Abstract
One common physiological phenomenon that is involved both in infectious and in malignant processes is the reduction in appetite: disease anorexia. An increase in plasma levels of leptin with inflammation is thought to be involved in this process. However, from an evolutionary perspective, in certain cases, it would be more adaptive for an internal parasite to stimulate the appetite of the host instead of causing its suppression. We tested whether a parasitic infection with the larvae of the helminth parasite Taenia taeniaformis affects the levels of appetite-regulating proteins, such as leptin, ghrelin and neuropeptide-Y (NPY) in wild yellow-necked mouse (Apodemus flavicollis). We found that infected mice had lower plasma levels of leptin and increased levels of NPY than the uninfected subjects. Ghrelin levels were not associated with the occurrence of the parasites; however, these levels strongly correlated with the levels of NPY. This study suggests a possible manipulation by parasitic larvae of appetite regulation in infected subjects.
Keywords: disease anorexia, parasite, appetite, appetite-regulating peptides, yellow-necked mouse
1. Introduction
The ability of parasites to cause behavioural changes in the host has been observed in a variety of host–parasite systems. These behavioural changes are considered to be adaptive for the parasite, since they may enhance the transmission of parasites between hosts and/or the probability that the parasite gets released in an appropriate location [1,2]. In trophic transmission cycles, the parasite often modifies the behaviour of the intermediate host in a way that enhances the possibility of the intermediate host to be preyed upon by the final host [3]. For example, rodents that are infected with Toxoplasma gondii show increased exploratory behaviour, activity and aggression, which makes them more conspicuous to the definitive host, the cat (Felis catus) [4,5].
The relationship between parasites and their hosts implies complicated biochemical coevolution. In many cases, the parasites are thought to secrete chemicals or induce the production of behaviour-altering chemicals in the host. Tapeworms from the family Taneidae, for example, can use host-synthesized cytokines as indirect growth factors for themselves [6]. Taneiids have also evolved structures that are similar to the steroid and peptide hormone receptors of higher vertebrates with binding properties and terminal effects similar to the hormonal metabolites synthesized by the host [6].
Taenia taeniaformis is a helminth parasite that is mostly found in the intestine of cats [7]. Rodents serve as intermediate hosts and are infected when ingesting the ova of T. taeniaformis from contaminated food or bedding. Taenia infections in rodents are considered to be clinically asymptomatic and ‘harmless’ [7].
A reduction in appetite is a common characteristic of many diseases [8]. Several inflammatory cytokines such as the tumour necrosis factor (TNF) and inteleukin (IL)-1 are associated with inflammatory conditions and can induce anorexia and loss of lean body mass. According to Sarraf et al. [8], administration of TNF and IL-1 in mice increases serum levels of the hormone leptin, which inhibits both appetite and adiposity. This suggests that leptin levels may be one mechanism by which disease anorexia is induced during acute inflammatory conditions.
Peptides, such as leptin, neuropeptide-Y (NPY) and ghrelin are well-known substances involved in the regulation of appetite. Leptin is mainly produced by white adipose tissue and circulates in the blood in levels proportional to the fat mass. Both central and peripheral administration of leptin inhibits appetite and adiposity [9]. Ghrelin is produced in the stomach and intestines and a similar administration of it stimulates appetite and adiposity [10]. A large part of the opposing effects of leptin and ghrelin are mediated by NPY that either increases or decreases the expression of these hormones [9,10].
In the present study, we investigated whether the parasitic larvae of T. taeniaformis manipulate the levels of appetite-regulating substances in their host. From an evolutionary perspective, such manipulation would be adaptive for T. taeniaformis larvae, whose future reproduction requires that their intermediate host is predated by final host, the cat, to manipulate the appetite of the intermediate host, in this way making it more explorative and conspicuous to cats.
2. Methods
Yellow-necked mice were caught by snap-traps around the city of Uppsala in Sweden. The corpses of the mice were weighed, measured and opened, and blood samples were collected around the heart. Samples were centrifuged and the serum preserved at −70°C. Serum samples from 14 adult reproductively active male mice infected with the parasitic larvae T. taeniaformis (displaying one single parasitic cyst) and 14 uninfected male mice were used to determine the plasma hormone levels. Mouse leptin (EZML-82K), NPY (EZRMNPY-27K) and ghrelin (EZRGRT-91K) ELISAs (Millipore, Billerica, MA, USA) were used to determine the plasma levels of hormones (results shown in the electronic supplementary material, ‘raw data’).
We tested for measurement error by using two samples from the same individual. Measurement error was then estimated through a one-way ANOVA with individuals as a random effect. With this approach, the within-individual (error) variance component is an estimate of the measurement error [11]. We present measurement error as the percentage of total variance.
When we tested for differences in mean hormone levels, we used a one-way ANOVA with body mass as a covariate. Since the relationship between leptin and body mass differed between the groups (see below), we used a separate-slopes model for this analysis and a model assuming homogeneity of slopes for NPY and ghrelin. We tested heterogeneity in variances and the normality of the within-group residuals and found no deviations; hence the assumptions of the test made are met.
3. Results
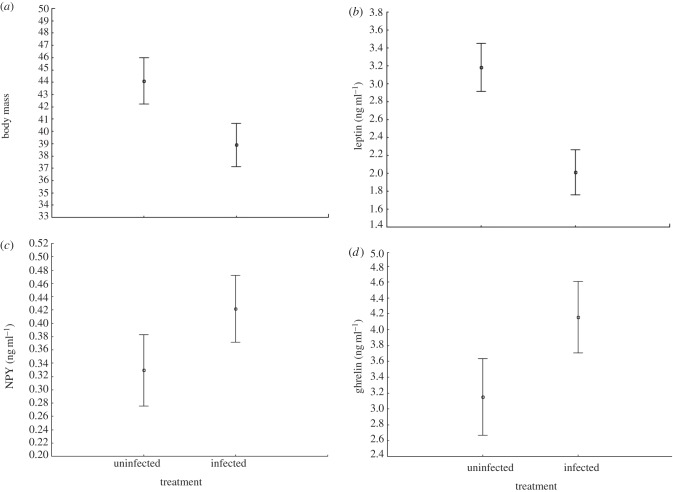
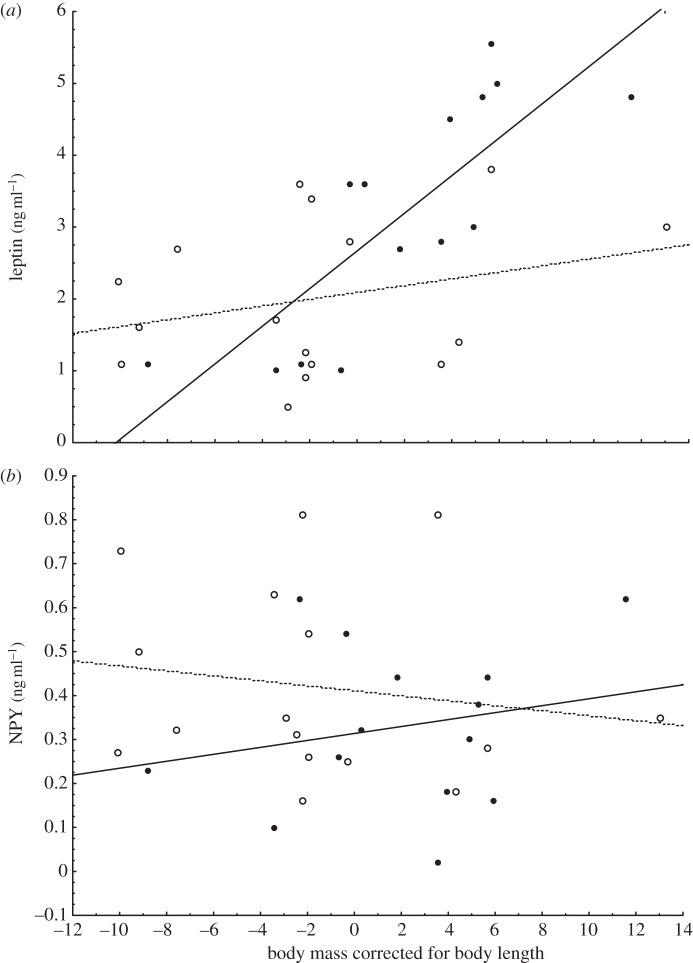
The measurement error was low for all three hormones (leptin 4.6%, NPY 2.3%, ghrelin 2.8%). Uninfected mice tended to be heavier than infected ones (F1,28 = 4.07, p = 0.053; figure 1a); they also demonstrated significantly higher plasma leptin levels than infected mice (F1,26 = 10.25, p = 0.0036; figure 1b), and tended to have lower levels of NPY (F1,26 = 3.59, p = 0.069; figure 1c). There was no significant difference in levels of ghrelin (F1,26 = 2.29, p = 0.14, figure 1d). The levels of leptin were not correlated with the levels of NPY in the uninfected mice (r = 0.18, p = 0.54; table 1), but the two hormones were significantly negatively correlated in the infected mice (r = −0.51, p = 0.044; table 1). The levels of leptin were strongly correlated with body mass in the control group (r = 0.84, p < 0.001), but not so in the infected group (r = 0.023, p = 0.39; table 1). This difference in correlation can be seen as a significant interaction between groups and covariate in the separate-slopes ANOVA (F2,26 = 12.81, p = 0.00013). The difference between the two groups with regard to the relationship between leptin, NPY and body mass is shown in figure 2. Since body mass is affected by structural components and by body fat content, we made a regression of body mass on body length and used the residuals as the dependent variable (r = 0.61, p < 0.001, n = 30, no differences between the groups). There was a strong positive relationship between leptin levels and the residuals in the uninfected group (r = 0.80, p = 0.001), but not in the infected group (r = 0.27, p = 0.31, test of difference, p = 0.055). There were no significant relationships between residuals and levels of NPY or ghrelin in either group.
Figure 1.
Mean differences (±s.e.) between groups in levels of (a) body mass, (b) leptin, (c) NPY and (d) ghrelin. Graphs for leptin, NPY and ghrelin are taken from an ANOVA with weight as a covariate (see text).
Table 1.
Correlations between hormone levels and body mass (p-values in brackets). Uninfected individuals are shown below the diagonal and infected individuals above the diagonal.
| NPY | leptin | ghrelin | body mass | |
|---|---|---|---|---|
| NPY | — | −0.51 (0.044) | 0.85 (<0.001) | −0.19 (0.48) |
| leptin | 0.18 (0.54) | — | −0.37 (0.16) | 0.23 (0.39) |
| ghrelin | 0.92 (<0.001) | 0.26 (0.38) | — | 0.018 (0.95) |
| body mass | 0.46 (0.1) | 0.84 (<0.001) | 0.44 (0.11) | — |
Figure 2.
Hormone levels in relation to body mass corrected for body length for infected (filled circles) and uninfected (open circles) mice. (a) Leptin. The relationship between hormone level and body mass is significant for uninfected mice (r2 = 0.62, p = 0.00054, solid line), but not for infected mice (r2 = 0.009, p = 0.30, dotted line). (b) NPY. The relationship between hormone levels and body mass was not significant for uninfected mice (r2 = 0.0059, p = 0.40, solid line), or infected mice (r2 = 0.0029, p = 0.84, dotted line).
To get a more complete picture, we performed a principal components analysis for each group. The first eigenvector accounted for 64.1 per cent of total variance in the uninfected group and 55.4 per cent in the infected group of mice (table 2). However, the leading eigenvector summarizing the pattern of correlations revealed large differences between the two groups (table 2). In the uninfected group, all parameters had positive loadings, meaning that heavy mice, in general, had high levels of hormones. In the group of infected mice, however, heavy mice had high levels of leptin but low levels of NPY and ghrelin (table 2). The second-largest eigenvector mirrored the differences in the first eigenvector insofar that in the uninfected group this vector describes individuals that are heavy with high levels of leptin but with low levels of NPY and ghrelin, whereas in the infected group the second vector describes individuals that are heavy and tend to have higher levels of the hormones. The same result was obtained when using the residuals from the body mass–body length regression instead.
Table 2.
The first two eigenvectors and associated eigenvalues (% of total variance) of the correlation matrices in table 1.
| uninfected |
infected |
|||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| NPY | 0.51 | −0.49 | −0.63 | 0.12 |
| leptin | 0.43 | 0.62 | 0.48 | 0.26 |
| ghrelin | 0.52 | −0.45 | −0.58 | 0.36 |
| body mass | 0.53 | 0.41 | 0.18 | 0.89 |
| eigenvalue % | 64.1 | 30.8 | 55.4 | 26.2 |
4. Discussion
In the present study, infected mice demonstrated lower average plasma levels of leptin and a tendency to higher plasma levels of NPY than uninfected mice; consequently, it is possible that infected rodents have higher levels of hunger and are more likely to show behaviours that make them more conspicuous to the predators than uninfected animals [12,13]. The infected group also tended to be lower in mass than the uninfected group, implying a possible effect of the parasite on the energy deposits and partly explaining the lower leptin levels. However, the classic positive correlation between leptin levels and body mass, apparent in uninfected animals, had disappeared in the group of infected animals, implying a possible effect of the parasite on leptin secretion. The levels of ghrelin were not influenced by the parasitic infection; however, as the levels of ghrelin are strongly influenced by food intake [14], it is possible that the differences in time after last meal between tested animals were too great to let us detect any robust trends in the plasma levels of ghrelin.
In order to persist, most parasites must evolve physiological pathways that allow them to interact with host physiology, including immunity. As a consequence many parasites have evolved the ability to manipulate the immune response of their hosts. Cytokines and chemokines are strongly involved in both innate and adaptive immune response. Not surprisingly, helminths have evolved many strategies to interfere with the neuroendocrine and immune systems of their hosts [15]. Leid & McConnell [16] showed that the larval stage of T. taeniformis is able to produce prostaglandin E2, which is known to inhibit the production of the cytokine IL-12 and in this way act as a TH2-cell promoting factor. Prostaglandin E2 also depresses the efferent migration of skin Langerhans cells to the draining lymph nodes and thereby inhibits an important step in the initiation of immunity [15]. This process could be one explanation for the lack of disease anorexia following the infection with the larvae of T. taeniaformis in our study, but it does not explain the observed decrease in the plasma leptin levels.
Optimality models predict that hungry animals should be more willing to take risks than satiated individuals [12,13]. Consequently, from an evolutionary perspective, it would be more adaptive for a parasite with trophic transmission cycles to stimulate appetite in its intermediate host in a way that enhances the possibility of the intermediate hosts to be preyed upon by the final host. From this study, it seems likely that the changes in levels of appetite-regulating substances in infected mice could serve to increase the transmission from the intermediate host to the final one.
Acknowledgements
We are thankful to the colleagues of M.L. who donated the mice they had caught in their homes and to Reija Dufva for the laboratory-related work.
References
- 1.Lefevre T., Thomas F. 2008. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect., Genet. Evol. 8, 504–519 10.1016/j.meegid.2007.05.008 (doi:10.1016/j.meegid.2007.05.008) [DOI] [PubMed] [Google Scholar]
- 2.Thomas F., Adamo S., Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Process. 68, 185–200 10.1016/j.beproc.2004.06.010 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 3.Klein S. L. 2003. Parasite manipulation of the proximate mechanisms that mediate social behavior in vertebrates. Physiol. Behav. 79, 441–449 10.1016/S0031-9384(03)00163-X (doi:10.1016/S0031-9384(03)00163-X) [DOI] [PubMed] [Google Scholar]
- 4.Holliman R. 1997. Toxoplasmosis, behaviour and personality. J. Infect. 35, 105–110 10.1016/S0163-4453(97)91380-3 (doi:10.1016/S0163-4453(97)91380-3) [DOI] [PubMed] [Google Scholar]
- 5.Webster J. P. 2001. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microb. Infect. 3, 1037–1045 10.1016/S1286-4579(01)01459-9 (doi:10.1016/S1286-4579(01)01459-9) [DOI] [PubMed] [Google Scholar]
- 6.Escobedo G., López-Griego L., Morales-Montor J. 2009. Neuroimmunoendocrine modulation in the host by helminth parasites: a novel form of host-parasite coevolution? Neuroimmunomodulation 16, 78–87 10.1159/000180262 (doi:10.1159/000180262) [DOI] [PubMed] [Google Scholar]
- 7.Jithendran K., Somvanshi R. 1998. Experimental infection of mice with Taenia taeniaformis eggs from cats – course of infection and pathological studies. Indian J. Exp. Biol. 36, 523. [PubMed] [Google Scholar]
- 8.Sarraf P., Frederich R. C., Turner E. M., Ma G., Jaskowiak N. T., Rivet D. J., Flier J. S., Lowell B. B., Fraker D. L., Alexander H. R. 1997. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J. Exp. Med. 185, 171–175 10.1084/jem.185.1.171 (doi:10.1084/jem.185.1.171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havel P. J. 2001. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp. Biol. Med. 226, 963–977 [DOI] [PubMed] [Google Scholar]
- 10.Bagnasco M., Kalra P. S., Kalra S. P. 2002. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology 143, 726–729 10.1210/en.143.2.726 (doi:10.1210/en.143.2.726) [DOI] [PubMed] [Google Scholar]
- 11.Merilä J., Björklund M. 1995. Fluctuating asymmetry and measurement error. Syst. Biol. 44, 97–101 10.2307/2413486 (doi:10.2307/2413486) [DOI] [Google Scholar]
- 12.Krebs J. R., Kacelnik A. 1991. Decision-making. In Behavioural ecology an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 105–136 Oxford, UK: Blackwell Scientific [Google Scholar]
- 13.Lõhmus M., Sundstrom L. F. 2004. Leptin and social environment influence the risk-taking and feeding behaviour of Asian blue quail. Anim. Behav. 68, 607–612 10.1016/j.anbehav.2003.12.019 (doi:10.1016/j.anbehav.2003.12.019) [DOI] [Google Scholar]
- 14.Diano S., Farr S. A., Benoit S. C., McNay E. C., da Silva I., Horvath B., Gaskin F. S., Nonaka N., Jaeger L. B., Banks W. A. 2006. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 9, 381–388 10.1038/nn1656 (doi:10.1038/nn1656) [DOI] [PubMed] [Google Scholar]
- 15.Maizels R., Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3, 733–744 10.1038/nri1183 (doi:10.1038/nri1183) [DOI] [PubMed] [Google Scholar]
- 16.Leid R., McConnell L. 1983. PGE2 generation and release by the larval stage of the cestode Taenia taeniaeformis. Prostagland. Leukot. Med. 11, 317–323 10.1016/0262-1746(83)90043-4 (doi:10.1016/0262-1746(83)90043-4) [DOI] [PubMed] [Google Scholar]