Abstract
Males of sexually cannibalistic spiders commonly mutilate parts of their paired genitals (palps) during copulation, which may result in complete emasculation or the ‘eunuch phenomenon’. In an orb-web nephilid spider, Nephilengys malabarensis, about 75 per cent of males fall victim to sexual cannibalism, and the surviving males become half-eunuchs (one palp emasculated) or full-eunuchs (both palps emasculated). While it has been shown that surviving eunuchs are better fighters compared with intact males when guarding the females with which they have mated, mechanisms behind eunuchs’ superior fighting abilities are unknown. The previously proposed ‘gloves-off’ hypothesis, attributing eunuchs’ enhanced locomotor endurance to the reduction in total body weight caused by genital mutilation, is plausible but has remained untested. Here, we tested the gloves-off hypothesis in N. malabarensis by comparing the time until exhaustion (i.e. endurance) of intact males with half- and full-eunuchs created experimentally. We found that by reducing body weight up to 4 per cent in half-eunuchs and 9 per cent in full-eunuchs through emasculation, endurance increases significantly in half-eunuchs (32%) and particularly strongly in full-eunuchs (80%). Our results corroborate the gloves-off hypothesis and further point towards the adaptive significance of male emasculation.
Keywords: genital mutilation, sexual cannibalism, Nephilengys malabarensis, orb-web spider, endurance
1. Introduction
Traditionally, males are seen as polygynous with limited investment in parental caring [1]. Nevertheless, examples of monogyny where males provide only sperm are common in insects and spiders. In the latter case, males mate with one or two females (spiders have paired genitalia) [2–4]. Theory predicts that monogamous males secure their paternity either by out-competing rivals in sperm competition, and/or by limiting the probability of female remating [2,5]. A variety of male behaviours thus co-occur with monogyny, including mate guarding, male sacrifice to a cannibalistic female, genital mutilation and plugging of female genitals, and mechanisms that increase the quantity of transferred sperm [4,6,7].
In spiders, male genital (palp) mutilation is common in monogynous/bigynous and highly sexually cannibalistic species [7], typically involving breakage of palp parts, which remain lodged within the female genitals. Such mating plugs may increase male paternity and reduce sperm competition [7]. A more extreme genital mutilation is total palp severance, also known as the ‘eunuch phenomenon’, which is limited to the families Nephilidae [8–12] and Theridiidae [13]. The end result of this behaviour is partially (‘half-eunuchs’ with a single remaining palp) or fully castrated males (‘full-eunuchs’) [11,12]. Because eunuchs are effectively sterile, emasculation seems maladaptive. Nevertheless, adaptive hypotheses for the eunuch phenomenon do exist, including the mating plug hypothesis [8], the better fighter hypothesis [8,12] and the remote copulation hypothesis [13–15]. These three hypotheses have been experimentally tested and supported in Nephilengys malabarensis, a highly sexually dimorphic and cannibalistic nephilid spider known for obligate self emasculation during mating and intense post-copulatory mate guarding in surviving eunuchs [12,14,15].
In a recent study, about 25 per cent of N. malabarensis males survived mating, became half-eunuchs (one palp emasculated) or full-eunuchs (both palps emasculated), continued guarding the females intensively, and these eunuchs were found to win contests against intact males [14]. However, proximate mechanisms behind better fighting abilities in eunuchs remain unknown. A previous study on Tidarren sisyphoides found that males were more agile and mobile showing higher endurance (time until exhaustion) after palp removal, because the palp represents a disproportionally high body weight share [16]. Tidarren sisyphoides males emasculate one palp before mating, whereas N. malabarensis males break off their palps during mating. It is unclear whether N. malabarensis eunuchs’ superior fighting abilities [14] are also directly related to increased endurance capacity after the removal of their disproportionately large palps [16], i.e. the ‘gloves-off’ hypothesis [12]. Therefore, we experimentally tested this hypothesis using N. malabarensis by comparing the physical stamina—or endurance capacity—of intact, half- and full-eunuch males.
2. Material and methods
(a). Study subjects
Subadult N. malabarensis males were collected in Singapore and housed in a laboratory under controlled environment, kept individually in vials and fed with Drosophila melanogaster twice a week [14]. They were monitored daily until adulthood to control for their virginity and post-maturity age (defined as days elapsed from the final moult until the day of trial).
(b). Experimental procedure
Virgin males with similar post-maturity age were divided into three groups at random: intact males (n = 9), half-eunuchs (n = 6) and full-eunuchs (n = 9). To obtain half- and full-eunuchs, we experimentally removed one or two palps from the males, respectively, using forceps and razor blade to tear off the palp tarsus under a light microscope. Our experimental severance mimics the location of natural emasculation [9]. To estimate the reduction of weight after palp removal, all males from half- and full-eunuch groups were weighed immediately before and after the experimental removal of the palp(s), while intact males were weighed twice.
To test the gloves-off hypothesis, we measured the time (min) until exhaustion (i.e. endurance) of males with two (intact), one (half-eunuch) and no (full-eunuch) palps following earlier studies [16]. For each trial, a test male (intact, half- or full-eunuch) was placed in a plastic box (20 × 15 × 11 cm). A trial began when the test male started to move. Continuous motion was ensured by disturbing the male with a paint brush whenever it stopped moving. As the trial proceeded, the test male became increasingly tired and hence reluctant to move. Repeated touches were needed to ensure constant motion. The trial ended as the test male remained stationary despite five continuous touches, when it was deemed exhausted. The time elapsed from the first move to the time of exhaustion was taken to represent endurance capacity [16]. All trials were recorded using a digital HD camcorder.
(c). Data analysis
We performed generalized linear models (GLM) to analyse the effects of three independent variables (number of palps, post-maturity age and male body weight before the tests) on endurance (for data, see electronic supplementary material, table S1). For potential confounders in determining the endurance of the males, male body weight before the tests and male post-maturity age were used as covariates. The maximal models, including all three factors were fitted to a normal distribution. The Akaike information criterion (AIC: the smallest is the best) was used to select the best model.
3. Results
Before palp removal, there was no significant difference in male body weight for individuals that were later used as intact males, half- and full-eunuchs (ANOVA: F2,21 = 2.07, p = 0.151). After palp removal, both palps on average represented 9 (±2)% of the whole male body weight, and the remaining palp accounted for 4 (±1)% (figure 1a), hence palp(s) represented a significant proportion of males’ body weight (Kruskal–Wallis test: 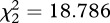 , N = 24, p < 0.0001). Full-eunuchs exhibited a significantly greater weight change than half-eunuchs and intact males (both comparisons: p < 0.05), but there was no significant difference in weight change between half-eunuchs and intact males (p = 0.193).
, N = 24, p < 0.0001). Full-eunuchs exhibited a significantly greater weight change than half-eunuchs and intact males (both comparisons: p < 0.05), but there was no significant difference in weight change between half-eunuchs and intact males (p = 0.193).
Figure 1.
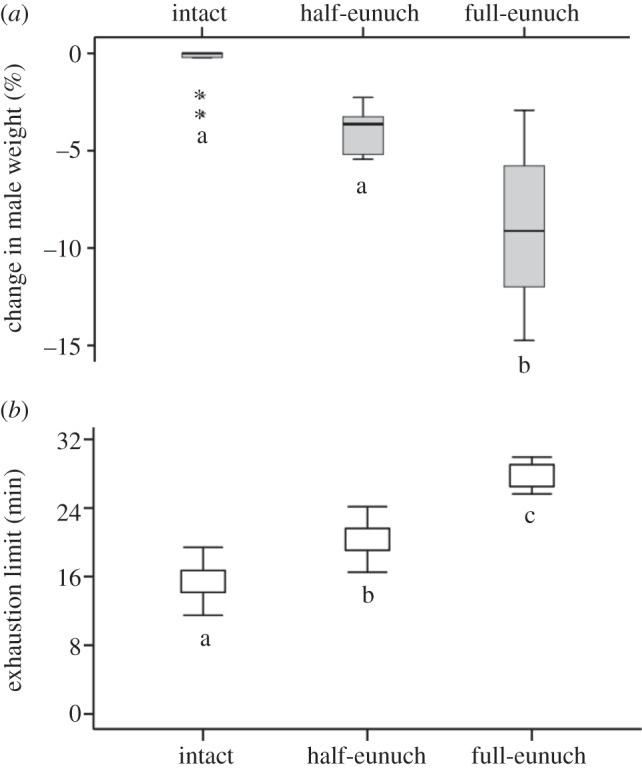
Change (%) in male body weight after the experimental removal of the palp(s) and the exhaustion limit (s) (i.e. endurance capacity) of intact males (n = 9), half- (n = 6) and full-eunuchs (n = 9). (a) Box plot of change (%) in male body weight after the palp removal. Boxes show median (line within the box) and upper (25%) and lower (75%) quartiles, whiskers indicate 5th and 95th percentiles, and starts are outliers. (b) Mean (±s.e.) exhaustion limit (min). Different letters below boxes indicate significant differences.
The number of palps (intact, half-eunuch and full-eunuch) significantly affected males’ endurance (Goodness of fit: AIC = 146.932; Omnibus test: χ2 = 26.396, d.f. = 4, p < 0.0001; table 1 and figure 1b). Full-eunuchs had significantly higher endurance capacities than half-eunuchs and intact males, whereas half-eunuchs had significantly higher endurance capacities than intact males (table 1). Half-eunuchs had endurance capacities that were 32 per cent greater than intact males, while full-eunuchs had endurance capacities that were 80 per cent greater than intact males. Male body weight before the tests, but not post-maturity age, had a significant effect on endurance capacities (table 1): lighter males had higher endurance capacities.
Table 1.
Results from a generalized linear model (GLM) testing the effects of three factors (the number of palps, male body weight before the tests and male post-maturity age) on the exhaustion limits (i.e. endurance). The number of palps was used as a categorical independent variable, while male body weight before the tests and male post-maturity age were used as covariates.
| exploratory factors | Wald χ2 | d.f. | p-value |
|---|---|---|---|
| number of palps | 40.053 | 2 | <0.0001 |
| full-eunuch (without palps) versus intact (with two palps) | 39.978 | 1 | <0.0001 |
| half-eunuch (with one palp) versus intact (with two palps) | 8.415 | 1 | 0.004 |
| male body weight before the tests | 5.039 | 1 | 0.025 |
| male post-maturity age | 1.163 | 1 | 0.281 |
4. Discussion
Palp removal produced significant reduction in male body weight in N. malabarensis and significantly enhanced physical stamina, thus increased endurance capacity. Hence, the gloves-off hypothesis [12] is supported as a possible mechanism behind eunuchs’ better fighting abilities [14].
While previous work supported the better fighter hypothesis explaining the eunuch adaptive significance in N. malabarensis [12], but [17], the mechanism underlying better fighting abilities remained unknown. A study of T. sisyphoides showed that males were more agile and mobile after palp removal, probably because palp(s) represent a disproportionally high total body weight share (ca 10%) [16]. Likewise, N. malabarensis palps represented 9 per cent of total male body weight; palp severance thus causes a significant body weight reduction. While our method of emasculation differed from the earlier study, the end effect on locomotor endurance is similar, and exaggerated in full-eunuchs in N. malabarensis.
Both studies point towards a functional conflict of possessing large palps, which are favourable for reproduction, but hinder effective locomotion. Emasculation significantly increased endurance of N. malabarensis males (32% in half-eunuchs and 80% in full-eunuchs), supporting the gloves-off prediction of palp removal enabling better locomotion abilities [14]. The significantly increased endurance of N. malabarensis after palp removal concurs with that observed in T. sisyphoides, where palp removal increased the distance travelled by 300 per cent [16]. While this better performance could also be due to palpal morphology actually interfering with locomotion on solid surfaces, our data, showing a significant reduction of eunuch's weight and his concurrent higher endurance, imply that palp weight bears significant physical costs and probably interferes with fighting abilities.
Increased endurance probably enables eunuchs to perform better in the contests with intact rivals [14,16]. Mating biology of N. malabarensis males consists of a plethora of mate-guarding and male–male agonistic behaviours [14] that are physically demanding; an elevated endurance will hence put eunuchs at an advantage over intact rivals. Enhanced endurance due to palp removal is plausibly the mechanism behind better fighting ability and hence mate-guarding success, but other mechanisms acting synergistically or in parallel could also be present. Mating and palp removal might co-occur with physiological changes that contribute to the enhanced aggressiveness of N. malabarensis eunuch males, but we currently lack such data. Male post-maturity age is unlikely to explain the observed endurance increase between males with different number of palps, because male age of different groups had no significant effect on the endurance (table 1).
In conclusion, while prior work has demonstrated that eunuch spiders are superior fighters, we here pinpoint a mechanism that enables eunuch's greater endurance. Our present results imply that palp weight poses significant physical costs to males, and thus support the gloves-off hypothesis.
Acknowledgements
The research was supported by the Ministry of Education (MOE) Academic Research Fund (AcRF) (R-154-000-435-112) to D.L., by the Slovenian Research Agency (J1-2063 and 1000-10-720023) and the Raffles Museum for Biodiversity Research Fellowship, National University of Singapore to M.K. and by Humboldt Return Fellowship to S.K.F. We thank Poh Moi Goh, Diego P. Araujo, Seok Ping Goh, Jun Hao Tang, Shichang Zhang, Mindy Tuan, Ganison s/o Rajamohan and Matjaž Gregorič for their support and help. Spiders were collected under research permit (NP/RP10-036) granted by the NParks.
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Fromhage L., Elgar M. A., Schneider J. M. 2005. Faithful without care: the evolution of monogyny. Evolution 59, 1400–1405 [PubMed] [Google Scholar]
- 3.Hosken D.J., Stockley P., Tregenza T., Wedell N. 2009. Monogamy and the battle of the sexes. Annu. Rev. Entomol. 54, 361–378 10.1146/annurev.ento.54.110807.090608 (doi:10.1146/annurev.ento.54.110807.090608) [DOI] [PubMed] [Google Scholar]
- 4.Schneider J. M., Fromhage L. 2010. Mongynous mating strategies in spiders. In Animal behaviour: evolution and mechanisms (ed. Kappeler P. M.), pp. 441–464 Berlin, Germany: Springer [Google Scholar]
- 5.Fromhage L., Houston A. I., McNamara J. M. 2008. A model for the evolutionary maintenance of monogyny in spiders. J. Theor. Biol. 250, 524–531 10.1016/j.jtbi.2007.10.008 (doi:10.1016/j.jtbi.2007.10.008) [DOI] [PubMed] [Google Scholar]
- 6.Uhl G., Nessler S., Schneider J. 2009. Securing paternity in spiders? A review on occurrence and effects of mating plugs and male genital mutilation. Genetica 138, 75–104 10.1007/s10709-009-9388-5 (doi:10.1007/s10709-009-9388-5) [DOI] [PubMed] [Google Scholar]
- 7.Simmons L. W. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 8.Kuntner M. 2005. A revision of Herennia (Araneae: Nephilidae: Nephilinae), the Australasian ‘coin spiders’. Invert. System. 19, 391–436 10.1071/IS05024 (doi:10.1071/IS05024) [DOI] [Google Scholar]
- 9.Kuntner M. 2007. A monograph of Nephilengys, the pantropical ‘hermit spider’ (Araneae, Nephilidae, Nephilinae). System. Entomol. 32, 95–135 10.1111/j.1365-3113.2006.00348.x (doi:10.1111/j.1365-3113.2006.00348.x) [DOI] [Google Scholar]
- 10.Kuntner M., Coddington J. A., Hormiga G. 2008. Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics 24, 147–217 10.1111/j.1096-0031.2007.00176.x (doi:10.1111/j.1096-0031.2007.00176.x) [DOI] [Google Scholar]
- 11.Kuntner M., Agnarsson I., Gregorič M. 2009. Nephilid spider eunuch phenomenon induced by female or rival male aggressiveness. J. Arachnol. 37, 266–271 10.1636/St08-67.1 (doi:10.1636/St08-67.1) [DOI] [Google Scholar]
- 12.Kuntner M., Kralj-Fišer S., Schneider J. M., Li D. 2009. Mate plugging via genital mutilation in nephilid spiders: an evolutionary hypothesis. J. Zool. 277, 257–266 10.1111/j.1469-7998.2008.00533.x (doi:10.1111/j.1469-7998.2008.00533.x) [DOI] [Google Scholar]
- 13.Knoflach B., van Harten A. 2001. Tidarren argo sp. nov. (Araneae: Theridiidae) and its exceptional copulatory behaviour: emasculation, male palp organ as a mating plug and sexual cannibalism. J. Zool. 254, 449–459 [Google Scholar]
- 14.Kralj-Fišer S., Gregorič M., Zhang S., Li D., Kuntner M. 2011. Eunuchs are better fighters. Anim. Behav. 81, 933–939 10.1016/j.anbehav.2011.02.010 (doi:10.1016/j.anbehav.2011.02.010) [DOI] [Google Scholar]
- 15.Li D., Oh J., Kralj-Fišer S., Kuntner M. 2012. Remote copulation: male adaptation to female cannibalism. Biol. Lett. 1, 1–3 10.1098/rsbl.2011.1202 (doi:10.1098/rsbl.2011.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos M., Irschick D. J., Christenson T. E. 2004. Overcoming an evolutionary conflict: removal of a reproductive organ greatly increases locomotor performance. Proc. Natl Acad. Sci. USA 101, 4883–4887 10.1073/pnas.0400324101 (doi:10.1073/pnas.0400324101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kralj-Fišer S., Kuntner M. 2012. Eunuchs as better fighters? Naturwissenschaften 99, 95–101 10.1007/s00114-011-0873-1 (doi:10.1007/s00114-011-0873-1) [DOI] [PubMed] [Google Scholar]


