Abstract
Mammalian torpor saves enormous amounts of energy, but a widely assumed cost of torpor is immobility and therefore vulnerability to predators. Contrary to this assumption, some small marsupial mammals in the wild move while torpid at low body temperatures to basking sites, thereby minimizing energy expenditure during arousal. Hence, we quantified how mammalian locomotor performance is affected by body temperature. The three small marsupial species tested, known to use torpor and basking in the wild, could move while torpid at body temperatures as low as 14.8–17.9°C. Speed was a sigmoid function of body temperature, but body temperature effects on running speed were greater than those in an ectothermic lizard used for comparison. We provide the first quantitative data of movement at low body temperature in mammals, which have survival implications for wild heterothermic mammals, as directional movement at low body temperature permits both basking and predator avoidance.
Keywords: body temperature, lizard, marsupial, running speed, torpor
1. Introduction
Locomotor performance is fundamental for many behaviours, including predator avoidance, territoriality, mating interactions and foraging, and therefore directly relates to an animal's fitness and survival [1,2]. Locomotor performance has been examined in detail with regard to size and maximum aerobic capacity in mammals, whereas temperature effects on running speed over a wide range of body temperatures (Tb) have been quantified only in ectotherms, such as lizards [1,3]. However, low Tb are also expressed in many heterothermic mammals and birds during torpor that is characterized by controlled and pronounced reductions of Tb and metabolism [4]. While torpor is the most effective means for energy conservation available to endotherms [4–7] and torpid animals can sense external stimuli [8], its widely assumed downside is a lack of directional movement [9], and thus an increased vulnerability to predation [10]. In contrast, recent studies have shown that small arid zone marsupials and elephant shrews (Macroscelidea), which regularly use torpor in the wild, are in fact capable of moving while torpid at low Tb. These mammals move to basking sites and expose themselves to solar radiation to passively rewarm from torpor, which minimizes the energetic costs of raising Tb at the end of a torpor bout [11–14]. Currently, there are no data on the effects of low Tb, characteristic of torpor, on running speed in mammals. A previous study on round-tailed ground squirrels (Spermophilus tereticaudus) revealed no difference in speed over a 11°C Tb range, however, the minimum Tb measured was 30°C [15], which is above that often used to define torpor (Tb < 30°C).
As locomotor function is crucial for movement in some torpid mammals, both when moving to basking sites and/or avoiding predators, we investigated running speed as a function of Tb in three small (11.7–35 g) dasyurid marsupials that use torpor and basking in the wild [14,16,17]. A similar-sized agamid lizard was examined to provide a comparison with an ectothermic species.
2. Material and methods
Our study animals were four kalutas (Dasykaluta rosamondae, mean body mass ± s.d.; 35.1 ± 2.0 g) trapped in Port Hedland, Western Australia (20°18′ S, 118°36′ E), six captive-bred dunnarts (Sminthopsis crassicaudata, 17.5 ± 1.9 g), five planigales (Planigale gilesi, 11.7 ± 1.3 g) captured at Kinchega National Park, New South Wales, Australia (32°32′ S, 142°17′ E) and three jacky lizards (Amphibolurus muricatus, 24.2 ± 8.7 g) caught near Armidale, New South Wales, Australia (30°32′ S, 151°40′ E).
All animals were run on an illuminated 5 m × 20 cm running track and were videotaped with a Samsung digital camera to determine running speeds (for detailed methods, see the electronic supplementary material). Running speed was recorded over a range of Tb that was measured using small implantable temperature-sensitive transmitters or using a thermocouple to measure the rectal/cloacal temperature.
Torpor was induced in the marsupials to obtain running speed at low Tb by exposing them to low ambient temperatures (Ta) and removing food overnight. To achieve the desired Tb in the lizards, animals were placed into temperature-controlled cabinets for more than 2 h with a Ta range of 5–38°C. For both mammals and lizards, Tb was measured immediately before each run, and running speed was recorded over a range of Tb, as the animal re-warmed. Maximum running speed was recorded for each Tb over a distance of high continuous running speed.
3. Results
All animals were able to move at low Tb (kalutas 17.9°C; dunnarts 15.3°C; planigales 14.8°C; lizards 8.4°C) and exhibited similar sigmoid curves of running speed against Tb (figure 1). Running speed was significantly affected by Tb (ANOVA with mixed procedure; kalutas: F18,22 = 24.14, p < 0.001; dunnarts: F22,41 = 57.82, p < 0.001; planigales: F25,51 = 154.13, p < 0.001; lizards: F24,19 = 38.77, p < 0.001); individuals of each species did not differ in their thermal response (ANOVA with mixed procedure; kalutas: F1,2 = 4.62, p = 0.16; dunnarts: F1,4 = 0.00, p = 0.96; planigales: F1,3 = 6.63, p = 0.08; lizards: F1,1 = 5.86, p = 0.25).
Figure 1.
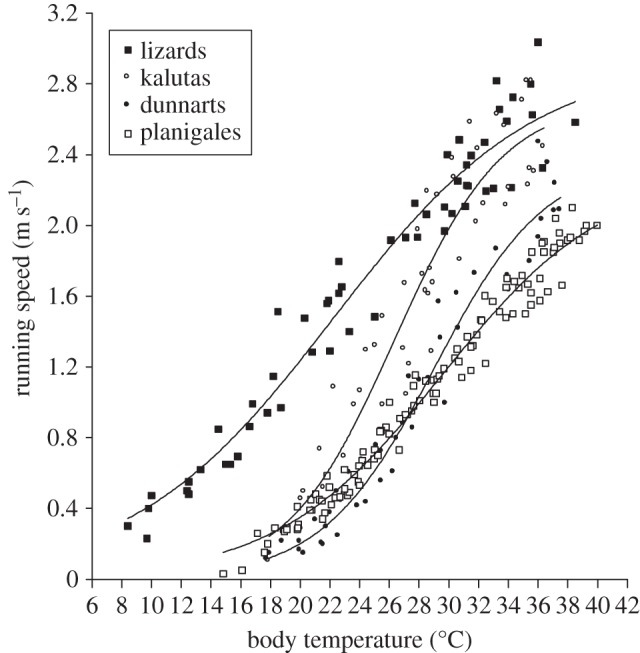
Running speeds over a range of body temperatures (Tb) of planigales, kalutas, dunnarts and lizards. Data points represent maximum values of individuals of a species at the measured Tb. Running speed against Tb was fitted with sigmoid curves: [planigales: y = 2.31/(1 − e−(x−29.53)/5.54); kalutas: y = 2.71/(1 + e−(x−26.37)/3.63); dunnarts: y = 2.38/(1 + e−(x−29.01)/3.77); lizards: y = 2.95/(1 + e−(x−22.21)/6.81)].
Maximum running speed ranged from 2 to 3 m s−1 and occurred between Tb 35°C and 40°C in all species. Although maximum running speeds in the small planigales were lower than in the larger kalutas and lizards (Tukey's, p < 0.05), running speed and body mass were only weakly correlated (linear regression; r2 = 0.44, p < 0.01), likely because of the small mass range. The effect of Tb on running speed differed significantly among species (ANOVA with mixed procedure; p < 0.05), with the exception of planigales and dunnarts (p > 0.05). At Tb 20°C, running speed differed significantly between the marsupials and the lizard (Tukey's, p < 0.05); in the marsupials, running speed was less than 0.5 m s−1, whereas in the lizards, running speed was approximately 1.5 m s−1. At Tb approximately 10°C, running speed in the lizards was less than 0.5 m s−1, similar to the marsupials at Tb approximately 20°C.
In all species, running speed at Tb 30–40°C showed a lower thermal dependence (low Q10 coefficients of 1.3–2.0) than at Tb 20–30°C (Q10 coefficients of 1.8–6.5; figure 2; paired t-test, p < 0.05). At Tb 30–40°C, Q10 were similar in most species, but differed in dunnarts and lizards (Tukey's, p < 0.05). In all species, the Q10 was higher at Tb 20–30°C in comparison with the Q10 at Tb 30–40°C and did not differ among the marsupials. However, the Q10 of kalutas and dunnarts at Tb 20–30°C were greater than those of the lizards (Tukey's, p < 0.05), whereas planigales and lizards were indistinguishable.
Figure 2.
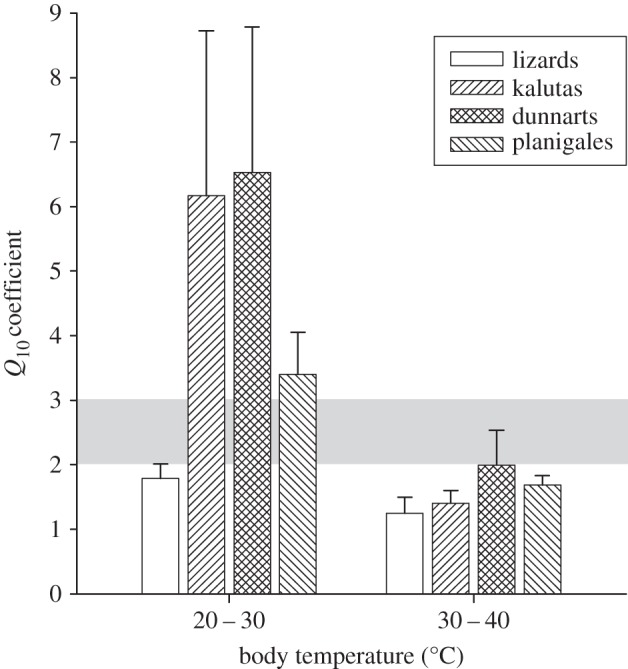
Q10 coefficients of running speed (mean ± s.d.) at Tb 20–30°C and Tb 30–40°C for planigales, kalutas, dunnarts and lizards. Shaded area corresponds to Q10 = 2–3.
4. Discussion
Our study provides the first quantitative data on locomotor performance at low Tb in mammals. We show that heterothermic mammals can move directionally while torpid at Tb as low as 14.8°C. The thermal response of locomotor performance we observed was qualitatively similar in marsupials and lizard, following sigmoid curves as in ectothermic organisms in general [18].
The maximum steepness of these sigmoid curves, with Q10 well beyond the generally accepted range for thermal dependence of biological functions (i.e. Q10 approx. 2–3), shows that running speed is not simply owing to temperature effects on generic biochemical reactions, but rather a complex interaction of neurological, muscular, metabolic and other functions affected differently by temperature [19]. The Q10 of maximum shortening velocity and maximum power output ranged between 1.7–2.4 in isolated mouse muscle between 20°C and 30°C [20], well below values we observed for running speed in our study. With regard to muscle performance of intact animals, important in the context of our study, temperature affects tetanic tension and neural transmission [21], but also affects muscle power output, thus limiting limb cycling frequency [22].
Although the temperature-dependence of locomotion in reptiles and mammals and maximum running speed was similar, functional differences were apparent. Running speeds of the mammals at Tb 20°C were similar to those of the lizards at Tb 10°C. Overall, running speed was less affected by Tb in the lizard (Q10 ≤ 2) than in mammals (Q10 1.8–6.5), and the lizard could move at lower Tb. This is likely related to the extent aerobic/anaerobic metabolism is used. Reptiles have high capacities for anaerobic metabolism enabling them to run at comparable speeds to mammals, but reptiles have less stamina [18]. By contrast, mammals rely heavily on aerobic metabolism and muscle activity is therefore dependent on a continuous blood supply. As cardiac function is strongly reduced at low Tb in heterothermic mammals [23], aerobic metabolism and running speed will be impaired.
Planigales were able to move at the lowest Tb measured, and running speed was less affected by Tb than in the other mammals, reflecting the lowest recorded Tb of 13.8°C observed in the species during basking [16]. To our knowledge, this is the lowest Tb at which purposeful and directional movement has been observed in any mammal in the wild. The difference in the minimum Tb at which movement was observed in the heterothermic mammals reflects different species-specific Tb minima that are metabolically defended during torpor [4,7]; in the lizard, it was the lowest Tb at which movement was recorded.
Reptiles must function effectively at low Tb to avoid predators and capture prey [24]. The main function of the ability by torpid mammals to move from shelters to the surface and seek sun exposure appears to be energy conservation, because small marsupials can save up to 80 per cent of arousal costs by basking [13]. Although basking potentially exposes animals to predators, these cool mammals do not, however, move far from their burrows or rock crevices and often stay motionless while basking [11,14]. They also remain highly alert and capable of astonishing mobility, including the ability to scale vertical cliffs [11,16] and therefore can escape from predators even at relatively low speed. Some other heterothermic mammals, such as bats, echidnas or bears forage even in the open at low Tb [6,25–27]. However, these species can avoid predation because they can either fly, have sharp spines or simply are fierce and large. Thus, it appears that movement at low Tb fulfils important functions in many heterothermic mammals, but its use is adjusted according to the predation pressure experienced in the wild.
Acknowledgments
The study was approved by the Animal Ethics Committee at the University of New England.
We thank Alex Riek for his help with the statistics, Paul McDonald for comments on the manuscript and Rob Hart for help with running the animals. We also thank Lisa Warnecke for allowing us to use her planigales, and Christine Cooper and Phil Withers for their kalutas. The work was supported by a grant from the ARC to F.G.
References
- 1.Bennett A. F., Dawson W. R. 1972. Aerobic and anaerobic metabolism during activity in the lizard Dipsosaurus dorsalis. J. Comp. Physiol. A 81, 289–299 10.1007/BF00693633 (doi:10.1007/BF00693633) [DOI] [Google Scholar]
- 2.Rezende E. L., Gomes F. R., Chappell M. A., Garland T., Jr 2009. Running behaviour and its energy cost in mice selectively bred for high voluntary locomotor activity. Physiol. Biochem. Zool. 82, 662–679 10.1086/605917 (doi:10.1086/605917) [DOI] [PubMed] [Google Scholar]
- 3.Garland T., Jr 1983. The relation between maximal running speed and body mass in terrestrial mammals. J. Zool. Lond. 199, 157–170 10.1111/j.1469-7998.1983.tb02087.x (doi:10.1111/j.1469-7998.1983.tb02087.x) [DOI] [Google Scholar]
- 4.Boyer B. B., Barnes B. M. 1999. Molecular and metabolic aspects of mammalian hibernation. Bioscience 49, 713–724 10.2307/1313595 (doi:10.2307/1313595) [DOI] [Google Scholar]
- 5.Körtner G., Brigham R. M., Geiser F. 2000. Metabolism: winter torpor in a large bird. Nature 407, 318. 10.1038/35030297 (doi:10.1038/35030297) [DOI] [PubMed] [Google Scholar]
- 6.Tøien Ø., Blake J., Edgar D. M., Grahn D. A., Heller H. C., Barnes B. M. 2011. Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331, 906–909 10.1126/science.1199435 (doi:10.1126/science.1199435) [DOI] [PubMed] [Google Scholar]
- 7.Geiser F. 2004. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274 10.1146/annurev.physiol.66.032102.115105 (doi:10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- 8.Pengelley E. T., Fisher K. C. 1968. Ability of the ground squirrel, Citellus lateralis, to be habituated to stimuli while in hibernation. J. Mammal. 49, 561–562 10.2307/1378234 (doi:10.2307/1378234) [DOI] [PubMed] [Google Scholar]
- 9.IUPS Thermal Commission. 2003. Glossary of terms for thermal physiology. J. Therm. Biol. 28, 75–106 10.1016/S0306-4565(02)00055-4 (doi:10.1016/S0306-4565(02)00055-4) [DOI] [Google Scholar]
- 10.Estók P., Zsebõk S., Siemers B. 2010. Great tits search for, capture, kill and eat hibernating bats. Biol. Lett. 6, 59–62 10.1098/rsbl.2009.0611 (doi:10.1098/rsbl.2009.0611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiser F., Goodship N., Pavey C. R. 2002. Was basking important in the evolution of mammalian endothermy? Naturwissenschaften 89, 412–414 10.1007/s00114-002-0349-4 (doi:10.1007/s00114-002-0349-4) [DOI] [PubMed] [Google Scholar]
- 12.Mzilikazi N., Lovegrove B. G., Ribble D. O. 2002. Exogenous passive heating during torpor arousal in free-ranging rock elephant shrews Elephantulus myurus. Oecologia 133, 307–314 10.1007/s00442-002-1052-z (doi:10.1007/s00442-002-1052-z) [DOI] [PubMed] [Google Scholar]
- 13.Geiser F., Drury R. L. 2003. Radiant heat affects thermoregulation and energy expenditure during rewarming from torpor. J. Comp. Physiol. B 173, 55–60 10.1007/s00360-002-0311-y (doi:10.1007/s00360-002-0311-y) [DOI] [PubMed] [Google Scholar]
- 14.Warnecke L., Turner J. M., Geiser F. 2008. Torpor and basking in a small arid zone marsupial. Naturwissenschaften 95, 73–78 10.1007/s00114-007-0293-4 (doi:10.1007/s00114-007-0293-4) [DOI] [PubMed] [Google Scholar]
- 15.Wooden K. M., Walsberg G. E. 2004. Body temperature and locomotor capacity in a heterothermic rodent. J. Exp. Biol. 207, 41–46 10.1242/jeb.00717 (doi:10.1242/jeb.00717) [DOI] [PubMed] [Google Scholar]
- 16.Warnecke L., Geiser F. 2009. Basking behaviour and torpor use in free-ranging Planigale gilesi. Aust. J. Zool. 57, 373–375 10.1071/ZO09097 (doi:10.1071/ZO09097) [DOI] [Google Scholar]
- 17.Körtner G., Rojas A. D., Geiser F. 2010. Thermal biology, torpor use and activity patterns of a small diurnal marsupial from a tropical desert: sexual differences. J. Comp. Physiol. B 180, 869–876 10.1007/s00360-010-0459-9 (doi:10.1007/s00360-010-0459-9) [DOI] [PubMed] [Google Scholar]
- 18.Bennett A. F. 1980. The thermal dependence of lizard behaviour. Anim. Behav. 28, 752–762 10.1016/S0003-3472(80)80135-7 (doi:10.1016/S0003-3472(80)80135-7) [DOI] [Google Scholar]
- 19.Rome L. C., Loughna P. T., Goldspink G. 1985. Temperature acclimation: improved sustained swimming performance in carp at low temperatures. Science 228, 194–196 10.1126/science.228.4696.194 (doi:10.1126/science.228.4696.194) [DOI] [PubMed] [Google Scholar]
- 20.Barclay C. J., Woledge R. C., Curtin N. A. 2010. Is the efficiency of mammalian (mouse) skeletal muscle temperature dependent? J. Physiol. 588, 3819–3831 10.1113/jphysiol.2010.192799 (doi:10.1113/jphysiol.2010.192799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullingham P. J., Lind A. R., Morton R. J. 1960. The maximal isometric tetanic tensions developed by mammalian muscle, in situ, at different temperatures. Q. J. Exp. Physiol. CMS 45, 142–156 [DOI] [PubMed] [Google Scholar]
- 22.Swoap S. J., Johnson T. P., Josephson R. K., Bennett A. F. 1993. Temperature, muscle power output and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J. Exp. Biol. 174, 185–197 [Google Scholar]
- 23.Geiser F., Baudinette R. V., McMurchie E. J. 1989. The effect of temperature on isolated perfused hearts of heterothermic marsupials. Comp. Biochem. Physiol. A 93, 331–335 10.1016/0300-9629(89)90046-7 (doi:10.1016/0300-9629(89)90046-7) [DOI] [PubMed] [Google Scholar]
- 24.Cury de Barros F., Eduardo de Carvalho J., Abe A. S., Kohlsdorf T. 2010. Fight versus flight: the interaction of temperature and body size determines antipredator behaviour in tegu lizards. Anim. Behav. 79, 83–88 10.1016/j.anbehav.2009.10.006 (doi:10.1016/j.anbehav.2009.10.006) [DOI] [Google Scholar]
- 25.Brice P. H., Grigg G. C., Beard L. A., Donovan J. A. 2002. Patterns of activity and inactivity in echidnas (Tachyglossus aculeatus) free-ranging in a hot dry climate: correlates with ambient temperature, time of day and season. Aust. J. Zool. 50, 461–475 10.1071/ZO01080 (doi:10.1071/ZO01080) [DOI] [Google Scholar]
- 26.Willis C. K. R., Brigham R. M. 2003. Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J. Comp. Physiol. B 173, 379–389 10.1007/s00360-003-0343-y (doi:10.1007/s00360-003-0343-y) [DOI] [PubMed] [Google Scholar]
- 27.Morrow G., Nicol S. C. 2009. Cool sex? Hibernation and reproduction overlap in the echidna. PLoS ONE 4, e6070. 10.1371/journal.pone.0006070 (doi:10.1371/journal.pone.0006070) [DOI] [PMC free article] [PubMed] [Google Scholar]


