 827–834 10.1111/j.1365-2664.2011.02006.x (doi:10.1111/j.1365-2664.2011.02006.x) [DOI] [Google Scholar]
- 11.Milner J. M., Nilsen E. B., Andreassen H. P. 2007. Demographic side effects of selective hunting in ungulates and carnivores. Conserv. Biol. 21, 36–47 10.1111/j.1523-1739.2006.00591.x (doi:10.1111/j.1523-1739.2006.00591.x) [DOI] [PubMed] [Google Scholar]
- 12.Bunnefeld N., Baines D., Newborn D., Milner-Gulland E. J. 2009. Factors affecting unintentional harvesting selectivity in a monomorphic species. J. Anim. Ecol. 78, 485–492 10.1111/j.1365-2656.2008.01500.x (doi:10.1111/j.1365-2656.2008.01500.x) [DOI] [PubMed] [Google Scholar]
- 13.Carlson S. M., Edeline E., Vøllestad L. A., Haugen T. O., Winfield I. J., Fletcher J. M., James J. B., Stenseth N. C. 2007. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius). Ecol. Lett. 10, 512–521 10.1111/j.1461-0248.2007.01046.x (doi:10.1111/j.1461-0248.2007.01046.x) [DOI] [PubMed] [Google Scholar]
- 14.Martínez M., Rodríguez-Vigal C., Jones O. R., Coulson T., San Miguel A. 2005. Different hunting strategies select for different weights in red deer. Biol. Lett. 1, 353–356 10.1098/rsbl.2005.0330 (doi:10.1098/rsbl.2005.0330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coltman D. W., O'Donoghue P., Hogg J. T., Festa-Bianchet M. 2005. Selection and genetic (co)variance in bighorn sheep. Evolution 59, 1372–1382 [PubMed] [Google Scholar]
- 16.Festa-Bianchet M., Lee R. 2009. Guns, sheep and genes; when and why trophy hunting may be a selective pressure. In Recreational hunting, conservation and rural livelihoods: science and practice (eds Dickson B., Hutton J., Adams B.). London, UK: Wiley-Blackwell [Google Scholar]
- 17.Coltman D. W., Festa-Bianchet M., Jorgenson J. T., Strobeck C. 2002. Age-dependent sexual selection in bighorn rams. Proc. R. Soc. Lond. B 269, 165–172 10.1098/rspb.2001.1851 (doi:10.1098/rspb.2001.1851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogg J. T., Forbes S. H. 1997. Mating in bighorn sheep: frequent male reproduction via a high-risk ‘unconventional’ tactic. Behav. Ecol. Sociobiol. 41, 33–48 10.1007/s002650050361 (doi:10.1007/s002650050361) [DOI] [Google Scholar]
- 19.Knox W. M. 2011. The antler religion. Wildl. Soc. Bull. 35, 45–48 10.1002/wsb.5 (doi:10.1002/wsb.5) [DOI] [Google Scholar]
- 20.Festa-Bianchet M., Jorgenson J. T., King W. J., Smith K. G., Wishart W. D. 1996. The development of sexual dimorphism: seasonal and lifetime mass changes in bighorn sheep. Can. J. Zool. 74, 330–342 10.1139/z96-041 (doi:10.1139/z96-041) [DOI] [Google Scholar]
- 21.Jorgenson J. T., Festa-Bianchet M., Gaillard J.-M., Wishart W. D. 1997. Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 78, 1019–1032 10.1890/0012-9658(1997)078[1019:EOASDA]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[1019:EOASDA]2.0.CO;2) [DOI] [Google Scholar]
- 22.Festa-Bianchet M., Coltman D. W., Turelli L., Jorgenson J. T. 2004. Relative allocation to horn and body growth in bighorn rams varies with resource availability. Behav. Ecol. 15, 305–312 10.1093/beheco/arh014 (doi:10.1093/beheco/arh014) [DOI] [Google Scholar]
- 23.Hengeveld P., Festa-Bianchet M. 2011. Harvest regulations and artificial selection on horn size in male bighorn sheep. J. Wildl. Manage. 75, 189–197 10.1002/jwmg.14 (doi:10.1002/jwmg.14) [DOI] [Google Scholar]
- 24.Crawley M. J. 2007. The R book. Chichester, UK: John Wiley [Google Scholar]
- 25.Pinheiro J. C., Bates D. M. 2000. Mixed-effects models in S and S-plus. 528 p New York, NY: Springer [Google Scholar]
- 26.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 27.Mysterud A., Tryjanowski P., Panek M. 2006. Selectivity of harvesting differs between local and foreign roe deer hunters: trophy stalkers have the first shot at the right place. Biol. Lett. 2, 632–635 10.1098/rsbl.2006.0533 (doi:10.1098/rsbl.2006.0533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garel M., Cugnasse J.-M., Maillard D., Gaillard J.-M., Hewison A. J. M., Dubray D. 2007. Selective harvesting and habitat loss produce long-term life history changes in a mouflon population. Ecol. Appl. 17, 1607–1618 10.1890/06-0898.1 (doi:10.1890/06-0898.1) [DOI] [PubMed] [Google Scholar]