Abstract
Population sizes and movement patterns of ungulate grazers and their predators have fluctuated dramatically over the past few centuries, largely owing to overharvesting, land-use change and historic management. We used δ13C and δ15N values measured from bone collagen of historic and recent gray wolves and their potential primary prey from Yellowstone National Park to gain insight into the trophic dynamics and nutrient conditions of historic and modern grasslands. The diet of reintroduced wolves closely parallels that of the historic population. We suggest that a significant shift in faunal δ15N values over the past century reflects impacts of anthropogenic environmental changes on grassland ecosystems, including grazer-mediated shifts in grassland nitrogen cycle processes.
Keywords: stable isotope, Yellowstone, Canis lupus, grasslands, historic
1. Introduction
Anthropogenic habitat modification, harvest of ungulate grazers and control of their predators over the past few centuries have caused dramatic fluctuations in the population sizes and movement patterns in many large terrestrial grazers. Grazers affect ecological structure and nutrient cycling in grasslands, so their loss or extreme reduction could have repercussions throughout the ecosystem [1,2].
Yellowstone National Park (YNP) in Wyoming illustrates this phenomenon. Populations of large grazers, including bison (Bison bison) and elk (Cervus elaphus), declined owing to human harvest during the late 1800s [3]. Predator reduction programmes led to the extirpation of gray wolves from YNP and surrounding areas by 1926. As part of a trend to restore habitats and ecosystems in some public areas, gray wolves (Canis lupus) were reintroduced to YNP in 1995 and 1996 [4]. In the context of carnivore restoration efforts, recent work has focused on understanding contemporary direct and indirect effects of wolves on Yellowstone prey populations and the ecosystem [5]. Implicit in these efforts is the assumption that the reintroduced wolves will fill the same ecological niche as the historic population.
Within an ecosystem, plant nitrogen isotope (δ15N) values generally reflect a combination of the δ15N values of their source nitrogen (N) pools and the isotopic legacy of N cycle processes [6]. Carbon (C) and N in animal collagen are derived from diet, and collagen δ13C and δ15N values reflect time-averaged diet over multiple years prior to death [7]. We analysed bone collagen δ13C and δ15N values from YNP fauna from two time periods in the past century when grazer population sizes were dramatically different [3]. We investigate whether food web isotope values may serve as a proxy for broader impacts of anthropogenic environmental changes on grassland ecosystems. Further, we estimate the diet of the historic and the modern wolves through comparison of the isotope composition of wolves with their known or putative prey to test the hypothesis that modern wolves consume the same prey as the extirpated population did historically.
2. Material and methods
Bone fragments from 14 historic and seven modern bison, 15 historic and 18 modern elk, two modern mule deer (Odocoileus hemionus), and six historic and 29 modern gray wolves from YNP were analysed (see electronic supplementary materials, table S1). The first interval is the early 1900s, when grazer populations were recovering from very low numbers in the late 1800s; the second is the late 1990s and early 2000s, when grazer numbers were high. Collagen was extracted and analysed for C and N stable isotopes as in Fox-Dobbs et al. [8]. Stable isotope compositions are referenced to Vienna Pee Dee Belemnite for C and air for N. The δ13C values from the historic material were corrected (−1.15‰) to account for temporal change in the δ13C value of atmospheric carbon dioxide [9].
Within-species changes in isotope values between time periods were assessed by one-way ANOVA. Relative contributions of putative prey to the diet of the recent wolf population were estimated using the Bayesian mixing model stable isotope analysis in R (SIAR) [10]. The only grazer available to historic wolves was elk (the small, historic bison population was fenced), and overlap between historic elk and wolf δ13C and δ15N distributions was assessed using MANOVA. High overlap suggests a predator–prey interaction. No historic mule deer specimens were available for this project. The same isotopic trophic differences (Δ13Cwolf-prey = 1.32 ± 0.6‰, Δ15Nwolf-prey = 4.64 ± 0.7‰) between wolf and prey collagen values were used in the modern and historic wolf dietary analyses [8].
3. Results
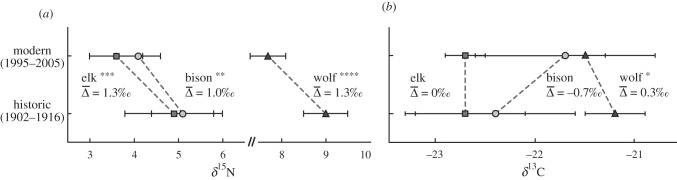
The δ15N values of grazers and wolves decreased significantly between the historic and contemporary intervals (one-way ANOVA): bison F1,19 = 10.9, p = 0.004; elk F1,31 = 19.5, p = 0.0001; and wolf F1,33 = 44.7, p < 0.0001 (figure 1a). Isotopic shifts that are similar in magnitude and occur simultaneously at multiple consumers and trophic levels suggest a change in the isotopic values of primary producers, not a change in consumer diets [11]. The absence of highly significant differences in faunal δ13C values between time periods (bison F1,19 = 3.6, p = 0.075; elk F1,31 = 0.13, p = 0.73; and wolf F1,33 = 5.0, p = 0.03; figure 1b) is important for two reasons. It supports the conclusion that we are not tracking changes in consumer diet composition, and it suggests that soil and plant δ13C values were not strongly regulated by the mechanism(s) responsible for the shift in faunal δ15N values.
Figure 1.
Mean (±s.d.) of (a) δ15N and (b) δ13C values (‰) for modern and historic YNP fauna. Mean differences in isotopic values between time periods (Δ) are included, and levels of significance are shown (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). On average, the estimated grazer (winter) population sizes on the northern range of YNP were greater than or equal to 10 times (bison) and greater than or equal to 2 times (elk) smaller during the historic versus modern time periods [3].
The SIAR analysis of modern YNP wolves suggested an average diet based primarily on elk (5% credibility interval of the posterior distribution = 80–98%, mean = 90%; figure 2a), as observed in winter wolf kill records [5]. Scat analyses suggest that summer wolf diet is more variable than winter diet, including a greater proportion of mule deer, and smaller mammals [12]. The same pattern of a primarily elk-based diet was deduced for the historic wolf population, because the distributions of isotopic values were indistinguishable between the two species (MANOVA, F2,18 = 0.72, p = 0.50; figure 2b). While we can be certain that bison were not part of wolf diet during the historic time period, we cannot rule out some contribution of mule deer. But based on a modern understanding of mule deer abundances and movement patterns, as well as their isotope values, mule deer were probably not a major fraction of historic wolf diet. Our data suggest that reintroduced wolves fill the same ecological niche as the historic wolves.
Figure 2.

Contributions of the potential prey to modern YNP wolf diet (a), as determined by a SIAR mixing model. Boxplots show the relative proportions of each prey with 95% (darkest grey), 75%, 25% and 5% (lightest grey) credibility intervals. Above/below each boxplot are estimates of prey contribution to wolf diet determined by SIAR model (5% credibility intervals) and observational records of winter wolf kills (1995–2004 average) [5]. Plot of historic YNP wolf and elk δ13C and δ15N values (‰) (b). Wolf isotopic values are shifted to account for trophic differences between wolf and prey collagen values [8].
4. Discussion
Recent work highlights the importance and role of the geohistorical perspective in modern ecology and conservation biology [13]. Yet, historical datasets are often bounded by taphonomic factors (natural and anthropogenic), and generally cannot be held to the same standards of evidence as neoecological datasets. Our approach is to catalogue dynamics that are well understood in comparable modern ecosystems, and use the principle of parsimony to suggest the most plausible mechanisms to explain historical data.
Because the overlap in habitat between YNP bison and elk occurs in grasslands, it is most likely that the shift in faunal δ15N values reflects changes in this habitat over the nineteenth century. Climatic factors, such as changes in temperature and precipitation in YNP over the last century [14], are less likely explanations for the shift in faunal δ15N values because they should also be recorded in plant, and thus grazer, δ13C values [15]. Furthermore, because bison and elk have seasonally different habitat preferences [16], it is doubtful that well-documented environmental changes in the greater YNP ecosystem (e.g. expansion of forests, reduction in riparian vegetation, occurrence of wildfires and introduced plant species; [3]), would be recorded similarly by both species’ δ15N values.
We focus on three anthropogenic factors that potentially affected YNP grassland δ15N values over the past century. First, sediment cores from regional lakes record the isotopic fingerprint (low δ15N values) of increased atmospheric deposition of anthropogenic N [17]. The isotopic signal of this change is less coherent in terrestrial ecosystems, and reported plant δ15N chronologies can increase or decrease over the same time period [18,19]. Second, rising atmospheric CO2 concentrations have been correlated to a decline in North American prairie foliar δ15N values, owing to an increase in ecosystem N storage (and decrease in plant available N) [20]. However, experimental data from YNP grasslands suggest that plant N and soil N cycling increase in plots where plant δ15N values decrease [1,2,21], all in response to higher grazer abundance. The third factor is the dramatic growth of native YNP grazer populations since 1900 (the winter number of bison has increased greater than or equal to 10 times, and elk greater than or equal to 2 times; [3]).
Plant and soil biogeochemical datasets collected from fenced exclosure plots suggest that grazers strongly affect N processes in YNP grasslands, and the temporal shift in faunal δ15N values we report is similar to plant δ15N values after several decades of grazer absence/presence [1]. Urine and dung deposition are a mechanisms by which grazers impact N processes, and urine addition in grazed plots results in lower grass δ15N values than in ungrazed plots at local and regional scales (mechanisms presented in earlier studies [1,22]). While our faunal data may serve as a proxy for changes in grazer–grassland interactions, it is difficult to fully disentangle the relative influences of grazer densities from baseline shifts driven by atmospheric conditions, because these factors have changed over the same timescale in YNP. Additional insight may be derived from similar datasets in the future, because YNP grazer population sizes are stabilized in part by the presence of reintroduced wolves, thus constraining this important variable.
Molecular work has documented the significant loss of genetic heritage in western North American large mammal populations owing to grazer overharvest and predator control over the past 150 years [23]. Our biogeochemical results now show that recent human activity may have permeated the ecosystem far beyond the large mammals. Because grazer populations were decimated across their historical range, this probably represents a widespread phenomenon. Our observation that reintroduced wolves rapidly filled the same dietary niche as historic wolves illustrate that reconstitution of ecological roles is possible within large mammal communities.
Acknowledgments
We thank the National Museum of Natural History and Yellowstone National Park for providing access to specimens. This project was supported by the National Science Foundation (OPP 0352634). Logistical support was provided by the Center for Conservation and Evolutionary Genetics, National Zoological Park, Smithsonian Institution.
References
- 1.Frank D. A., Evans R. D. 1997. Effects of native grazers on grassland N cycling in Yellowstone National Park. Ecology 78, 2238–2248 10.1890/0012-9658(1997)078[2238:EONGOG]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[2238:EONGOG]2.0.CO;2) [DOI] [Google Scholar]
- 2.Frank D. A., Groffman P. M. 1998. Ungulate versus landscape control of soil C and N processes in grasslands of Yellowstone National Park. Ecology 79, 2229–2241 10.1890/0012-9658(1998)079[2229:UVLCOS]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[2229:UVLCOS]2.0.CO;2) [DOI] [Google Scholar]
- 3.National Research Council. 2002. Ecological dynamics on Yellowstone's northern range. Washington, DC: National Academies Press [Google Scholar]
- 4.Bangs E. E., Fritts S. H. 1996. Reintroducing the gray wolf to central Idaho and Yellowstone National Park. Wildl. Soc. B. 24, 402–413 [Google Scholar]
- 5.Smith D. W. 2005. Ten years of Yellowstone wolves, 1995–2005. Yellowstone Sci. 13, 7–33 [Google Scholar]
- 6.Högberg P. 1997. Tansley Review No. 95 15N natural abundance in soil-plant systems. New Phytol. 137, 179–203 10.1111/j.1469-8137.1997.tb01211.x (doi:10.1111/j.1469-8137.1997.tb01211.x) [DOI] [PubMed] [Google Scholar]
- 7.Koch P. L. 2007. Isotopic study of the biology of modern and fossil vertebrates. In Stable isotopes in ecology and environmental science, 2 edn. (eds Michener B., Lajtha K.), pp. 99–154 Boston, MA: Blackwell Publishing [Google Scholar]
- 8.Fox-Dobbs K., Bump J. K., Peterson R. O., Fox D. L., Koch P. L. 2007. Carnivore-specific stable isotope variables and variation in the foraging ecology of past and present wolf populations: case studies from Isle Royale, Minnesota and La Brea. Can. J. Zool. 85, 458–471 [Google Scholar]
- 9.Chamberlain C. P., et al. 2005. Pleistocene to recent dietary shifts in California condors. Proc. Natl Acad. Sci. USA 102, 16 707–16 711 10.1073/pnas.0508529102 (doi:10.1073/pnas.0508529102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parnell A. C., Inger R., Bearhop S., Jackson A. L. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5, e9672. 10.1371/journal.pone.0009672 (doi:10.1371/journal.pone.0009672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bump J. K., Fox-Dobbs K., Bada J. L., Koch P. L., Peterson R. O., Vucetich J. A. 2007. Stable isotopes, ecological integration, and environmental change: wolves record atmospheric carbon isotope trend better than tree rings. Proc. R. Soc. B 274, 2471–2480 10.1098/rspb.2007.0700 (doi:10.1098/rspb.2007.0700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahler D. R., Smith D. W., Guernsey D. S. 2006. Foraging and feeding ecology of the gray wolf (Canis lupus): lessons from Yellowstone National Park, Wyoming, USA. J. Nutr. 136, 1923S–1926S [DOI] [PubMed] [Google Scholar]
- 13.Dietle G. P., Flessa K. W. 2011. Conservation paleobiology: putting the dead to work. Trends Ecol. Evol. 26, 30–37 10.1016/j.tree.2010.09.010 (doi:10.1016/j.tree.2010.09.010) [DOI] [PubMed] [Google Scholar]
- 14.Rocky Mountain Climate Organization 2011. Greater Yellowstone in peril: the threats of climate disruption. See http://www.rockymountainclimate.org/images/YellowstoneInPeril.pdf [Google Scholar]
- 15.Heaton T. H. E. 1999. Spatial, species, and temporal variations in the13C/12C ratios of C3 plants: implications for palaeodiet studies. J. Archaeol. Sci. 26, 637–649 10.1006/jasc.1998.0381 (doi:10.1006/jasc.1998.0381) [DOI] [Google Scholar]
- 16.Singer F. J., Norland J. E. 1994. Niche relationships within a guild of ungulate species in Yellowstone National Park, Wyoming, following release from artificial controls. Can. J. Zool. 72, 1383–1394 10.1139/z94-183 (doi:10.1139/z94-183) [DOI] [Google Scholar]
- 17.Holtgrieve G. W., et al. 2011. A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the northern hemisphere. Science 334, 1545–1548 10.1126/science.1212267 (doi:10.1126/science.1212267) [DOI] [PubMed] [Google Scholar]
- 18.Bukata A. R., Kyser K. R. 2007. Carbon and nitrogen isotope variations in tree-rings as records of perturbations in regional carbon and nitrogen cycles . Environ. Sci. Technol. 41, 1331–1338 10.1021/es061414g (doi:10.1021/es061414g) [DOI] [PubMed] [Google Scholar]
- 19.Hietz P., Turner B. L., Wanek W., Richter A., Nock C. A., Wright S. J. 2011. Long-term change in the nitrogen cycle of tropical forests. Science 334, 664–666 10.1126/science.1211979 (doi:10.1126/science.1211979) [DOI] [PubMed] [Google Scholar]
- 20.McLauchlan K. K., Ferguson C. J., Wilson I. E., Ocheltree T. W., Craine J. M. 2010. Thirteen decades of foliar isotopes indicate declining nitrogen availability in central North American grasslands. New Phytol. 187, 1135–1145 10.1111/j.1469-8137.2010.03322.x (doi:10.1111/j.1469-8137.2010.03322.x) [DOI] [PubMed] [Google Scholar]
- 21.Frank D. A. 2008. Ungulate and topographic control of nitrogen: phosphorus stoichiometry in a temperate grassland; soils, plants and mineralization rates. Oikos 117, 591–601 10.1111/j.0030-1299.2008.16220.x (doi:10.1111/j.0030-1299.2008.16220.x) [DOI] [Google Scholar]
- 22.Frank D. A., Evans R. D., Tracy B. F. 2004. The role of ammonia volatilization in controlling the natural 15N abundance of a grazed grassland. Biogeochemistry 68, 169–178 10.1023/B:BIOG.0000025736.19381.91 (doi:10.1023/B:BIOG.0000025736.19381.91) [DOI] [Google Scholar]
- 23.Leonard J. A., Vilà C., Wayne R. K. 2005. Legacy lost: genetic variability and population size of extirpated US gray wolves (Canis lupus). Mol. Ecol. 14, 9–17 10.1111/j.1365-294X.2004.02389.x (doi:10.1111/j.1365-294X.2004.02389.x) [DOI] [PubMed] [Google Scholar]