Abstract
Plant productivity is predicted to increase in boreal forests owing to climate change, but this may depend on whether N inputs from biological N-fixation also increases. We evaluated how alteration of climatic factors affects N input from a widespread boreal N-fixer, i.e. cyanobacteria associated with the feather moss Pleurozium schreberi. In each of 10 forest stands in northern Sweden, we established climate-change plots, including a control (ambient climate) plot and three plots experiencing a +2°C temperature increase, an approximately threefold reduction in precipitation frequency, and either 0.07, 0.29 or 1.16 times normal summer precipitation. We monitored N-fixation in these plots five times between 2007 and 2009, and three times in 2010 after climate treatments ended to assess their recovery. Warmer temperatures combined with less frequent precipitation reduced feather moss moisture content and N-fixation rates regardless of total precipitation. After climate treatments ended, recovery of N-fixation rates occurred on the scale of weeks to months, suggesting resilience of N-fixation to changes in climatic conditions. These results suggest that modelling of biological N-inputs in boreal forests should emphasize precipitation frequency and evaporative water loss in conjunction with elevated temperature rather than absolute changes in mean precipitation.
Keywords: bryophytes, cyanobacteria, climate change, climate warming, nitrogen fixation, precipitation
1. Introduction
Boreal forests are a significant sink of global carbon (C) [1], and play an important role in the global C cycle [2]. Several models predict that climate change will result in increased net primary productivity (NPP) in boreal ecosystems [3,4]. Given that boreal ecosystems receive very low levels of anthropogenic nitrogen (N)-deposition [5], it is increasingly recognized that over long timescales productivity may be constrained if a corresponding increase in biological N-fixation does not co-occur [6–8]. Therefore, understanding how global change factors directly influence N-fixation in boreal forests is needed to understand and model potential long-term changes in NPP [5,9,10].
In this study, we evaluated the effect of altered precipitation and temperature on N-fixation by cyanobacteria associated with the feather moss, Pleurozium schreberi (hereafter referred to as a feather moss association), which is one of the main sources of N inputs to boreal forests [11]. Climate change in northern latitudes is expected to result in warmer mean annual temperatures and regional changes in precipitation frequency and quantity [12]. Research has shown that predicted temperature increases in boreal forests during the next century (i.e. 2–5°C) are likely to have a positive effect on N-fixation by feather moss associations [13]. Furthermore, this N-fixation is known from greenhouse experiments to be limited by moisture availability [13,14]. Boreal forests in North America, Asia and Europe are predicted to experience higher annual quantities of precipitation within the next century, primarily in the winter, with variable changes in summer precipitation occurring across regions, and with individual precipitation events likely to be larger and less frequent [12]. Few experimental field data exist to understand how reduced frequency and quantity of summer precipitation may affect N-fixation rates [14,15], which are needed to effectively model N and C balances in boreal forests in response to climate change [6,7].
We performed an experiment using 10 forest sites in northern Sweden to assess the potential effect of altered precipitation on feather moss N-fixation rates. We created experimental plots representing different climate-change scenarios where (relative to the control) all experimental plots experienced a 2°C temperature increase, less frequent summer precipitation, and one of three different precipitation quantities (0.07, 0.29, and 1.16 times mean summer precipitation). We hypothesized (i) that a simultaneous decrease in precipitation frequency and increase in temperature would reduce moss-associated N-fixation rates; and (ii) that a reduction in the quantity of summer precipitation in conjunction with reduced precipitation frequency and increased temperature would reduce N-fixation rates.
2. Material and methods
We used 10 forest stands near Arvidsjaur, Sweden (65°35′–66°07′ N, 17°15′–19°26′ E). The stands form a post-fire chronosequence and were divided into two age classes, ‘young’ and ‘old’, which had a mean age of 62 and 282 years at the start of the experiment, respectively. In June 2007, we established four 1 × 1 m plots within each of the 10 stands, consisting of a control (unmanipulated) and three plots fitted with transparent Plexiglass covers mounted 30 cm above the plot surface during the growing season each year from 2007 to 2009, which caused the mean moss surface temperature to increase by 2°C, and a minor reduction in light (less than 13% photosynthetically active radiation reduction). The three warming plots within each stand were randomly assigned to one of three precipitation levels (0.05, 0.25, and 1.0 times mean precipitation from June 1 to September 30; SMHI, Sweden) from 1961 to 2000. Because actual summer rainfall during the experimental period deviated slightly from the 40 year mean, the actual precipitation quantities were 0.07, 0.29 and 1.16 times the control plots (hereafter the 0.07, 0.29 and 1.16 treatments). Precipitation levels were divided into biweekly applications that reduced precipitation frequency approximately three-fold (table 1).
Table 1.
Macroclimatic variables at the study area during June, July and August 2007–2010 (SMHI, Sweden).
| year | June | July | August | June–August |
|---|---|---|---|---|
| temperature (°C) |
mean | |||
| 2007 | 11.9 | 13.5 | 12.0 | 12.5 |
| 2008 | 10.9 | 14.0 | 10.4 | 11.8 |
| 2009 | 10.6 | 13.6 | 13.1 | 12.4 |
| 2010 | 9.2 | 12.0 | 11.4 | 10.9 |
| precipitation (mm) | total | |||
| 2007 | 8.4 | 112.9 | 58.8 | 180.1 |
| 2008 | 89.3 | 50.3 | 93.4 | 233.0 |
| 2009 | 38.0 | 110 | 61.1 | 209.1 |
| 2010 | 58.4 | 83.6 | 22.5 | 164.5 |
| precipitation frequency (days month−1 > 1 mm) | mean | |||
| 2007 | 2 | 13 | 11 | 8.7 |
| 2008 | 13 | 5 | 12 | 10.0 |
| 2009 | 5 | 11 | 10 | 8.7 |
| 2010 | 9 | 14 | 9 | 10.7 |
We used the acetylene reduction method [16] to estimate the potential N-fixation rates of mosses immediately after fully rehydrating them. Mosses were collected in each plot five times during treatment implementation (September 2007, and June and September 2008, 2009), and three times in the year after treatments ended (June, July and September 2010) to assess whether N-fixation recovered following drought. Additionally, during the September sampling period each year, we collected two 50 mm diameter moss cores from each plot to estimate moss moisture content.
Data were analysed using three-factor ANOVAs, with climate treatments, stand age class and time as main factors, using nested repeated-measures ANOVAs in SPSS (v. 19.0). This analysis was able to identify main and interactive effects of the three factors, and was followed by individual two-factor nested ANOVAs at each time, and followed by post hoc S–N–K tests assessing pair-wise climate treatment differences. Detailed methodology and supporting data are provided in the electronic supplementary material, appendix S1 and S2.
3. Results
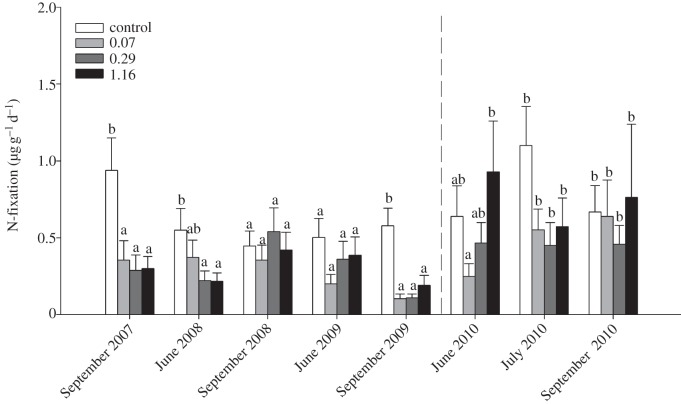
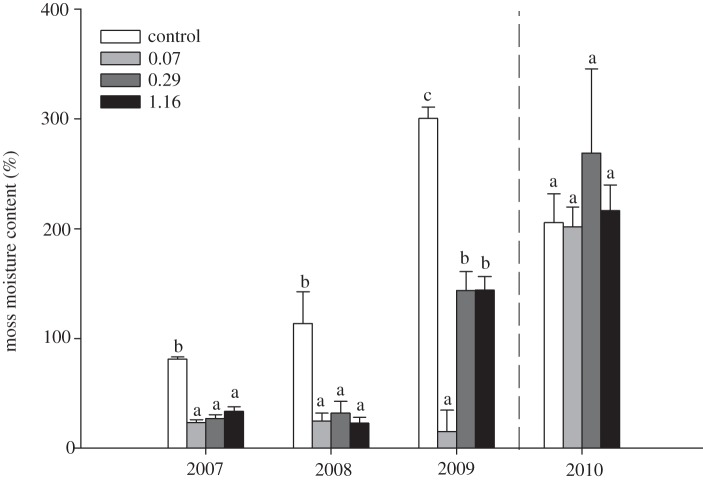
The data show that N-fixation rates were significantly affected by the climate treatments, by forest age class and by sampling time, whereas significant interactive effects between these factors were never found (table 2). All climate treatment plots had reduced N-fixation rates relative to the control on the first and fifth sampling date (September 2007, 2009), and in the 0.29 and 1.16 moisture treatments relative to the control on the second sampling date (June 2008; figure 1). The significant effect of stand age class was due to higher N-fixation rates occurring in old stands relative to young stands (data not presented). We also found that moss moisture content was significantly reduced relative to the control in all climate treatment plots in 2007 and 2008, whereas in 2009 moss moisture content of the 0.29 and 1.16 moisture treatments was higher than the 0.07 treatment, but still lower than the control (table 2 and figure 2). During the recovery period (i.e. during 2010), the moisture content of the mosses did not differ between the treatments (table 2 and figure 2).
Table 2.
Results from ANOVA analysis evaluating the effect of climate treatments (C), stand age (A), sampling time (T) and their interactive effects, on biological N-fixation and feather moss moisture content during treatment and recovery periods. ES denotes effect size. Italic indicates p < 0.05.
| N-fixation rate |
moss moisture content |
|||||||
|---|---|---|---|---|---|---|---|---|
| F-value | d.f. | p-value | ES | F-value | d.f. | p-value | ES | |
| treatment period (2007–2009) | ||||||||
| climate treatment (C) | 9.005 | 3 | <0.001 | 0.144 | 44.513 | 3 | <0.001 | 0.582 |
| age class (A) | 11.367 | 1 | <0.001 | 0.066 | 0.423 | 1 | 0.517 | 0.004 |
| sampling time (T) | 3.293 | 4 | 0.013 | 0.076 | 128.860 | 2 | <0.001 | 0.729 |
| C × A | 0.887 | 3 | 0.449 | 0.016 | 0.734 | 3 | 0.534 | 0.022 |
| C × T | 1.517 | 12 | 0.123 | 0.102 | 5.006 | 6 | <0.001 | 0.238 |
| A × T | 0.452 | 4 | 0.771 | 0.011 | 0.532 | 2 | 0.589 | 0.011 |
| C × A × T | 0.702 | 12 | 0.748 | 0.050 | 0.245 | 6 | 0.960 | 0.015 |
| recovery period (2010) | ||||||||
| climate treatment (C) | 1.863 | 3 | 0.131 | 0.055 | 1.614 | 3 | 0.205 | 0.131 |
| age class (A) | 6.204 | 1 | 0.014 | 0.061 | 6.418 | 1 | 0.016 | 0.167 |
| sampling time (T) | 0.364 | 2 | 0.699 | 0.008 | — | — | — | |
| C × A | 1.327 | 3 | 0.270 | 0.040 | 0.842 | 3 | 0.481 | 0.073 |
| C × T | 0.763 | 6 | 0.601 | 0.045 | — | — | — | |
| A × T | 1.331 | 2 | 0.269 | 0.027 | — | — | — | |
| C × A × T | 0.185 | 6 | 0.980 | 0.011 | — | — | — | |
Figure 1.
The mean nitrogen (N)-fixation rate associated with feather mosses as influenced by climate treatments, including a control and three climate-change plots receiving +2°C warming, three times reduced precipitation frequency, and either 0.07, 0.29 or 1.16 times normal summer precipitation, during the treatment period (2007–2009), and recovery period (2010). Within each sampling time, different letter above bars (a or b) indicates significant pair-wise differences (α = 0.05).
Figure 2.
The mean moisture content of feather mosses as influenced by climate treatments, as described in figure 1. Within each sampling time, different letter above bars (a, b or c) indicates significant post hoc pair-wise post hoc differences (α = 0.05).
4. Discussion
Our aim was to evaluate how reductions in summer precipitation frequency and quantity that are predicted to occur in combination with climate warming are likely to influence N-fixation by boreal feather moss associations. In support of our first hypothesis, the data show that all climate-change treatments resulted in a mean decrease in N-fixation rates relative to the control in three of the five sampling periods (table 2 and figure 1), showing an adverse effect of combined elevated temperature and reduced precipitation frequency. Contrary to our second hypothesis, the data show that N-fixation rates never differed between the three climate-change treatments. Because temperature increases greater than 2°C have been shown to increase N-fixation rates in feather mosses when moisture is not limiting [13], and precipitation quantity had no effect, these results show that reductions in precipitation frequency can strongly impair N-fixation rates [14]. This response was probably due to the combined effect of prolonged desiccation periods between re-wetting events, and enhanced evaporative water loss owing to warming-enhanced vapour pressure deficits. In support of this, measurements of the moisture content of feather mosses made during the September sampling period each year (i.e. two weeks after the previous water application) showed that the water content of the two highest precipitation treatments (0.29 and 1.16) never differed during the three years, and that the lowest precipitation treatment only once differed from the two highest water treatments (i.e. in 2009). These data suggest that the combined effects of warmer temperatures and less frequent precipitation, regardless of total precipitation quantity, strongly influence the hydration status of the feather mosses, which in turn exerts a strong control on their N-fixation rates.
Our data also provide insights into the resilience of N-fixation in response to climate variability. During the course of the experiment, N-fixation of the control plots varied substantially, and twice (September 2008 and June 2009) we found no difference between the climate-change treatments and the control (figure 1). The absence of treatment effects at these times may have been due to dry periods in July 2008 and June 2009 (table 1) that temporarily reduced the N-fixation capacity of the feather moss associations in the control plots. During the final year of the study when climate treatments were removed, we found carryover treatment effects on N-fixation rates in June 2010, with the two highest precipitation treatments recovering more rapidly than the lowest precipitation treatment. By July, differences between the treatments were no longer present (figure 1), suggesting that N-fixation of the feather moss associations had fully recovered. These data suggest that the N-fixation capacity associated with feather mosses can be temporarily impaired following anomalous climate events, with recovery occurring over timescales of weeks or months.
The negative effect that our climate-change treatments had on N-fixation is inconsistent with studies for many other N-fixing organisms showing that N-fixation rates often increase as temperatures increase to 25°C, which is the kinetic optimum of the nitrogenase enzyme [17]. The results instead show over a wide range of forest stand types that feather moss moisture content exerts a dominant control on N-fixation rates. This finding is consistent with several studies in Arctic ecosystems [18,19], but has been poorly described in boreal forests. These data are highly relevant for predicting and modelling how biological N-fixation may respond to climate change in boreal forests and suggest that reductions in summer precipitation frequency and enhanced evaporative water loss associated with climate warming may override the effect that altered precipitation quantity or elevated temperature may have on N-fixation [13].
Acknowledgements
We thank H. Gustafsson and M. Karlsson for help with field and laboratory work and acknowledge the Swedish Research Council FORMAS for funding this work.
References
- 1.Schlesinger W. H. 1977. Carbon balance in terrestrial detritus. Annu. Rev. Ecol. Syst. 8, 51–81 10.1146/annurev.es.08.110177.000411 (doi:10.1146/annurev.es.08.110177.000411) [DOI] [Google Scholar]
- 2.Bonan G. B. 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 10.1126/science.1155121 (doi:10.1126/science.1155121) [DOI] [PubMed] [Google Scholar]
- 3.Cramer W., et al. 2001. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob. Change Biol. 7, 357–373 10.1046/j.1365-2486.2001.00383.x (doi:10.1046/j.1365-2486.2001.00383.x) [DOI] [Google Scholar]
- 4.Friedlingstein P., Fung I., Holland E., John J., Brasseur G., Erickson D., Schimel D. 1995. On the contribution of CO2 to the missing biospheric sink. Glob. Biogeochem. Cycles 9, 541–556 10.1029/95GB02381 (doi:10.1029/95GB02381) [DOI] [Google Scholar]
- 5.Gundale M. J., Deluca T. H., Nordin A. 2011. Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob. Change Biol. 17, 2743–2753 10.1111/j.1365-2486.2011.02407.x (doi:10.1111/j.1365-2486.2011.02407.x) [DOI] [Google Scholar]
- 6.Hungate B. A., Dukes J. S., Shaw M. R., Luo Y. Q., Field C. B. 2003. Nitrogen and climate change. Science 302, 1512–1513 10.1126/science.1091390 (doi:10.1126/science.1091390) [DOI] [PubMed] [Google Scholar]
- 7.Gerber S., Hedin L. O., Oppenheimer M., Pacala S. W., Shevliakova E. 2010. Nitrogen cycling and feedbacks in a global dynamic land model. Glob. Biogeochem. Cycles 24, 1–15 10.1029/2008GB003336 (doi:10.1029/2008GB003336) [DOI] [Google Scholar]
- 8.Jain A., Yang X. J., Kheshgi H., McGuire A. D., Post W., Kicklighter D. 2009. Nitrogen attenuation of terrestrial carbon cycle response to global environmental factors. Glob. Biogeochem. Cycles 23, GB4028 10.1029/2009GB003519 (doi:10.1029/2009GB003519) [DOI] [Google Scholar]
- 9.Hungate B. A., Stiling P. D., Dijkstra P., Johnson D. W., Ketterer M. E., Hymus G. J., Hinkle C. R., Drake B. G. 2004. CO2 elicits long-term decline in nitrogen fixation. Science 304, 1291 10.1126/science.1095549 (doi:10.1126/science.1095549) [DOI] [PubMed] [Google Scholar]
- 10.Esser G., Kattge J., Sakalli A. 2011. Feedback of carbon and nitrogen cycles enhances carbon sequestration in the terrestrial biosphere. Glob. Change Biol. 17, 819–842 10.1111/j.1365-2486.2010.02261.x (doi:10.1111/j.1365-2486.2010.02261.x) [DOI] [Google Scholar]
- 11.DeLuca T.H., Zackrisson O., Nilsson M-C., Sellstedt A. 2002. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419, 917–920 10.1038/nature01051 (doi:10.1038/nature01051) [DOI] [PubMed] [Google Scholar]
- 12.IPCC 2007. Intergovermental panel on climate change fourth assessment report (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.). New York, NY and Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Gundale M. J., Nilsson M., Bansal S., Jäderlund A. 2012. The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol. 194, 453–463 10.1111/j.1469-8137.2012.04071.x (doi:10.1111/j.1469-8137.2012.04071.x) [DOI] [PubMed] [Google Scholar]
- 14.Jackson B. G., Martin P., Nilsson M.-C., Wardle D. A. 2011. Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency. Oikos 120, 570–581 10.1111/j.1600-0706.2010.18641.x (doi:10.1111/j.1600-0706.2010.18641.x) [DOI] [Google Scholar]
- 15.Gundale M. J., Gustafsson H., Nilsson M. C. 2009. The sensitivity of nitrogen fixation by a feathermoss-cyanobacteria association to litter and moisture variability in young and old boreal forests. Can. J. Forest Res. 39, 2542–2549 10.1139/X09-160 (doi:10.1139/X09-160) [DOI] [Google Scholar]
- 16.Schöllhorn R., Burris R. H. 1967. Acetylene as a competitive inhibitor of nitrogen fixation. Proc. Natl Acad. Sci. USA 58, 213–218 10.1073/pnas.58.1.213 (doi:10.1073/pnas.58.1.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houlton B. Z., Wang Y. P., Vitousek P. M., Field C. B. 2008. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454, 327–330 10.1038/nature07028 (doi:10.1038/nature07028) [DOI] [PubMed] [Google Scholar]
- 18.Stewart K. J., Coxson D., Siciliano S. D. 2011. Small-scale spatial patterns in N2-fixation and nutrient availability in an arctic hummock–hollow ecosystem. Soil Biol. Biochem. 43, 133–140 10.1016/j.soilbio.2010.09.023 (doi:10.1016/j.soilbio.2010.09.023) [DOI] [Google Scholar]
- 19.Zielke M., Solheim B., Spjelkavik S., Olsen R. A. 2005. Nitrogen fixation in the high arctic: role of vegetation and environmental conditions. Arctic, Antarctic Alpine Res. 37, 372–378 10.1657/1523-0430(2005)037[0372:NFITHA]2.0.CO;2 (doi:10.1657/1523-0430(2005)037[0372:NFITHA]2.0.CO;2) [DOI] [Google Scholar]