Abstract
Plants under attack by pathogens and pests can mount a range of inducible defences, encompassing both chemical and structural changes. Although few reports exist, it appears that plants responding to pathogen or herbivore attack, or chemical defence elicitors, may produce progeny that are better able to defend themselves against attack, compared with progeny from unthreatened or untreated plants. To date, all research on transgenerational effects of biotic stress has been conducted on dicotyledenous plants. We examined the possibility that resistance induced by application of chemical defence elicitors to the monocot plant barley, could be passed on to the progeny. Plants were treated with acibenzolar-S-methyl (ASM) or saccharin, and grain harvested at maturity. Germination was unaffected in seed collected from plants treated with saccharin, while germination was reduced significantly in seed collected from ASM-treated plants. The subsequent growth of the seedlings was not significantly different in any of the treatments. However, plants from parents treated with both ASM or saccharin exhibited significantly enhanced resistance to infection by Rhynchosporium commune, despite not being treated with elicitor themselves. These data hint at the possibility of producing disease-resistant plants by exposing parent plants to chemical elicitors.
Keywords: Hordeum vulgare L., Rhynchosporium commune, induced resistance, priming, transgenerational effects
1. Introduction
Prior inoculation with necrotizing pathogens or treatment with various agents is well known to induce resistance in plants [1]. The induced plant is then able to resist attack by virulent pathogens due to an increased ability to express defences rapidly following challenge. This is possible because first, all plants possess the genetic machinery to defend themselves [1], and second, the inducing treatment either activates defences directly and/or it conditions the plant to express defences rapidly upon pathogen challenge, a phenomenon referred to as priming [2]. Various types of induced resistance have been described, including systemic acquired resistance (SAR), where prior inoculation with a necrotizing pathogen or treatment with certain chemicals (e.g. acibenzolar-S-methyl; ASM), provides protection in distal plant parts to subsequent infection [3]. SAR is thought to be effective mainly against biotrophic pathogens [4] and is controlled by a signalling pathway that depends on accumulation of salicylic acid (SA) and the defence regulatory protein NPR1 [5].
Because seeds develop in a maternal environment, and the maternal environment might predict the progeny's environmental conditions, it is conceivable that parents might further enhance their net reproductive success by endowing their progeny with phenotypes to deal with potential hazards, such as parasitism or predation. Indeed, such maternal effects have been reported previously from both animal and plant systems [6,7] and have been viewed as potentially adaptive [6,8,9]. Tobacco plants inoculated with tobacco mosaic virus induced SAR in the selfed progeny of the infected plants compared with the progeny of the uninfected plants [10], while experiments by Agrawal et al. [11] showed that non-lethal exposure of an animal (Daphnia cucullata) to carnivores and a plant (Raphanus raphanistrum) to herbivores induced defences but also resulted in the attacked organisms producing offspring that were better defended than offspring from non-threatened parents. Subsequent work by Molinier et al. [12] showed that in Arabidopsis thaliana treated with ultraviolet-C radiation or flagellin, somatic homologous recombination of a transgenic reporter was increased in the treated population and persisted in the subsequent, untreated generations. Recently, three separate studies demonstrated the transgenerational persistence of pathogen-, herbivore- and chemical-induced resistance in A. thaliana [13–15]. In contrast, earlier work found that SAR induced by the blue mould pathogen, Peronospora tabacina, did not transfer to the offspring via seeds, although plants derived from tissue culture from various parts of induced plants were systemically protected against subsequent challenged with P. tabacina [16].
To date, the small number of studies examining transgenerational effects of pathogen-, herbivore- and chemically induced resistance have used dicotyledenous plants. Here, we report on the transgenerational effects of treating a monocotyledonous plant, barley, with the chemical elicitors ASM and saccharin, the latter having been shown to induce resistance to pathogen infection in barley and broad bean [17,18].
2. Material and methods
Seeds of barley (Hordeum vulgare L. cv Cellar) were sown in pots in Fisons Levington compost and grown in a glasshouse at 18°C with a 16 h photoperiod (190 µmol m−2 s−1 provided by 400 W mercury vapour lamps). Plants were used for experiments when the fourth leaf was fully formed. They were sprayed with saccharin (1 mM in distilled water containing 0.01% Tween 20) or ASM (as Bion; 1 mM) using a hand-held sprayer and were sprayed twice: when the fourth leaf had emerged fully and when the flag leaf had emerged. Controls were treated with distilled water containing 0.01 per cent Tween 20. Flowering started three weeks after the final elicitor treatment. Plants (10 per treatment) were then grown until maturity and grain from each treatment was conditioned in preparation for production of a second generation by placing in a cold room at 4°C for 3 days.
Conditioned seeds from the first generation were germinated by placing 100 seeds per treatment onto moist filter paper in Petri dishes and incubating them in the dark at 18°C. The number of germinating seeds was counted after 5 and 7 days. Seeds were considered to have germinated when the radical protruded through the seed coat.
To determine the effects of treatments on growth of second-generation progeny, plants (10 per treatment) were harvested two and four weeks after germination, for determination of whole shoot dry weights. These were used to calculate relative growth rates (RGR, g g–1 d–1) using
where W1 and W2 are plant dry weights at time points t1 and t2, respectively.
To determine whether progeny from parents treated with elicitors expressed induced resistance, plants (10 per treatment) grown as described above were used for experiments when the third leaf was emerging. All leaves were inoculated with Rhynchosporium commune as described in Walters et al. [18] and infection intensity assessed on all three leaves 21 days after inoculation by determining the per cent leaf area exhibiting symptoms. Saccharin content of barley seeds and first leaves of germinated seedlings was determined using the spectrophotometric method described by Weinert et al. [19] (detection limit = 1.5 × 10−5 M) using the extraction method for soluble sugars described by Bingham et al. [20].
Data were subjected to ANOVA using the GenStat Release 11.1 statistical program and comparison of treatment means performed using Fisher's protected least significant difference (LSD) test. Data from this paper have been deposited in the Dryad repository (doi:10.5061/dryad.9hh83).
3. Results
Seed produced by plants treated with elicitors in the first generation were collected, conditioned and used in germination and plant growth tests. Compared with seed collected from untreated first-generation plants, germination was not affected in seed from parents treated with saccharin (figure 1a). By contrast, a significant reduction in germination was obtained with seed from parents treated with ASM (figure 1a). When plant growth was examined, although RGR of plants from saccharin-treated parents was increased and RGR of progeny from ASM-treated parents was reduced, these changes were not statistically significant (figure 1b).
Figure 1.
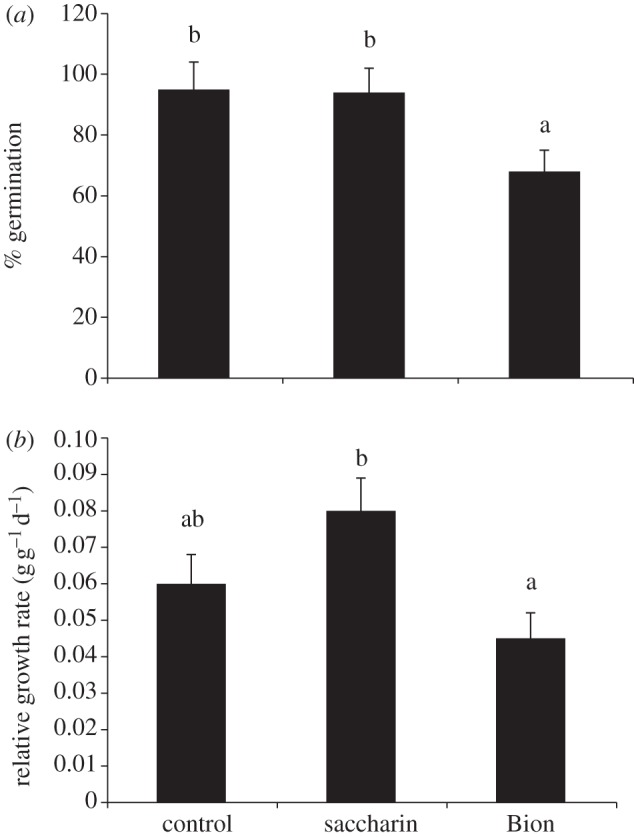
Effects of treating barley with saccharin or ASM on growth parameters in the progeny. Seed germination (a) and relative growth rate (RGR) of young plants (b). Bars with different letters are significantly different at p < 0.05 (Fisher's LSD).
Second-generation plants at the three-leaf stage were inoculated with R. commune in order to determine whether they would express enhanced resistance to infection. These second-generation plants did not receive any elicitor treatment; only their parents had been treated with elicitors. Remarkably, plants from saccharin- and ASM-treated parents exhibited significantly less infection than did controls. These reductions in R. commune infection were observed on all three leaves (figure 2). Saccharin was not detected in barley seed or in first leaves of germinated seedlings.
Figure 2.
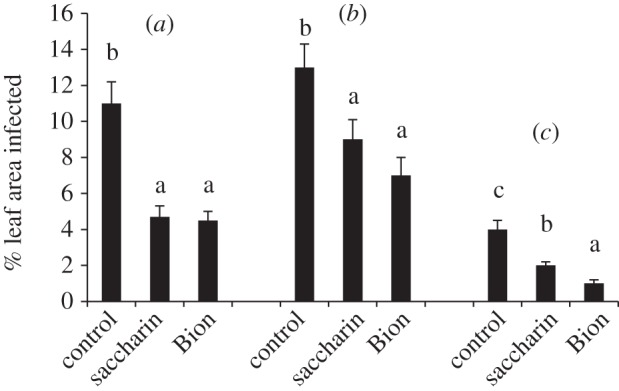
Effects of treating barley with saccharin or ASM on resistance of the progeny to infection by R. secalis. Progeny received no treatment with elicitors, but were inoculated with R. secalis. Per cent leaf area infected was then determined on leaves (a) one, (b) two and (c) three. Bars with different letters are significantly different at p < 0.05 (Fisher's LSD).
4. Discussion
Previous work has shown that parents in which resistance had been induced, produced progeny capable of expressing enhanced resistance to infection or herbivory. For example, three recent studies provided a clear demonstration of the transgenerational effects of pathogen-, herbivore- and chemical-induced resistance in the dicotyledenous model plant A. thaliana [13–15]. The results presented in this paper represent the first report of transgenerational effects on pathogen infection resulting from treatment of a monocotyledonous plant (barley) with chemical elicitors. In terms of germination and growth responses, the effects depended on the elicitor used in the parental generation. Thus, in progeny from saccharin-treated parents, there was no effect on seed germination or plant growth rate, whereas in progeny from ASM-treated parents, seed germination, but not plant growth rate was reduced. It seems possible that the reduced germination observed in progeny from ASM-treated parents could be related to the reduced seed weights produced by the parents (data not shown). Interestingly however, subsequent growth of the progeny was not significantly different from the controls. However, irrespective of the effects on germination and growth of the progeny, progeny from parents treated with saccharin and ASM exhibited enhanced resistance to infection by R. commune. It is possible that these reductions in R. commune infection in the progeny are due to carry over effects such as accumulation of the elicitors in barley seed and subsequent activation of induced resistance in the young seedlings. Although we can provide no data on ASM in seeds from treated barley in our experiments, ASM is known to be rapidly metabolized in plants with little apical translocation [21], and indeed, following extensive field trials, no evidence could be found for ASM accumulation in cotton seed [22]. However, we were able to determine saccharin concentrations in plant tissue, but could not detect saccharin in seeds produced by plants treated with saccharin. This suggests that the effects observed in the barley progeny are transgenerational. The mechanisms responsible for these effects in barley are not known, but future work should examine chromatin re-modelling as a basis for the possible epigenetic effects of elicitor treatment [23].
The data presented here show that resistance induced in a major monocotyledonous crop plant by chemical elicitors can be passed from one generation to the next, thereby conferring enhanced resistance to pathogen infection. There is now an increasing body of evidence to show that a plant's capacity for priming is important in its struggle to deal with abiotic and biotic stresses. Inheritance of this primed state is likely to contribute to enhanced adaptation of the progeny to the prevailing environment. It also raises the possibility of producing seed already primed for enhanced pathogen defence. Given the vulnerability of plants to pathogens in the early stages of growth, in-built protection during this period could represent a very useful addition to crop protection practice.
Acknowledgements
This work is financially supported by the Rural and Environment Science and Analytical Services Division of the Scottish Government. This study was conducted as part of the RERAD-funded work-package on barley pathology (WP1.4). We are grateful to Duncan McKenzie of Syngenta for the kind gift of Bion.
References
- 1.Walters D. R., Fountaine J. M. 2009. Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J. Agric. Sci. 147, 523–535 10.1017/S0021859609008806 (doi:10.1017/S0021859609008806) [DOI] [Google Scholar]
- 2.Goellner K., Conrath U. 2008. Priming: it's all the world to induced resistance. Eur. J. Plant Pathol. 121, 233–242 10.1007/s10658-007-9251-4 (doi:10.1007/s10658-007-9251-4) [DOI] [Google Scholar]
- 3.Ryals J. A., Neuenschwander U. H., Willits M. G., Molina A., Steiner H. Y., Hunt M. D. 1996. Systemic acquired resistance. Plant Cell 8, 1809–1819 10.2307/3870231 (doi:10.2307/3870231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann. Rev. Phytopath. 43, 205–227 10.1146/annurev.phyto.43.040204.135923 (doi:10.1146/annurev.phyto.43.040204.135923) [DOI] [PubMed] [Google Scholar]
- 5.Dong X. N. 2004. NPR1, all things considered. Curr. Opin. Plant Biol. 7, 547–552 10.1016/j.pbi.2004.07.005 (doi:10.1016/j.pbi.2004.07.005) [DOI] [PubMed] [Google Scholar]
- 6.Mousseau T. A., Fox C. W. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press [Google Scholar]
- 7.Roach D. A., Wulff R. D. 1987. Maternal effects in plants. Ann. Rev. Ecol. Syst. 18, 209–236 10.1146/annurev.ecolsys.18.1.209 (doi:10.1146/annurev.ecolsys.18.1.209) [DOI] [Google Scholar]
- 8.Thiede D. A. 1998. Maternal inheritance and its effect on adaptive evolution: a quantitative genetic analysis of maternal effects in a natural plant population. Evolution 52, 998–1015 10.2307/2411232 (doi:10.2307/2411232) [DOI] [PubMed] [Google Scholar]
- 9.Tollrian R. 1995. Predator-induced morphological defences: costs, life history shifts, and maternal effects in Daphnia pulex . Ecology 76, 1691–1705 10.2307/1940703 (doi:10.2307/1940703) [DOI] [Google Scholar]
- 10.Roberts D. A. 1983. Acquired resistance to tobacco mosaic virus transmitted to the progeny of hypersensitive tobacco. Virology 124, 161–163 10.1016/0042-6822(83)90299-4 (doi:10.1016/0042-6822(83)90299-4) [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A. A., Laforsch C., Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60–63 10.1038/43425 (doi:10.1038/43425) [DOI] [Google Scholar]
- 12.Molinier J., Ries G., Zipfel C., Hohn B. 2006. Transgeneration memory of stress in plants. Nature 442, 1046–1049 10.1038/nature05022 (doi:10.1038/nature05022) [DOI] [PubMed] [Google Scholar]
- 13.Luna E., Bruce T. J. A., Roberts M. R., Flors V., Ton J. 2012. Next-generation systemic acquired resistance. Plant Physiol. 158, 844–853 10.1104/pp.111.187468 (doi:10.1104/pp.111.187468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmann S., De Vos M., Casteel C. L., Tian D., Halitschke R., Sun J. Y., Agrawal A. A., Felton G. W., Jander G. 2012. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 158, 854–863 10.1104/pp.111.187831 (doi:10.1104/pp.111.187831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slaughter A., Daniel X., Flors V., Luna E., Hohn B., Mauch-Mani B. 2012. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 158, 835–843 10.1104/pp.111.191593 (doi:10.1104/pp.111.191593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuzun S., Kuć J. 1987. Persistence of induced systemic resistance to blue mold in tobacco plants derived via tissue culture. Phytopathology 77, 1032–1035 [Google Scholar]
- 17.Boyle C., Walters D. R. 2006. Saccharin-induced resistance to powdery mildew in barley: effects on growth and phenylpropanoid metabolism. Plant Pathol. 55, 82–91 10.1111/j.1365-3059.2005.01281.x (doi:10.1111/j.1365-3059.2005.01281.x) [DOI] [Google Scholar]
- 18.Walters D. R., Paterson L., Walsh D. J., Havis N. D. 2009. Priming for plant defense in barley provides benefits only under high disease pressure. Physiol. Mol. Plant Pathol. 73, 95–100 10.1016/j.pmpp.2009.03.002 (doi:10.1016/j.pmpp.2009.03.002) [DOI] [Google Scholar]
- 19.Weinert P. L., Pezza H. R., de Oliveira J. E., Pezza L. 2004. A simplified method for routine analysis of saccharin in commercial non-calorific sweeteners. J. Agric. Food Chem. 52, 7788–7792 10.1021/jf0402781 (doi:10.1021/jf0402781) [DOI] [PubMed] [Google Scholar]
- 20.Bingham I. J., Bengough G. A., Rees R. M. 2010. Soil compaction–N interactions in barley: root growth and tissue composition. Soil Tillage Res. 106, 241–246 10.1016/j.still.2009.10.008 (doi:10.1016/j.still.2009.10.008) [DOI] [Google Scholar]
- 21.Scarponi L., Buonaurio R., Martinetti L. 2001. Persistence and translocation of a benzothiadiazole derivative in tomato plants in relation to systemic acquired resistance against Pseudomonas syringae pv. tomato. Pest Manag. Sci. 57, 262–268 10.1002/ps.285 (doi:10.1002/ps.285) [DOI] [PubMed] [Google Scholar]
- 22.Australian Pesticide and Veterinary Medicines Authority (APVMA) 2007. Evaluation of the new active acibenzolar-S-methyl in the product ‘Bion plant activator seed treatment’. Canberra, Australia: APVMA [Google Scholar]
- 23.Van den Burg H. A., Takken F. L. W. 2009. Does chromatin remodelling mark systemic acquired resistance? Trends Plant Sci. 14, 286–294 10.1016/j.tplants.2009.02.003 (doi:10.1016/j.tplants.2009.02.003) [DOI] [PubMed] [Google Scholar]


