Abstract
The comb jelly Mertensia ovum, widely distributed in Arctic regions, has recently been discovered in the northern Baltic Sea. We show that M. ovum also exists in the central Baltic but that the population consists solely of small-sized larvae (less than 1.6 mm). Despite the absence of adults, eggs were abundant. Experiments revealed that the larvae were reproductively active. Egg production and anticipated mortality rates suggest a self-sustaining population. This is the first account of a ctenophore population entirely recruiting through larval reproduction (paedogenesis). We hypothesize that early reproduction is favoured over growth to compensate for high predation pressure.
Keywords: Mertensia ovum, comb jelly, reproduction, paedogenesis
1. Introduction
Reproduction before metamorphosis in the larval stage owing to delayed somatic growth (neoteny) or precocious maturation (paedogenesis) is known among amphibians and parthenogenetic insects [1]. In some extreme cases, natural populations consist exclusively of larvae [1].
Marine ctenophores are similarly capable of reproduction in the larval stage [2–5]. While larvae are normally defined as a non-reproductive developmental stage before metamorphosis, larval reproduction in ctenophores has been shown for both metamorphosing (Lobata) and non-metamorphosing (Cydippida) orders in their early life stage less than ca 2.7 mm [3–5]. In the cydippid Pleurobrachia spp., larval gonad structures are different from adult gonads and first reproduction has been described at a minimum size of 0.4 mm [3]. After larvae of both major orders reach a threshold size, reproduction ceases, animals rapidly grow and then become reproductive again as adults (6–10 mm) [4,5]. While metamorphosis in amphibians generally involves a habitat shift [6], ctenophore paedogenesis is linked to early age reproduction being favoured over growth [4,5] and has been hypothesized to compensate for high mortality [2–5]. However, this hypothesis has never been confirmed at population level in nature.
Recently, the Arctic cydippid ctenophore Mertensia ovum was discovered in the Baltic Sea possibly as a relict population form the former ice age [7]. While Arctic specimens measure up to 90 mm, M. ovum in the northern Baltic remain small (less than 6.5 mm) [7,8]. Here, we describe for the first time that a ctenophore population is recruiting solely through larval reproduction (paedogenesis). The lack of larger-sized specimens is hypothesized to be due to high predation pressure.
2. Material and methods
Zooplankton sampling was conducted (13 monthly cruises) during 2009/2010 in the central Baltic Sea (figure 1), with vertical (90 µm HYDRO-BIOS Kiel, Germany, 0.25 m−2 midi-MultiNet) hauls in five depth-strata from 70, 200 and 180 m for stations 1 to 3. At station 4, the total water column (17 m) was sampled without depth-resolution.
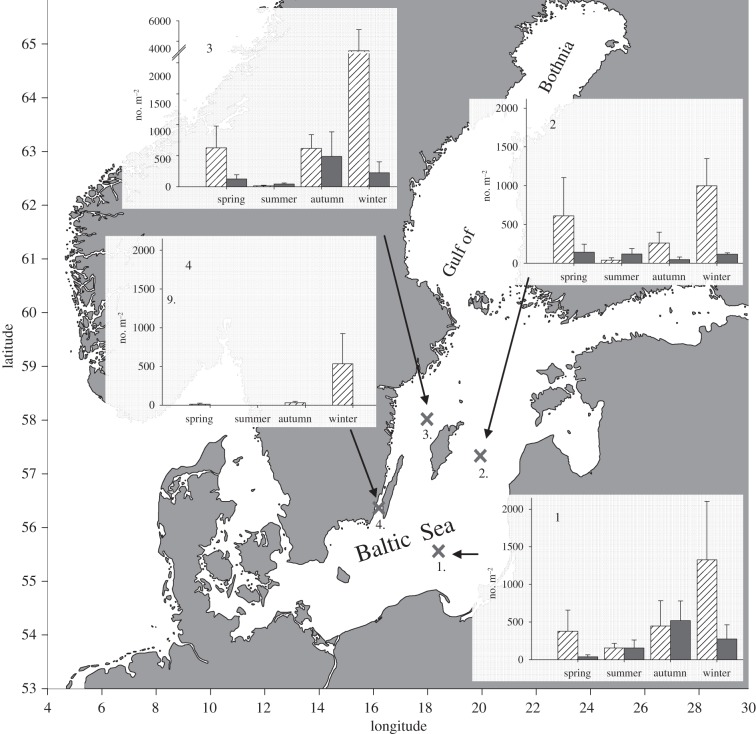
Figure 1.
Integrated seasonal Mertensia ovum larvae (bars with stripes) and egg (black bars) distribution in the central Baltic Sea, 2009/2010 (pooled for season ±s.d.).
Ctenophore eggs/larvae were measured, either live or after 2 per cent acidified Lugol preservation. Live versus preserved animal sizes (n = 1114) were compared. Preserved sizes were 75 ± 12.2% of unpreserved ones and were multiplied by 1.33 to correct for this shrinkage.
Ctenophore DNA from dried samples (n = 121) was verified using species-specific primers for the ITS-1 region of the ribosomal-RNA genes for M. ovum and invasive Mnemiopsis leidyi, respectively [9]. Analyses used standard PCR protocols (electronic supplementary material). All eggs are assumed to be M. ovum since no other ctenophore larvae (in the study of Gorokhova et al. [7, present study]) have been confirmed for this area.
For reproduction experiments (October 2009, station 2), larvae were collected with vertical 300 µm (MultiNet) tows (90–50 m) and incubated individually in 20 ml tissue-culture trays (Nunc Roskilde, Denmark) with 20 µm filtered water at 7 ± 1°C, salinity of 7. For 24 animals (0.54–1.33 mm), eggs were counted after 48 h with sizes based on averages (before/after the experiment). Regression analysis was performed for estimating size-dependent egg production.
Expected egg abundance at each station was estimated from the observed ctenophore size distribution and the size-dependent egg production, multiplied by the hatching time (T, days), excluding station 4 where eggs were never observed. Hatching time for M. leidyi eggs is 7 days (7°C, C. Jaspers 2010, unpublished data), and we assume the same for M. ovum. From this, expected egg abundance (squared metre) at each station was computed assuming no mortality. With no egg mortality, this computation should yield a larger observed egg abundance than expected at half the stations, on average. To test the hypothesis of zero mortality, the fraction of such stations was compared with 50 per cent using binomial statistics (electronic supplementary material).
3. Results
Ctenophores were present throughout the year (figure 1). Molecular analysis confirmed that the individuals sampled were M. ovum.
Population sizes peaked during winter with maximum abundances recorded at the deep-water, northernmost station 3. Ctenophores were not observed at the shallow-water station 4 during summer when water temperatures were high (ca 17°C). Mertensia ovum showed a difference in depth distribution with season, residing deeper during warm seasons, and distributed throughout the water column or in surface waters during cold seasons (electronic supplementary material, figure S1). Hence, the temperature range where larvae were observed was low (−0.3°C to 11.6°C).
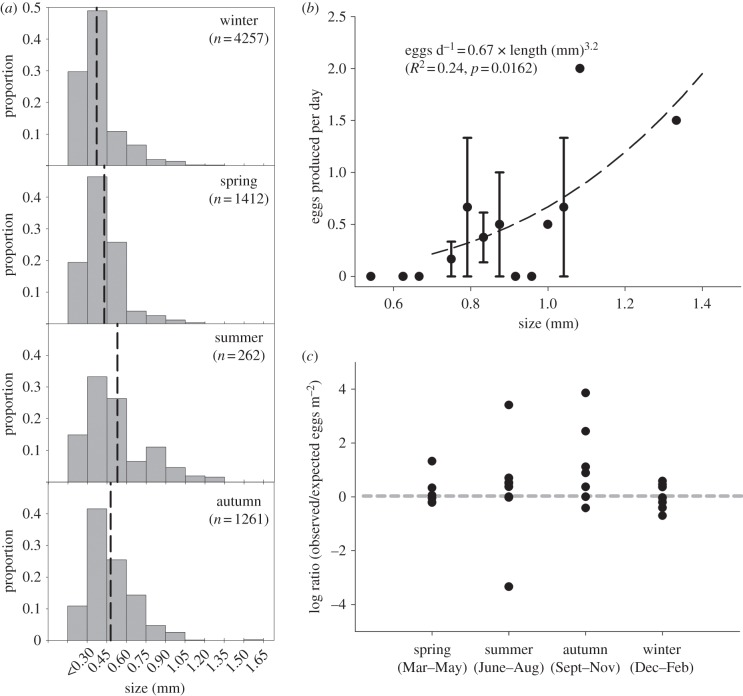
Size distributions differed significantly between seasons with the largest sizes during summer (figure. 2a). However, throughout the year and area, ctenophores were very small: 95 per cent of the population were less than 1.1 mm and the largest specimen found was 1.6 mm (n = 7192).
Figure 2.
(a) Seasonal size distribution of Mertensia ovum in the central Baltic (0.15 mm size bins, averages (dashed lines), log-transformed lengths significantly differed with season (one-way ANOVA F3,7845 = 821, p < 0.0001) (b) size-dependent egg production at 7 °C, October 2009 (average sizes ±s.e.) (c) log ratio of observed to expected egg abundance with zero observations substituted by 0.1 (log ratio = 0, no egg mortality). The fraction of stations where expected egg abundance exceeds observed egg abundance does not differ significantly from 50% (p = 0.36).
High numbers of ctenophore eggs were observed (figure 1). While larvae abundances were highest in winter, most eggs were present in autumn. Highest egg density was observed for the southernmost station 1, while at the shallow-water station 4 eggs were never found.
Reproduction experiments revealed that M. ovum greater than 0.75 mm produced eggs and production increased significantly with size (figure 2b). Overall, nine animals produced 22 eggs in 48 h−1, while 14 animals (0.54–1.04 mm) did not.
The fraction of stations with lower egg abundance than expected did not deviate significantly from 50 per cent, either for the entire sampling period or within seasons; hence egg mortality can be hypothesized to be zero (figure 2c).
4. Discussion
Our data show for the first time that a ctenophore population in nature consists entirely of small, larval size classes (less than 1.6 mm) throughout the year. We argue that our observations are consistent with a self-sustaining population maintained entirely through larval reproduction.
First, it is unlikely that the population in the central Baltic is supplied by advection from the Gulf of Bothnia in the north. In the north, M. ovum individuals are four times larger but their densities are only 1–8% compared with the central Baltic and they occur primarily at depth greater than 50 m [7]. Since the average residence time of water in the Gulf of Bothnia is 6 years and the southward advection mainly consists of surface water [10], drift recruitment is unlikely to be important.
Second, M. ovum larvae greater than 0.75 mm produce eggs at significant rates. Minimum size for larval reproduction in Pleurobrachia spp. is 0.4 mm [3], and 1.2 mm P. bachei produces eight eggs per day at 15°C [5]. The lobate M. leidyi (2 mm) produces up to 14 eggs per day at 22°C at high food concentrations [4]. Mertensia ovum (less than 1.3 mm) produces up to two eggs day. Considering the variation in temperature [11] and sizes, larval reproductive rates are similar for the different species and confirm our observation of reproducing larvae in the Baltic.
Finally, the observed egg production is sufficient to maintain the population in the face of likely mortality rates. The requirement for population maintenance is that the net reproductive rate R0 ≥ 1 for simultaneous hermaphrodites. Assuming an egg hatching time of T1, that it takes time T2 to grow to reproductive size whereupon growth is replaced by egg production at a constant rate (m), and that mortalities in the egg (δ1) and post-hatch (δ2) stages are constant, then R0 = exp(−δ1T1)(m/δ2)exp(−δ2T2) [12]. Using temperature-corrected maximum growth rates reported for M. leidyi larvae [11,13], it would take M. ovum about 8 days to reach 1 mm size at 7°C. If we insert the numbers relevant to our October reproduction experiments (δ1 = 0, T2 = 8 d, m = 0.7 d−1), then R0 > 1, as long as the mortality is less than 0.174 d−1. Faster growth and reproduction during summer allows for higher mortality, and conversely during winter. In general, mortality rates of similarly sized pelagic organisms are similar or less [14] and the observed larval fecundity is thus consistent with a self-sustaining population.
Why does M. ovum population in the central Baltic consists of only larvae that are 1–2 orders of magnitude smaller in length (3–6 orders in mass) than in the Arctic [7,8]? The temperature–size rule predicts that individual sizes within a species increase with decreasing temperatures, but a temperature difference as observed here of 10°C predicts only a 30 per cent difference in individual masses [15]. Similarly, marine species are often smaller in brackish water systems like the Baltic [16], but not to the degree observed here. Also, the largest M. ovum were observed in the least saline Gulf of Bothnia [7]. Therefore, neither temperature nor salinity can explain the observed differences.
Life-history theory predicts that the optimum age of maturation decreases with increasing juvenile mortality [17]. The trade-off is between maturing early at a small size and low fecundity but high chance of surviving to maturity versus maturing late at a large size and high reproduction but lower chance of reaching maturity. Our observation of early reproduction in M. ovum is consistent with the suggestion of high juvenile mortality. We substantiate this by a simple calculation. If juvenile growth in mass is a power function of time with an exponent of c = 2 as in the initial phase of the von Bertalanffy growth model [14], egg-production rate is proportional to individual mass (or length3, figure 2b), and growth ceases subsequent to start of egg production [4,5], then the maturation time yielding the highest R0 is c/δ2 [12]. Assuming δ2 = 0.17 d−1 as has been shown for less than 19 day larvae of the ctenophore Pleurobrachia bachei [18] and as calculated above, implies a development time of 12 days, close to the estimated time required to reach reproductive size. Higher temperatures may imply shorter maturation time but probably also higher mortality, and vice versa for lower temperatures.
Why would mortality be higher in the Baltic than in the Arctic where M. ovum are much bigger? We have no strongly substantiated explanation of this but note that planktivorous fish, potential predators on M. ovum, are abundant in the central Baltic Sea [19], and, more generally, that the relative significance of pelagic versus demersal fish increases from Arctic to temperate and tropical ecosystems [20]. The Baltic relict population of M. ovum [7] has had several thousand years to adapt to the local predation pressure.
In amphibians, a wide range of life-history strategies exist including facultative and obligate metamorphosis [1], and neoteny has been shown to be a response to density-dependent processes in newts with adult and larval reproduction occurring at the same age [6]. In extreme cases, natural populations are ‘trapped’ in the larval stage [1]. In insects, paedogenesis has been linked to optimum utilization of food patches, leading to short generation times in response to food availability [1]. In contrast, M. ovum paedogenesis is suggested to be caused by high predation pressure. However, very little is known about the potential predators on ctenophores in the Baltic, and this suggestion should be tested, e.g. through diet analyses and experiments with and without the presence of predators.
Acknowledgements
The project was funded by BONUS (BAZOOCA: project no. 210-2008-1882/-1889).
References
- 1.Gould S. J. 1977. Ontogeny and phylogeny, 1st edn London, UK: Belknap [Google Scholar]
- 2.Chun C. 1892. Die Disoogenie, eine Form der geschlechtlichen Zeugung In Festschrift zum siebzigsten Geburtstage (ed. Leuckarts R.), pp. 77–108 Leipzig, Germany: Engelmann [Google Scholar]
- 3.Garbe A. 1901. Untersuchung über die Entstehung der Geschlechtsorgane bei den Ctenophoren. Z. wiss. Zool. 69, 472–491 [Google Scholar]
- 4.Martindale M. Q. 1987. Larval reproduction in the ctenophore Mnemiopsis mccradyi (order Lobata). Mar. Biol. 94, 409–414 10.1007/BF00428247 (doi:10.1007/BF00428247) [DOI] [Google Scholar]
- 5.Hirota J. 1972. Laboratory culture and metabolism of the planktonic ctenophore, Pleurobrachia bachei. In Biological oceanography of the North Pacific (ed. Takenouti A. Y.), pp. 465–484 Tokyo, Japan: Idemitsu-Shoten [Google Scholar]
- 6.Harris R. N. 1987. Density-dependent paedomorphosis in the salamander Notophthalmus viridescens . Ecology 68, 705–712 10.2307/1938476 (doi:10.2307/1938476) [DOI] [Google Scholar]
- 7.Gorokhova E., Lehtiniemi M., Viitasalo-Frosen S., Haddock S. H. D. 2009. Molecular evidence for the occurrence of ctenophore Mertensia ovum in the northern Baltic Sea and implications for the status of the Mnemiopsis leidyi invasion. Limnol. Oceanogr. 54, 2025–2033 10.4319/lo.2009.54.6.2025 (doi:10.4319/lo.2009.54.6.2025) [DOI] [Google Scholar]
- 8.Matsumoto G. 1991. Functional morphology and locomotion of the Arctic ctenophore Mertensia ovum (Fabricius) (Tentaculata: Cydippida). Sarsia 76, 177–185 [Google Scholar]
- 9.Reusch T. B. H., Bolte S., Sparwel M., Moss A., Javidpour J. 2010. Microsatellites reveal origin and genetic diversity of Eurasian invasions by one of the world's most notorious marine invader, Mnemiopsis leidyi. Mol. Ecol. 19, 2690–2699 10.1111/j.1365-294X.2010.04701.x (doi:10.1111/j.1365-294X.2010.04701.x) [DOI] [PubMed] [Google Scholar]
- 10.Myrberg K., Andrejev O. 2006. Modelling of the circulation, water exchange and water age properties of the Gulf of Bothnia. Oceanologia 48, 55–74 [Google Scholar]
- 11.Hansen P. J., Bjornsen P., Hansen B.W. 1997. Zooplankton grazing and growth: scaling within the 2–2,000-µm body size range. Limnol. Oceanogr. 42, 687–704 10.4319/lo.1997.42.4.0687 (doi:10.4319/lo.1997.42.4.0687) [DOI] [Google Scholar]
- 12.Kiørboe T., Hirst A. 2008. Optimum development time in pelagic copepods. Mar. Ecol. Prog. Ser. 367, 15–22 10.3354/meps07572 (doi:10.3354/meps07572) [DOI] [Google Scholar]
- 13.Baker L., Reeve M. 1974. Laboratory culture of lobate ctenophore Mnemiopsis with notes on feeding and fecundity. Mar. Biol. 26, 57–62 10.1007/BF00389086 (doi:10.1007/BF00389086) [DOI] [Google Scholar]
- 14.Kiørboe T. 2008. A mechanistic approach to plankton ecology, 1st edn Oxford, UK: Princeton University Press [Google Scholar]
- 15.Forster J., Hirst A., Atkinson D. 2011. How do organisms change size with changing temperature? Funct. Ecol. 25, 1024–1031 10.1111/j.1365-2435.2011.01852.x (doi:10.1111/j.1365-2435.2011.01852.x) [DOI] [Google Scholar]
- 16.Kautsky N., Tedengren M. 1992. Ecophysiological strategies in Baltic Sea invertebrates. In Proc. 12th Baltic Marine Biologists Symp. 25–30 August 1991, Helsingør, Denmark, pp. 91–96 Fredensborg, Denmark: Olsen & Olsen [Google Scholar]
- 17.Stearns S. C. 1992. The evolution of life histories, 1st edn Oxford, UK: Oxford University Press [Google Scholar]
- 18.Hirota J. 1974. Quantitative natural history of Pleurobrachia bachei in La Jolla Bight. Fish. Bull. 72, 295–335 [Google Scholar]
- 19.Casini M., Lovgren J., Hjelm J., Cardinale M., Molinero J. C., Kornilovs G. 2008. Multi-level trophic cascades in a heavily exploited open marine ecosystem. Proc. R. Soc. B 275, 1793–1801 10.1098/rspb.2007.1752 (doi:10.1098/rspb.2007.1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen H., Curtis M. 1980. Difference in energy flows through major components of subarctic, temperate and tropical marine shelf ecosystems. Dana 1, 53–64 [Google Scholar]