Abstract
The behaviour of fossil organisms can typically be inferred only indirectly, but rare fossil finds can provide surprising insights. Here, we report from the Eocene Messel Pit Fossil Site between Darmstadt and Frankfurt, Germany numerous pairs of the fossil carettochelyid turtle Allaeochelys crassesculpta that represent for the first time among fossil vertebrates couples that perished during copulation. Females of this taxon can be distinguished from males by their relatively shorter tails and development of plastral kinesis. The preservation of mating pairs has important taphonomic implications for the Messel Pit Fossil Site, as it is unlikely that the turtles would mate in poisonous surface waters. Instead, the turtles initiated copulation in habitable surface waters, but perished when their skin absorbed poisons while sinking into toxic layers. The mating pairs from Messel are therefore more consistent with a stratified, volcanic maar lake with inhabitable surface waters and a deadly abyss.
Keywords: Testudines, Carettochelyidae, Carettochelys insculpta, taphonomy, ethology
1. Introduction
The behavioural repertoire of fossil organisms is difficult to assess, because the vast majority of body fossils do not reveal much about ethology. The behavioural characteristics of most fossil organisms must therefore be inferred indirectly using, among others, trace fossils [1], taphonomy [2], functional morphology [3], comparison to closest living relatives [4] or phylogenetic inference [5]. The fossil record nevertheless occasionally yields material that documents behaviours directly. Particularly famous examples include fish that choked on large prey items [6] or dinosaurs that died while fighting [7] or while brooding their nests [8]. One reason why these fossils are so rare is because organisms typically do not perish while undertaking daily routines. Fossilized organisms that directly document particular behaviours therefore have the potential to reveal the taphonomic circumstances that lead to their sudden death. We here report on the spectacular finding of fossilized mating pairs of the carettochelyid turtle Allaeochelys crassesculpta at the Eocene Messel Pit in Germany, the first vertebrate fossil to directly document mating behaviour. We furthermore discuss the implications that these highly unusual fossils have for the taphonomic settings of the fossil lake that preserved them.
2. Material and results
The UNESCO World Heritage Messel Pit Fossil Site between Darmstadt and Frankfurt, Germany is notable for its diverse assemblage of fossil animals and plants preserved in Eocene (Early Lutetian, MP11, approx. 47 Ma) black oil shale [9,10]. The site has yielded a great number of rare fossils of outstanding taxonomic value [11,12] and specimens with exquisite soft-tissue preservation, including colours [13]. It is now agreed that the organic-rich sediments were deposited at the bottom of a deep, volcanic maar lake [9,10], but there is little consensus regarding the taphonomic conditions that led to the unusual accumulations of vertebrates, in particular numerous birds and bats. Some taphonomic models suggest that poisonous gases such as carbon dioxide were periodically emitted from the maar lake and caused the death of vertebrates living in and around the lake [14]. Another hypothesis suggests that episodic cyanobacterial blooms poisoned animals that drank from surface waters [15].
The carettochelyid turtle A. crassesculpta [16] is a notable element of the Messel fauna, not only because it represents the only known occurrence of complete fossil carettochelyid skeletons worldwide [17], but also because many individuals are found in pairs (figure 1). Systematic excavations throughout Messel Pit that have been undertaken for more than three decades by the Hessisches Landesmuseum Darmstadt (HLMD) and the Senckenberg Forschungsinstitut und Naturmuseum (SMF) have yielded 51 specimens of A. crassesculpta of which 12 individuals as preserved in pairs (HLMD Me 7593, 14919/20, 14998/99; SMF ME 2034, 2449, 2566). Additional pairs are known from the Royal Belgian Institute of Natural Sciences (IRSNB Me R10), the Staatliches Museum für Naturkunde Karlruhe (SMNK 2348) and the Wyoming Dinosaur Center (WDC CMG 69, see electronic supplementary material). The occurrence and prevalence of A. crassesculpta couples throughout the lake is absolutely unique among macrofossils recovered from Messel, given that two or more individuals of the same taxon within the same layer are not otherwise to be found among tens of thousands of documented finds (N.M. & S.F.K.S. 2012, personal observation).
Figure 1.
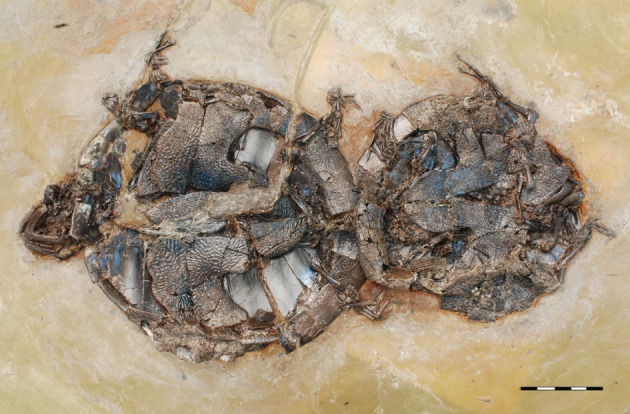
SMF ME 2449, one of nine mating pairs of the carettochelyid turtle Allaeochelys crassesculpta found at Messel Pit Fossil Site. The male (to the right) is about 20% smaller than the female and can be recognized by its relatively longer tail and lack of plastral kinesis. Scale bar, 5 cm.
Our study of the available material provides for the first time positive anatomical evidence that the A. crassesculpta pairs represent male and female individuals and not a random co-occurrence of turtles. As in the majority of living turtles, including the extant relative Carettochelys insculpta [18], male individuals have measurably elongate tails that protrude beyond the margin of the shell, whereas females possess relatively shorter tails that barely reach the shell margin (see figure 2a–c and electronic supplementary material). Furthermore, females show evidence of plastral kinesis along the hyoplastral/xiphiplastral suture (figure 2d,e). Although this important specialization has not been reported from C. insculpta, it is present in an eclectic mix of other extant turtles [18] and perhaps facilitated oviposition of large eggs by this small turtle of 20–25 cm. Males are on average 17 per cent smaller than the females with which they are coupled (see electronic supplementary material).
Figure 2.
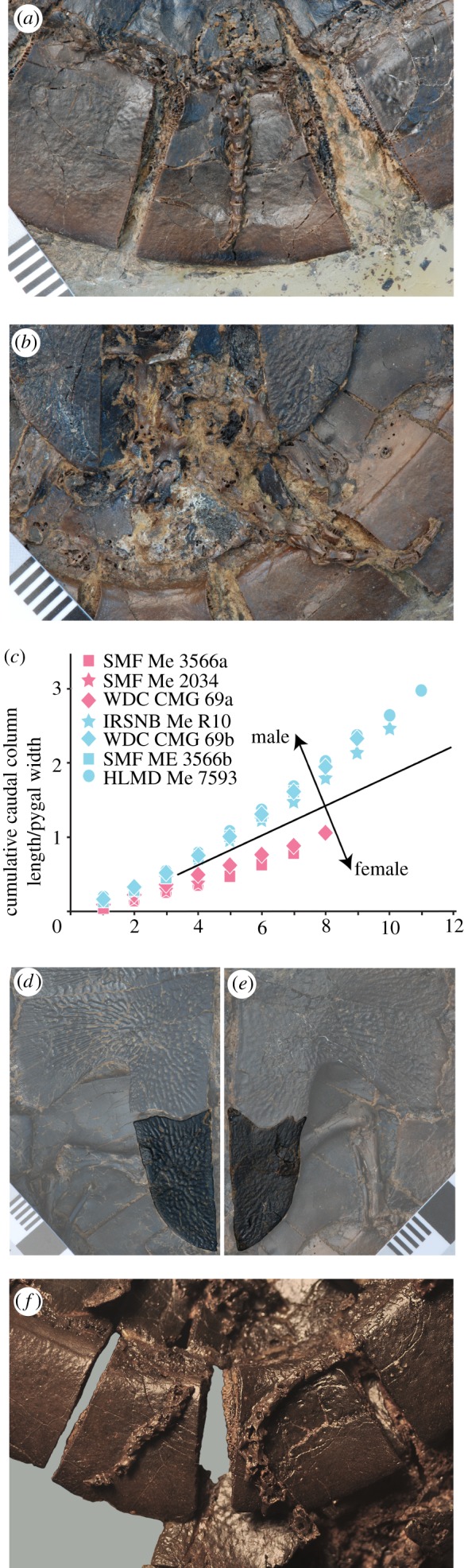
Sexual dimorphism in the carettochelyid turtle A. crassesculpta. SMF ME 3566, ventral view of the tail of (a) a female and (b) a male individual. Note that the caudal vertebrae of the male are notably long and that the tail would have terminated beyond the posterior margin of the shell, if extended. The tail of the female, by contrast, consists of shorter vertebrae and terminates near the margin of the shell. (c) Quantitative differences between the relative tail length of male and female individuals (see electronic supplementary material). The general trend is already apparent starting with the most terminal caudal vertebra. SMF ME 3566, detailed view of the xiphiplastron of (d) a female and (e) a male individual. Note that the hyoplastral/xiphiplastral suture is straight in females. This allows for plastral kinesis, which is useful in oviposition. The same suture is sinuous in male individuals and therefore locked. (f) WDC CMG 69, detailed view of the tails of male and female individuals. The elongate tail of the male to the right is wrapped below the carapace of the female and both tails are aligned.
Seven of the nine available pairs are in direct contact with one another along the posterior margins of their shells. In the two most telling specimens (IRSNB Me R10, WDC CMG 69) the tails of the male individuals wrap below the carapace of the female and are aligned with those of the female (see figure 2f and electronic supplementary material).
3. Discussion
The prevalence of A. crassesculpta couples had lead previous researchers to conclude that these animals perished while mating [15,19], but no explicit anatomical evidence was provided that would allow confirming this assertion. We are able to corroborate that all A. crassesculpta couples consist of two individuals only, and we demonstrate for the first time that all couples contain one male and one female individual and that the tails of some males are aligned with those of the female. We therefore confirm that these animals indeed perished while mating and that they are the only known vertebrate fossils to be preserved in the act of mating. This further highlights the world-class status of the Messel locality but raises the question of how so many individuals could repeatedly fall into the same death trap.
Turtles of the clade Trionychia (Trionychidae + Carettochelyidae) are unique among extant turtles by having almost fully lost the reptilian scales that cover the skin of their relatives [18,20]. Among trionychids this loss is coupled with the skin acting as a respiratory membrane that allows animals to absorb oxygen from the water and remain submerged for extended amounts of time [21,22]. The presence of this external respiratory membrane, however, is disadvantageous under anoxic conditions, because carbon dioxide and dissolved poisons are absorbed as well [23]. Although the physiological properties of carettochelyid skin have not yet been studied, the gross similarity with trionychid skin, the sister group relationship with trionychids [20], the prevalence of blood vessels near the surface of the skin [24], and exclusive presence of the extant pig-nosed turtle in oxygenated waters [18,24] allow the justified inference that extant and extinct carettochelyids also respire with their skin, and may have been in danger in a lake with poisonous waters.
Some tendencies are apparent for the reproductive behaviour of fresh water turtles: aquatic turtles universally mate in the water and the male mounts the female from the rear [18]. In highly aquatic taxa, such as the fossil A. crassesculpta, males tend to be smaller than females and extensive courtship precedes copulation [25]. Finally, once the male successfully mounts the female, the couple will often freeze in their position before separating [18]. If mounting occurs in the open water, the mating couple is likely to thereby sink to considerable depths.
The spectacular preservation of numerous fossilized mating couples at Messel has important implications regarding the taphonomic settings of the locality. Up to now, two primary models have been proposed to explain the unusually high occurrence of fossil vertebrate skeletons in the lake sediments. The more traditional model suggests that volcanic gases were periodically emitted from the lake and thereby caused the death of the animals that lived in and around the lake [18]. A more recent, competing hypothesis suggests that episodic cyanobacterial blooms in the summer months rapidly poisoned the animals that drank from the surface waters of the lake, in particular terrestrial mammals, or bats and birds that drank before returning to their roosts [15]. In our opinion, it is implausible that the A. crassesculpta couples found at Messel would actively swim, court and finally mate in poisonous surface waters or ingest poisonous surface waters only while mating. The rich accompanying fauna of freshwater aquatic animals, a complete lack of cyanobacterial fossils, and the absence of layers with mass accumulations [9,10] furthermore speak against poisonous surface waters. We propose, instead, that the turtles initiated copulation in habitable surface waters, perished when their skin started to absorb poisons while sinking during their embrace into deeper portions of the lake made toxic from the build up of volcanic gases or decay of organic matter, and fully or partially separated once they reached the bottom of the lake. The mating pairs from Messel are therefore more consistent with a stratified, volcanic maar lake with inhabitable surface waters and a deadly abyss.
Acknowledgements
We thank B. Pohl, A. Folie and E. Frey for access to material in their care, and A. Vogel for help with photography. J. Corsini, S. Ladevèze, T. Lehmann, T. Lyson, M. Rabi, K. Smith, V. Volpato and three anonymous reviewers provided useful comments. This study was funded by SNF grant no. 31003A_127053 to T.M.S.
References
- 1.Seilacher A. 2007. Trace fossil analysis. Berlin, Germany: Springer [Google Scholar]
- 2.Lyman R. L. 1994. Vertebrate taphonomy. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Lauder G. V. 1995. On the inference of function from structure. In Functional morphology in vertebrate paleontology (ed. Thomason J. J.), pp. 1–18 New York, NY: Cambridge University Press [Google Scholar]
- 4.Wolfe J. A. 1995. Paleoclimatic estimates from Tertiary leaf assemblages. Annu. Rev. Earth Planet. Sci. 23, 119–142 10.1146/annurev.ea.23.050195.001003 (doi:10.1146/annurev.ea.23.050195.001003) [DOI] [Google Scholar]
- 5.Gauthier J. A., Nesbitt S., Schachner E., Bever G. S., Joyce W.G. 2011. The bipedal stem crocodilian Poposaurus gracilis: inferring function in fossils and innovation in archosaur locomotion. Bull. Peabody Mus. Nat. Hist. 52, 107–126 10.3374/014.052.0102 (doi:10.3374/014.052.0102) [DOI] [Google Scholar]
- 6.Frey E., Tischlinger H. 2012. The Late Jurassic pterosaur Rhamphorhynchus, a frequent victim of the ganoid fish Aspidorhynchus? PLoS ONE 7, e31945 10.1371/journal.pone.0031945 (doi:10.1371/journal.pone.0031945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kielan-Jaworowska Z., Barsbold R. 1972. Narrative of the Polish–Mongolian palaeontological expeditions 1967–1971. Palaeontol. Polon. 27, 5–13 [Google Scholar]
- 8.Norell M. A., Clark J. M., Chiappe L. M., Dashzeveg D. 1995. A nesting dinosaur. Nature 378, 774–776 10.1038/378774a0 (doi:10.1038/378774a0) [DOI] [Google Scholar]
- 9.Schaal S., Ziegler W. 1992. Messel—an insight into the history of life and of the earth. Oxford, UK: Clarendon [Google Scholar]
- 10.von Koenigswald W., Storch G. 1998. Messel, ein Pompeji der Paläontologie. Sigmaringen, Germany: Thorbecke [Google Scholar]
- 11.Wedmann S., Bradler S., Rust J. 2007. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc. Natl Acad. Sci. USA 104, 565–569 10.1073/pnas.0606937104 (doi:10.1073/pnas.0606937104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller J., Hipsley C. A., Head J. J., Kardjilov N., Hilger A., Wuttke M., Reisz R. R. 2011. Eocene lizard from Germany reveals amphisbaenian origins. Nature 473, 364–367 10.1038/nature09919 (doi:10.1038/nature09919) [DOI] [PubMed] [Google Scholar]
- 13.Vinther J., Briggs D. E. G., Clarke J., Mayr G., Prum R. O. 2009. Structural coloration in a fossil feather. Biol. Lett. 6, 128–131 10.1098/rsbl.2009.0524 (doi:10.1098/rsbl.2009.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzen J. L., Köster A. 1994. Die eozänen Tiere von Messel—ertrunken, erstickt oder vergiftet? Nat. Mus. 124, 91–97 [Google Scholar]
- 15.von Koenigswald W., Braun A., Pfeiffer T. 2004. Cyanobacteria and seasonal death: a new taphonomic model for the Eocene Messel lake. Paläont. Z. 78, 417–424 [Google Scholar]
- 16.Harrassowitz H. 1922. Die Schildkrötengattung Anosteira von Messel bei Darmstadt und ihre stammesgeschichtliche Bedeutung. Abh. Hess. Geol. Landesanst. Darmstadt 6, 137–238 [Google Scholar]
- 17.Joyce W. G., Klein N., Mörs T. 2004. Carettochelyine turtle from the Neogene of Europe. Copeia 2004, 406–411 10.1643/CH-03-172R (doi:10.1643/CH-03-172R) [DOI] [Google Scholar]
- 18.Ernst C. H., Barbour R. W. 1989. Turtles of the world. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 19.Rietschel S. 1998. Schildkröten bei der Paarung? In Messel, ein Pompeji der Paläontologie (eds von Koenigswald W., Storch G.), pp. 44–45 Sigmaringen, Germany: Thorbecke [Google Scholar]
- 20.Meylan P. A. 1988. Peltochelys Dollo and the relationships among the genera of the Carettochelyidae (Testudines: Reptilia). Herpetologica 44, 440–450 [Google Scholar]
- 21.Ultsch G. R., Herbert C. V., Jackson D. C. 1984. The comparative physiology of diving in North American freshwater turtles. I. Submergence tolerance, gas exchange and acid–base balance. Physiol. Zool. 57, 620–631 [Google Scholar]
- 22.Bagatto B., Henry R. P. 1999. Exercise and forced submergence in the pond slider (T. scripta) and softshell turtle (A. ferox): influence on bimodal gas exchange, diving behaviour and blood acid–base status. J. Exp. Biol. 202, 267–278 [DOI] [PubMed] [Google Scholar]
- 23.Reese S. A., Jackson D. C., Ultsch G. R. 2003. Hibernation in freshwater turtles: softshell turtles (Apalone spinifera) are the most intolerant of anoxia among North American species. J. Comp. Phys. B 173, 263–268 [DOI] [PubMed] [Google Scholar]
- 24.Schulze-Westrum T. 1963. Die Papuaschildkröte aus Neuguinea. Nat. Mus. 93, 119–127 [Google Scholar]
- 25.Berry J. F., Shine R. 1980. Sexual size dimorphism and sexual selection in turtles (order Testudines). Oecologia 44, 185–191 10.1007/BF00572678 (doi:10.1007/BF00572678) [DOI] [PubMed] [Google Scholar]


