Abstract
Previous studies using thermal imaging have suggested that face and body temperature increase during periods of sexual arousal. Additionally, facial skin temperature changes are associated with other forms of emotional arousal, including fear and stress. This study investigated whether interpersonal social contact can elicit facial temperature changes. Study 1: infrared images were taken during a standardized interaction with a same- and opposite-sex experimenter using skin contact in a number of potentially high–intimate (face and chest) and low–intimate (arm and palm) locations. Facial skin temperatures significantly increased from baseline during the face and chest contact, and these temperature shifts were larger when contact was made by an opposite-sex experimenter. Study 2: the topography of facial temperature change was investigated in five regions: forehead, periorbital, nose, mouth and cheeks. Increased temperature in the periorbital, nose and mouth regions predicted overall facial temperature shifts to social contact. Our findings demonstrate skin temperature changes are a sensitive index of arousal during interpersonal interactions.
Keywords: thermal signature, arousal, face, sex difference
1. Introduction
A variety of affective states, such as aggression [1] and emotional arousal [2–5], have been shown to elicit thermal responses in the face suggesting that skin temperature may be indicative of affective/emotional states. For example, fear has been shown to cause a rapid (300 ms post-stimulus) increase in temperature in the periorbital region, with simultaneous cheek temperature decreases [6,7]. Stress in infants, caused by maternal separation, results in decreased forehead temperature [8,9]; while stress in adults (e.g. lying, performing difficult mental tasks) causes increases in skin temperature in the forehead [10] and periorbital regions [11], and temperature decreases in the jaw area [12].
Merla & Romani [12] tested facial thermal responses to stress, pain and sexual arousal/excitement in a male cohort. Pain and stress caused a decrease in facial temperature (particularly in the perioral region) while sexual arousal caused a temperature increase owing to increased facial blood perfusion rates, particularly in the forehead, mouth and lip regions. Thermographic measures in all conditions were correlated with other physiological measures of arousal (galvanic skin response, penile turgidity). These results suggest that specific thermal signatures may exist in relation to specific types of emotional arousal.
This study aimed to explore whether temperature changes occur during interpersonal social contact in the absence of any direct emotional manipulation. In study 1, we (i) measured thermal responses in response to social contact in a standardized setting, and (ii) determined if any such responses differ for inter- and intrasex social contact. In study 2, we assessed the topography of facial temperature changes in response to heterosexual social contact.
2. Material and methods
(a). Equipment
Thermal responses were measured using a Testo (881-1) thermal imager (FPA 160 × 120 a.Si, spectral range: 8–14 μm, thermal sensitivity (NETD): less than 80 mK, 1 frame per 75 s). Object emissivity was set at 0.98, the standard value for skin [13]. The camera captured a frontal view of the participant's head and chest. To preserve ecological validity, in study 1 the camera was placed out of direct view of the participant (distance of 1 m). In study 2, the camera was positioned 0.5 m from the face and participants were instructed to look at the camera and avoid movement whenever possible.
(b). Study 1
(i). Participants
Sixteen heterosexual, Caucasian women (age: M = 21 years, s.d. = 1.9, range = 19–24); six currently using hormonal contraceptives. Written consent to capture thermal images was obtained from all participants.
(ii). Procedure
During a 20-min acclimatization period, participants completed a demographics questionnaire and a filler task (viewing a series of emotionally neutral faces). The experiment was conducted in two separate, counterbalanced conditions; one involved interaction with a same-sex experimenter, the other with an opposite-sex experimenter (both peer-aged). During each condition, skin contact was made at different body locations (face × 3, arm × 3, palm, chest) using a handheld device that flashes light onto the skin under the guise of measuring skin colour (2 s of contact at each location). These measurements provided a standardized form of social interaction. Participants were given 15 min between conditions during which time a distracter task was given.
(iii). Image analysis
A set of six thermal images per condition was selected for analysis: two baseline images (averaged) taken prior to any experimenter contact, and the first image captured during facial contact (left cheekbone), outer arm contact (at the elbow), palm contact and chest contact (top of the sternum). All images were selected via visual inspection. To account for breathing artefacts, we ensured that participants' mouths were closed in all images selected. For each image, the maximum temperature within the facial region was assessed using Testo analysis software.
(c). Study 2
(i). Participants
Twenty-three heterosexual, Caucasian women (age: M = 20.3 years, s.d. = 1.9, range = 18–25); nine currently using hormonal contraceptives.
(ii). Procedure
The procedure for study 2 was the same as that of study 1, except only the opposite-sex experimenter interacted with the participant. Following interaction, all participants reported: excitement, embarrassment, discomfort, stress, sexual arousal and overall stimulation felt using a five-point Likert scale (table 1 for response distributions).
Table 1.
Self-reported arousal distributions. Values indicate the number of participant ratings given at each level of the Likert scale.
| response value |
|||||
|---|---|---|---|---|---|
| none |
high | ||||
| arousal type | 1 | 2 | 3 | 4 | 5 |
| excitement | 11 | 7 | 3 | 1 | 1 |
| embarrassment | 10 | 9 | 1 | 3 | 0 |
| discomfort | 10 | 8 | 4 | 1 | 0 |
| stress | 13 | 9 | 1 | 0 | 0 |
| sexual arousal | 19 | 1 | 2 | 1 | 0 |
| overall stimulation | 10 | 1 | 7 | 4 | 1 |
(iii). Image analysis
For each participant, a baseline image (before any contact) and the image captured immediately after contact were analysed to determine temperature changes resulting from social contact. Using a similar analysis to that of Pavlidis [7], images were converted to greyscale bitmap images. A thermal scale of 20–40°C was mapped to the 0–255 RGB range. In order to account for movement artefacts across images, facial structure was mapped using Psychomorph [14]. All faces were three-point aligned based on interpupillary and mouth distances which allowed for accurate assessment of facial temperature (via pixel value averaging) in five topographical regions of interest (ROIs): forehead, periorbital region, nose, mouth and cheeks (figure 1).
Figure 1.

Example greyscale thermal image with delineation mapping and five ROIs displayed: (A) forehead region, (B) periorbital region, (C) nasal region, (D) mouth region, (E) cheek regions (averaged).
3. Results
Facial temperature changes were calculated for each contact location by subtracting the baseline facial temperature from the facial temperature during experimenter contact. Subsequent data analyses reflects facial temperature.
(a). Study 1
(i). Does social contact cause a detectable temperature shift?
Overall temperature shift was calculated by averaging the changes for each of the four measurement locations. Social contact caused a significant increase in facial temperature (t16 = 2.78, p = 0.013), with an average change of 0.1°C across conditions (s.e.m. = 0.035°C).
(ii). Does experimenter sex affect temperature shift?
A paired comparison t-test confirmed that baseline temperatures did not differ between conditions (t16 = 0.058, p = 0.954;  ,
, 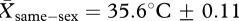 ). A repeated measures ANOVA was run with experimenter sex (two levels) and temperature change during measurement at various locations (four levels) acting as within-subject factors. Because oral contraceptive use can impact thermoregulation [15], it was included as a between-subject factor (Npill-users = 6); but no effect of pill use was found on observed thermal changes (F1,15 = 0.92, p = 0.354, m.s.e. = 0.17,
). A repeated measures ANOVA was run with experimenter sex (two levels) and temperature change during measurement at various locations (four levels) acting as within-subject factors. Because oral contraceptive use can impact thermoregulation [15], it was included as a between-subject factor (Npill-users = 6); but no effect of pill use was found on observed thermal changes (F1,15 = 0.92, p = 0.354, m.s.e. = 0.17, 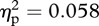 ).
).
The location of experimenter contact had a significant effect on facial skin temperature (Greenhouse–Geisser correction used for sphericity; F1.7,25.3 = 9.59, p = 0.001, m.s.e. = 0.11, 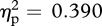 ). Pairwise comparisons using Bonferroni correction (to control for multiple testing error) revealed that facial temperature during chest contact was significantly higher than that observed during either outer arm (p = 0.02) or palm (p = 0.006) contact. Peak facial temperature during facial contact was marginally higher than that of the outer arm measure (p = 0.084) and significantly higher than during the palm measurement (p = 0.041). Facial temperatures did not differ between face and chest contact, or between outer arm and palm contact (all p > 0.95). A significant interaction was detected between experimenter sex and contact location (F3,45 = 4.60, p = 0.007, m.s.e. = 0.02,
). Pairwise comparisons using Bonferroni correction (to control for multiple testing error) revealed that facial temperature during chest contact was significantly higher than that observed during either outer arm (p = 0.02) or palm (p = 0.006) contact. Peak facial temperature during facial contact was marginally higher than that of the outer arm measure (p = 0.084) and significantly higher than during the palm measurement (p = 0.041). Facial temperatures did not differ between face and chest contact, or between outer arm and palm contact (all p > 0.95). A significant interaction was detected between experimenter sex and contact location (F3,45 = 4.60, p = 0.007, m.s.e. = 0.02, 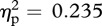 ). Peak facial temperature tended to be higher when interacting with an opposite-sex experimenter than a same-sex experimenter during face and chest contact, while no differences were observed during palm or outer arm contact (figure 2). There was no main effect of experimenter sex (F1,15 = 0.32, p = 0.58, m.s.e. = 0.29,
). Peak facial temperature tended to be higher when interacting with an opposite-sex experimenter than a same-sex experimenter during face and chest contact, while no differences were observed during palm or outer arm contact (figure 2). There was no main effect of experimenter sex (F1,15 = 0.32, p = 0.58, m.s.e. = 0.29, 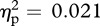 ), and all pill-use interactions failed to reach significance (all F
< 1.6, p > 0.21).
), and all pill-use interactions failed to reach significance (all F
< 1.6, p > 0.21).
Figure 2.

Change in facial temperature (°C) at each location of experimenter contact. Error bars represent s.e.m. Black bars, opposite-sex experimenter; grey bars denote same-sex experimenter.
(b). Study 2
Again, facial temperature significantly increased overall during social contact (t22 = 3.99, p = 0.001; 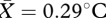 , s.e.m. = 0.07). There was no effect of pill use on observed thermal changes (F1,19 = 0.02, p = 0.893). After ensuring that a similar thermal response to that described in study 1 was present in the current dataset, temperature changes in each ROI were entered into a regression model to determine which facial regions were driving the observed change. The overall model was significant (r2 = 0.92, F5,22 = 36.4, p < 0.001). Average temperature increase in the periorbital (
, s.e.m. = 0.07). There was no effect of pill use on observed thermal changes (F1,19 = 0.02, p = 0.893). After ensuring that a similar thermal response to that described in study 1 was present in the current dataset, temperature changes in each ROI were entered into a regression model to determine which facial regions were driving the observed change. The overall model was significant (r2 = 0.92, F5,22 = 36.4, p < 0.001). Average temperature increase in the periorbital (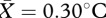 , s.e.m. = 0.07), mouth (
, s.e.m. = 0.07), mouth (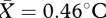 , s.e.m. = 0.08) and nose (
, s.e.m. = 0.08) and nose (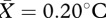 , s.e.m. = 0.10) regions accurately predicted overall facial temperature change (table 2). Although temperature increased in the forehead (
, s.e.m. = 0.10) regions accurately predicted overall facial temperature change (table 2). Although temperature increased in the forehead (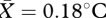 , s.e.m. = 0.09) and cheek regions (
, s.e.m. = 0.09) and cheek regions ( , s.e.m. = 0.09), these did not significantly predict overall facial temperature change. Outside of the face region, we also measured temperature changes in the chest area (at the top of the sternum); an independent t-test indicated that temperature increased in this region as well (t22 = 3.29, p = 0.003,
, s.e.m. = 0.09), these did not significantly predict overall facial temperature change. Outside of the face region, we also measured temperature changes in the chest area (at the top of the sternum); an independent t-test indicated that temperature increased in this region as well (t22 = 3.29, p = 0.003, 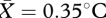 , s.e.m. = 0.11). Spearman's correlations were performed to investigate the relationship between self-reports of psychological reaction of social contact and temperature changes. None of the arousal categories (excitement, embarrassment, discomfort, stress, sexual arousal or overall stimulation) were significantly related to overall facial temperature change or change in any specific ROI (all p > 0.16). Self-reported sexual arousal showed a marginal positive correlation with temperature increase in the periorbital region (rs = 0.40, p = 0.058), however due to multiple testing this effect is likely to be small.
, s.e.m. = 0.11). Spearman's correlations were performed to investigate the relationship between self-reports of psychological reaction of social contact and temperature changes. None of the arousal categories (excitement, embarrassment, discomfort, stress, sexual arousal or overall stimulation) were significantly related to overall facial temperature change or change in any specific ROI (all p > 0.16). Self-reported sexual arousal showed a marginal positive correlation with temperature increase in the periorbital region (rs = 0.40, p = 0.058), however due to multiple testing this effect is likely to be small.
Table 2.
Regression model predicting overall facial temperature change based on 5 ROI. R2 = 0.92 (p < 0.001).
| region | B | s.e. B | β |
|---|---|---|---|
| forehead | −0.11 | 0.11 | −0.13 |
| periorbital | 0.48 | 0.20 | 0.46* |
| nose | 0.20 | 0.07 | 0.27* |
| mouth | 0.42 | 0.13 | 0.49** |
| cheeks | 0.01 | 0.09 | 0.01 |
*p < 0.05.
**p < 0.01.
4. Discussion
We find that tactile contact elevates facial temperature, even when touch is an incidental part of laboratory procedure. Study 1 indicated that this thermal response was dependent on the location of contact (i.e. larger for contact with more ‘personal’ locations) and the reaction was most pronounced for contact with an opposite-sex peer. Study 2 indicated that the main regions involved in this thermal reaction are the periorbital region, nose and mouth. This pattern of reactivity along the midline facial features parallels that seen in Merla & Romani's [12] study of thermal changes during sexual arousal in men.
Whether the changes measured in this study are detectable by others is currently unknown. If such changes in facial temperature during social contact are detectable (by observers or the individual), they could act as social cues; temperature changes may be evident to observers directly through touch, or indirectly through sight or smell. For example, because temperature changes are due to changes in blood flow, they may be visible via concurrent skin colour changes. Slight increases in facial skin redness are perceived as more attractive [16,17], so it may be the case that temperature changes impact perceived attractiveness—although whether or not the skin temperature changes in interactions such as those studied here lead to detectable changes in redness and attractiveness remains to be determined. Moreover, the skin temperature changes may be detectable to the individual and alter their behavioural reactivity. The detectability of thermal changes by observers and/or the individual remains an interesting area of research that has yet to be explored.
In summary, we present evidence for measurable immediate physiological reactions to social contact. Thermal imaging offers new possibilities in the study of psychological responses to social interactions and is of particular interest in the context of mating signals.
Acknowledgements
The authors thank D. Re for his assistance in data collection.
References
- 1.O'Kane B. L., Sandick P., Shaw T., Cook M. 2004. Dynamics of human thermal signatures. Paper presented at the InfraMation Proceedings, Las Vegas, NV. [Google Scholar]
- 2.Nhan B. R., Chau T. 2010. Classifying affective states using thermal infrared imaging of the human face. Biomed. Eng. IEEE Trans. 57, 979–987 10.1109/TBME.2009.2035926 (doi:10.1109/TBME.2009.2035926) [DOI] [PubMed] [Google Scholar]
- 3.Nozawa A., Tacano M. 2009. Correlation analysis on alpha attenuation and nasal skin temperature. J. Stat. Mech. Theory Exp. 01, P01007. 10.1088/1742-5468/2009/01/P01007 (doi:10.1088/1742-5468/2009/01/P01007) [DOI] [Google Scholar]
- 4.Shearn D., Bergman E., Hill K., Abel A. 1990. Facial coloration and temperature responses in blushing. Psychophysiology 27, 687–693 10.1111/j.1469-8986.1990.tb03194.x (doi:10.1111/j.1469-8986.1990.tb03194.x) [DOI] [PubMed] [Google Scholar]
- 5.Zajonc R. B., Murphy S. T., Inglehart M. 1989. Feeling and facial efference: implications of the vascular theory of emotion. Psychol. Rev. 96, 395–416 10.1037/0033-295X.96.3.395 (doi:10.1037/0033-295X.96.3.395) [DOI] [PubMed] [Google Scholar]
- 6.Levine J. A., Pavlidis I., Cooper M. 2001. The face of fear. Lancet 357, 1757. 10.1016/S0140-6736(00)04936-9 (doi:10.1016/S0140-6736(00)04936-9) [DOI] [Google Scholar]
- 7.Pavlidis I., Levine J., Baukol P. 2000. Thermal imaging for anxiety detection. Proceedings of the IEEE Workshop on Computer Vision Beyond the Visible Spectrum: Methods and Applications (CVBVS 2000), pp. 104–109 Washington, DC: IEEE Computer Society.
- 8.Mizukami K., Kobayashi N., Ishii T., Iwata H. 1990. First selective attachment begins in early infancy: a study using telethermography. Infant Behav. Dev. 13, 257–271 10.1016/0163-6383(90)90034-6 (doi:10.1016/0163-6383(90)90034-6) [DOI] [Google Scholar]
- 9.Mizukami K., Kobayashi N., Iwata H., Ishii T. 1987. Telethermography in infant's emotional behavioural research. Lancet 330, 38–39 10.1016/S0140-6736(87)93068-6 (doi:10.1016/S0140-6736(87)93068-6) [DOI] [PubMed] [Google Scholar]
- 10.Puri C., Olson L., Pavlidis I., Levine J., Starren J. 2005. StressCam: non-contact measurement of users’ emotional states through thermal imaging. Proceedings of CHI extended abstracts on human factors in computing systems, pp. 1725–1728 New York, NY: ACM. (doi:10.1145/1056808.1057007)
- 11.Pavlidis I., Levine J. 2002. Thermal image analysis for polygraph testing. Eng. Med. Biol. Mag. IEEE 21, 56–64 10.1109/MEMB.2002.1175139 (doi:10.1109/MEMB.2002.1175139) [DOI] [PubMed] [Google Scholar]
- 12.Merla A., Romani G. L. 2007. Thermal signatures of emotional arousal: a functional infrared imaging study. Paper presented at the 29th annual international conference of the IEEE (EMBS), Lyon, France 10.1109/IEMBS.2007.4352270 (doi:10.1109/IEMBS.2007.4352270) [DOI] [PubMed] [Google Scholar]
- 13.Steketee J. 1973. Spectral emissivity of the skin and pericardium. Phys. Med. Biol. 18, 686–694 10.1088/0031-9155/18/5/307 (doi:10.1088/0031-9155/18/5/307) [DOI] [PubMed] [Google Scholar]
- 14.Tiddeman B., Burt M., Perrett D. I. 2001. Prototyping and transforming facial textures for perception research. IEEE Comput. Graph. Appl. 21, 42–50 10.1109/38.946630 (doi:10.1109/38.946630) [DOI] [Google Scholar]
- 15.Charkoudian N., Johnson J. M. 1997. Modification of active cutaneous vasodilation by oral contraceptive hormones. J. Appl. Physiol. 83, 2012–2018 [DOI] [PubMed] [Google Scholar]
- 16.Stephen I. D., Coetzee V., Law-Smith M., Perrett D. I. 2009. Skin blood perfusion and oxygenation colour affect perceived human health. PLoS ONE 4, e5083. 10.1371/journal.pone.0005083 (doi:10.1371/journal.pone.0005083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Re D. E., Whitehead R. D., Xiao D., Perrett D. I. 2011. Oxygenated-blood colour change thresholds for perceived facial redness, health, and attractiveness. PLoS ONE 6, e17859. 10.1371/journal.pone.0017859 (doi:10.1371/journal.pone.0017859) [DOI] [PMC free article] [PubMed] [Google Scholar]


