Abstract
Invasive species are known to affect native species in a variety of ways, but the effect of acoustic invaders has not been examined previously. We simulated an invasion of the acoustic niche by exposing calling native male white-banded tree frogs (Hypsiboas albomarginatus) to recorded invasive American bullfrog (Lithobates catesbeianus) calls. In response, tree frogs immediately shifted calls to significantly higher frequencies. In the post-stimulus period, they continued to use higher frequencies while also decreasing signal duration. Acoustic signals are the primary basis of mate selection in many anurans, suggesting that such changes could negatively affect the reproductive success of native species. The effects of bullfrog vocalizations on acoustic communities are expected to be especially severe due to their broad frequency band, which masks the calls of multiple species simultaneously.
Keywords: invasive species, bioacoustics, noise pollution, Anura, Amphibia, Brazil
1. Introduction
Biological invasions contribute to biodiversity loss, ecosystem degradation and impairment of ecosystem services worldwide [1]. Indeed, invasive species are associated with over 50 per cent of the recent extinctions of animal species for which causes can be compiled [2]. They also provide invaluable insights into ecosystem functioning and evolutionary processes that complement and inform broader scale studies in systematics and biogeography [3].
Interactions between native and invasive species have been studied from a variety of perspectives; however, the potential consequences of invasion of the acoustic niche have not been explored: how do native species respond when a new acoustic competitor appears? Many animal species employ acoustic signals to attract and assess potential mates and evaluate rivals. Environmental sounds can impede acoustic communication by attenuating and degrading signals and reducing signal-to-noise ratio [4]. In response, acoustic species can rapidly modulate signal parameters, including rate, timing, amplitude, and frequency, which can result in altered female mate preference and decreased reproductive success [5,6]. In addition to the environmental noises that have been studied previously [7], sounds produced by invasive species might also cause native species to modulate acoustic signals.
To study the effects of an acoustic invader on native callers, we simulated an acoustic invasion by exposing calling native male white-banded tree frogs (Hypsiboas albomarginatus) to invasive American bullfrog (Lithobates catesbeianus) advertisement calls. Based on previous studies of the effect of environmental noise on anurans [8–10], we predicted immediate changes in signal rate, and we also tested for changes in other gross-temporal calling patterns and spectral properties.
2. Material and methods
(a). Study area and focal species
The study was conducted from November to December 2009 at three permanent ponds in an Atlantic forest relict in Serra do Itajaí National Park, Blumenau, Brazil (27°03′ S, 49°05′ W). Pond areas were 306–16 310 m2. The park road was 500 m from the closest pond, and we did not record any anthropogenic sounds during the experiments.
White-banded tree frogs (H. albomarginatus) occur in the Atlantic forest and breed in ponds near forest edges. Breeding males emit advertisement calls of one or two multi-pulsed notes with harmonics at 1060–1430 Hz and 2720–2780 Hz or 2280–2850 Hz [11]. American bullfrogs (L. catesbeianus) are native to eastern North America but are currently widespread in the Atlantic forest [12]. During the study period, bullfrogs were active in the region and we observed calling males, eggs, tadpoles and non-calling individuals in similar ponds also inhabited by H. albomarginatus. However, in four field expeditions in 2009 and 2010, we failed to detect tadpoles or post-metamorphic bullfrogs at or near any of the study ponds. Bullfrog advertisement calls cover a broad frequency band (90 to more than 4000 Hz) with energy peaks at 200–400 Hz and 1000–2000 Hz [13].
(b). Playback experiment
To assess calling patterns of invasive individuals in southern Brazil, we recorded 5 min of continuous calling by 10 solitary bullfrogs at Novo Treviso, Faxinal do Soturno municipality (29°34′ S, 53°26′ W). Solitary males emitted three to seven consecutive calls with intervals of 15 s to several minutes. We randomly selected a train of notes emitted by a single male (128 mm snout–vent length, recorded at 20.7°C) of average size [14]. Playback experiments were divided into three consecutive periods: 5 min silence, 5 min stimulus and 5 min silence. The stimulus consisted of nine trains of five bullfrog advertisement calls (6.6 s, 187.5 Hz dominant frequency, 234.4 Hz central frequency) separated by 30 s intervals. The stimulus was broadcast at a sound pressure level of 85 dB (C-weighting) measured at 1 m distance, which is equal to the mean amplitude we observed in the field, as calibrated using a portable sound pressure level meter (Instrutemp, ITDEC-4000).
We searched for calling male tree frogs from 21.00 to 0.00 h. Once a focal male was located, we placed the speaker at 1 m distance, 10 cm above the water and directed towards the caller. We waited 3–5 min before initiating the experiment. We recorded calls of focal males (n = 10) using a digital audio recorder (Marantz PMD670) and a directional microphone (Sennheiser M66-K6P) placed 1 m from the caller. Focal males were separated by at least 10 m and were captured following each experiment. Specimens were deposited in the Coleção de Anfíbios e Répteis, Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul (MCT 11561–11571).
(c). Acoustic analysis
The 15 min recordings of 10 individuals were examined using Raven Pro v. 1.4 [15]. Spectrograms were constructed using 16 bit resolution, 22 Hz sampling rate and 256 point fast Fourier transform. We displayed and counted the calls emitted in each of the three time periods and calculated the signal rate (calls per minute). To measure call parameters, we randomly sampled 20 calls in each period (60 calls per individual, see the electronic supplementary material) and estimated duration (s), inter-signal interval (time between sequential calls, s), dominant frequency (Hz) and centre frequency (Hz) [16].
Differences in call parameters were tested by ANOVA through randomization tests treating individuals as blocks and the period as a fixed factor. We assumed the null hypothesis that any call or signal rate of a given individual could be emitted in any period and used 1000 permutations and pseudo-Fratio statistics as test criteria [17]. To determine which periods differed, we used pairwise contrasts calculated using only the vectors and sample units of the groups involved in the pair under test and therefore not requiring correction of p-values. We used Multiv v. 2.4.2 [18] for all statistical tests.
3. Results
Bullfrog calls had no effect on tree frog signal rate (F = 0.004, p = 0.968) or inter-call interval (F = 0.004, p = 0.289), but signal duration was affected by the stimulus (F = 0.021, p = 0.002; figure 1). Signal duration did not differ significantly between pre-stimulus and stimulus periods (F = 0.001, p = 0.522) but was significantly shorter in post-stimulus than pre-stimulus (F = 0.018, p = 0.013) and stimulus periods (F = 0.025, p = 0.002).
Figure 1.
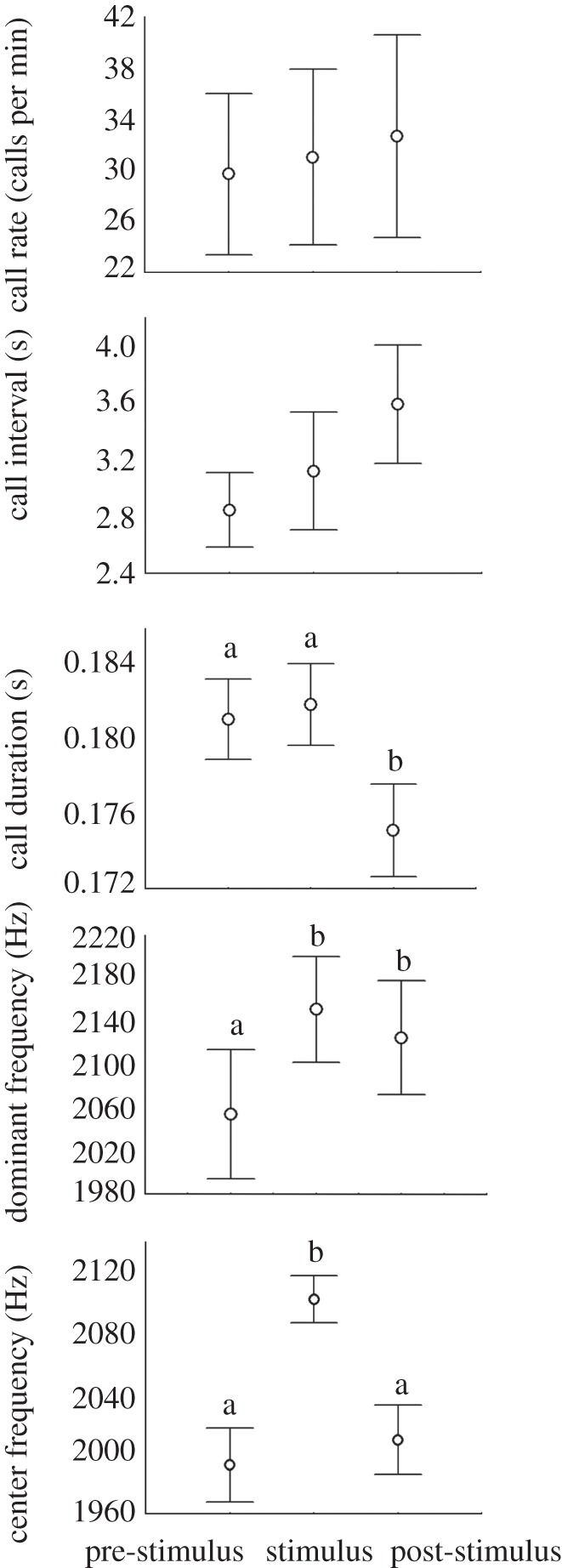
Main effects (means and s. e.) of American bullfrog advertisement calls on white-banded tree frog advertisement calls. During the stimulus period, tree frogs increased the dominant and centre frequencies. During the post-stimulus period, call duration decreased while the dominant frequency remained higher than in the pre-stimulus period. Letters indicate groups that are statistically different from one another.
Bullfrog calls caused tree frogs to call at higher frequencies (figure 2), with a significant effect on the dominant and centre frequencies of tree frog calls (F = 0.030, p = 0.001; F = 0.048, p = 0.001, respectively). Relative to the pre-stimulus period, the dominant frequency increased during the stimulus (F = 0.037, p = 0.001) and then decreased but remained significantly higher than in the pre-stimulus period (F = 0.021, p = 0.004). The difference between the dominant frequencies of the stimulus and post-stimulus periods was not significant (F = 0.004, p = 0.204). Centre frequencies differed only during the stimulus (F = 0.048, p = 0.001), being significantly different from both the pre- (F = 0.055, p = 0.001) and post-stimulus periods (F = 0.061, p = 0.001).
Figure 2.
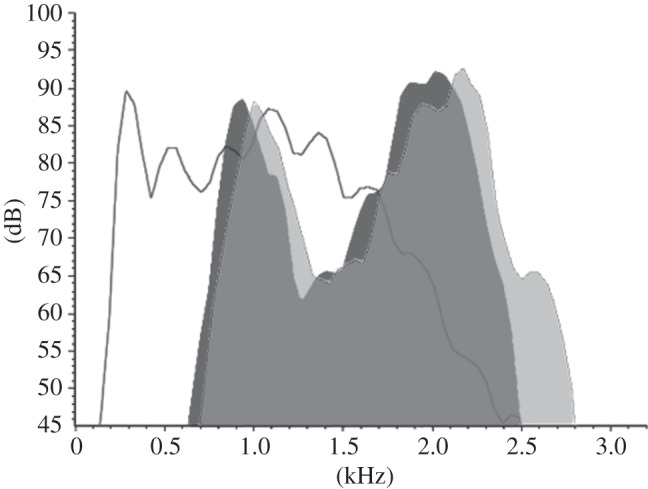
Power spectra (256 FFT) of a broadcast American bullfrog advertisement call (white), and white-banded tree frog calls emitted before (dark grey) and during (pale grey) the stimulus period. Tree frog calls recorded at 22°C; male snout-vent length 53.5 mm.
4. Discussion
In this study, we have demonstrated that acoustic invaders can affect native species in the acoustic niche, causing changes in the spectral properties of signals used to attract mates and repel rivals. Previous studies of responses to other kinds of environmental sounds reported significant alterations in signal rate [8–10]. In contrast, we found the signal rate of native tree frogs to be unaffected by invasive bullfrog calls. Instead, tree frogs immediately shifted calls to significantly higher frequencies. A similar spectral shift was reported for two Australian frogs in response to long-term exposure to traffic noise [19]. Such altered frequencies could be energetically suboptimal [20], which might explain the decrease in signal duration after exposure to bullfrog calls. Insofar as the advertisement call is the primary basis of mate selection [21,22], such changes could negatively impact the reproductive success of native species.
The mass of amplectant white-banded tree frogs is significantly correlated, which suggests that females choose males of proportional size [22]. This choice is probably guided by the dominant frequency of the male's advertisement call, which, as in many other anurans, is inversely proportional to body mass [11]. Given that tree frogs increase call frequencies in response to bullfrog calls, the resulting advertisement calls could affect pair formation by providing false cues of male condition.
In the light of the immediate response of calling tree frogs to a simulated invasion of a single bullfrog, full-scale acoustic invasions, which often involve choruses of up to five bullfrogs (C. Both 2010, personal observation), are likely to have major impacts on the structure of acoustic communities. The effects of bullfrog vocalizations on acoustic communities are expected to be especially severe due to their broad frequency band, which masks the calls of multiple species simultaneously. Biological invasions involve diverse interactions between native and invasive species, which makes it difficult to identify the causes of alterations in native species and communities. Simulated acoustic invasions allow the effects of invasive species on native individuals and whole communities to be rigorously tested by allowing researchers to experimentally isolate and control variables in natural environments.
Acknowledgements
This study was authorized by the Instituto ‘Chico Mendes’ de Conservação da Biodiversidade (licence 23009-1).
This study was supported by grants from the Rufford Small Grants Foundation (ref. 34.09.09) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (procs. 305473/2008-5 and 476789/2009-5 to T.G. and a doctoral fellowship to C.B.). We thank the staff of Parque Nacional da Serra do Itajaí for logistic support and Tiago Dalcin for his help in field activities. The manuscript benefitted from careful reviews by Ariel Rodríguez and an anonymous referee.
References
- 1.Pyšek P., Richardson D. M. 2010. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Res. 35, 25–55 10.1146/annurev-environ-033009-095548 (doi:10.1146/annurev-environ-033009-095548) [DOI] [Google Scholar]
- 2.Clavero M., García-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110. 10.1016/j.tree.2005.01.003 (doi:10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 3.Strauss S. Y., Webb C. O., Salamin N. 2006. Exotic taxa less related to native species are more invasive. Proc. Natl Acad. Sci. USA 103, 5841–5845 10.1073/pnas.0508073103 (doi:10.1073/pnas.0508073103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumm H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440 10.1111/j.0021-8790.2004.00814.x (doi:10.1111/j.0021-8790.2004.00814.x) [DOI] [Google Scholar]
- 5.Bee M. A. 2009. Finding a mate at a cocktail party: spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim. Behav. 75, 1781–1791 10.1016/j.anbehav.2007.10.032 (doi:10.1016/j.anbehav.2007.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bee M. A., Swanson E. M. 2007. Auditory masking of anuran advertisement calls by road traffic noise. Anim. Behav. 74, 1765–1776 10.1016/j.anbehav.2007.03.019 (doi:10.1016/j.anbehav.2007.03.019) [DOI] [Google Scholar]
- 7.Laiolo P. 2010. The emerging significance of bioacoustics in animal species conservation. Biol. Conserv. 143, 1635–1645 10.1016/j.biocon.2010.03.025 (doi:10.1016/j.biocon.2010.03.025) [DOI] [Google Scholar]
- 8.Schwartz B. Y. J. J., Wells K. D. 1983. An experimental study of acoustic interference between two species of neotropical treefrogs. Anim. Behav. 31, 181–190 10.1016/S0003-3472(83)80187-0 (doi:10.1016/S0003-3472(83)80187-0) [DOI] [Google Scholar]
- 9.Sun J. W. C., Narins P. M. 2005. Anthropogenic sounds differentially affect amphibian call rate. Biol. Conserv. 121, 419–427 10.1016/j.biocon.2004.05.017 (doi:10.1016/j.biocon.2004.05.017) [DOI] [Google Scholar]
- 10.Kaiser K., Scofield D. G., Alloush M., Jones R. M., Marczak S., Martineau K., Oliva M. A. 2011. When sounds collide: the effect of anthropogenic noise on a breeding assemblage of frogs in Belize, Central America. Behaviour 148, 215–232 10.1163/000579510X551660 (doi:10.1163/000579510X551660) [DOI] [Google Scholar]
- 11.Giasson L. O. M., Haddad C. F. B. 2006. Social interactions in Hypsiboas albomarginatus (Anura: Hylidae) and the significance of acoustic and visual signals. J. Herpetol. 40, 171–180 10.1670/205-05A.1 (doi:10.1670/205-05A.1) [DOI] [Google Scholar]
- 12.Both C., Lingnau R., Santos A., Jr, Madalozzo B., Lima L. P., Grant T. 2011. Widespread occurrence of the American bullfrog, Lithobates catesbeianus (Shaw, 1802) (Anura: Ranidae), in Brazil. South Am. J. Herpetol. 6, 127–134 10.2994/057.006.0203 (doi:10.2994/057.006.0203) [DOI] [Google Scholar]
- 13.Capranica R. R. 1968. The vocal repertoire of the bullfrog (Rana catesbeiana). Behaviour 31, 302–324 10.1163/156853968X00306 (doi:10.1163/156853968X00306) [DOI] [Google Scholar]
- 14.Kaefer I. L., Boelter R. A., Cechin S. Z. 2007. Reproductive biology of the invasive bullfrog Lithobates catesbeianus in southern Brazil. Ann. Zool. Fennici 44, 435–444 [Google Scholar]
- 15.Bioacoustic Research Program. 2011. Raven Pro: interactive sound analysis software (v. 1.4). Ithaca, NY: The Cornell Lab of Ornithology; See http://www.birds.cornell.edu/raven [Google Scholar]
- 16.Charif R. A., Waack A. M., Strickman L. M. 2010. Raven Pro v. 1.4 user's manual. Ithaca, NY: The Cornell Lab of Ornithology [Google Scholar]
- 17.Anderson M. J. 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 58, 626–639 10.1139/cjfas-58-3-626 (doi:10.1139/cjfas-58-3-626) [DOI] [Google Scholar]
- 18.Pillar V. D. 2006. Multiv: multivariate exploratory analysis, randomization testing and bootstrap resampling. Universidade Federal do Rio Grande do Sul. See http://ecoqua.ecologia.ufrgs.br/ecoqua/MULTIV.html [Google Scholar]
- 19.Parris K. M., Velik-Lord M., North J. M. A., Function L. 2009. Frogs call at a higher pitch in traffic noise. Ecol. Soc. 14, 25 [Google Scholar]
- 20.Bosch J., De la Riva I. 2004. Are frog calls modulated by the environment? An analysis with anuran species from Bolivia. Can. J. Zool. 82, 880–888 10.1139/Z04-060 (doi:10.1139/Z04-060) [DOI] [Google Scholar]
- 21.Ryan M. J. 1988. Constraints and patterns in the evolution of anuran acoustic communication. In The evolution of the amphibian auditory system (eds Fritzsch B., Ryan M., Wilczynski W., Walkowiak W., Hetherington T.), pp. 637–677 New York, NY: John Wiley & Sons, Inc [Google Scholar]
- 22.Giasson L. O. M., Haddad C. F. B. 2007. Mate choice and reproductive biology of Hypsiboas albomarginatus (Anura: Hylidae) in the Atlantic forest, southeastern Brazil. South Am. J. Herpetol. 2, 157–164 10.2994/1808-9798(2007)2 (doi:10.2994/1808-9798(2007)2) [DOI] [Google Scholar]


