Abstract
Signals in intraspecific communication should be inherently honest; otherwise the system is prone to collapse. Theory predicts, however, that honest signalling systems are susceptible to invasion by cheats, the extent of which is largely mediated by fear of reprisal. Cuttlefish facultatively change their shape and colour, an ability that evolved to avoid predators and capture prey. Here, we show that this ability is tactically employed by male mourning cuttlefish (Sepia plangon) to mislead conspecifics during courtship in a specific social context amenable to cheating 39 per cent of the time, while it was never employed in other social contexts. Males deceive rival males by displaying male courtship patterns to receptive females on one side of the body, and simultaneously displaying female patterns to a single rival male on the other, thus preventing the rival from disrupting courtship. The use of tactical deception in such a complex communication network indicates that sociality has played a key role in the cognitive evolution of cephalopods.
Keywords: cuttlefish, courtship, game theory, cognition, evolution
1. Introduction
The old adage that cheaters never prosper is far from applicable in the animal kingdom. Crying wolf is a form of deception where an individual sounds an alarm call to distract conspecifics while they monopolize a resource [1,2]. Although this type of deception provides a short-term gain to the perpetrator, theory predicts that for a signalling system to function effectively, honesty must remain the dominant mode of communication [3]. However, models and empirical data now show that many of these ‘honest’ signalling networks are prone to invasions from cheats [4]. Dawkins & Guilford [5] have argued that cheating is probably ubiquitous in most communication networks. The proportion of cheats in a population generally remains low, because the cheat's bluff is occasionally called and the costs of being caught cheating may be considerable (e.g. if the cheater is forced to fight).
The signal receiver concept of animal communication now accepts that signals that are directed at specific receivers are seldom private and are detected and acted upon by unintended recipients that may include heterospecifics. For example, while the mating calls of male Tungara frogs are aimed at potential females, they are also detected by predatory bats [6]. Thus, signalling occurs in the context of a complex social communication network consisting of signallers, receivers and bystanders. There is ample work illustrating that the signallers themselves are aware of this network and adjust their signal content accordingly (e.g. the audience effect; [7]). Few have considered the implications of individuals sending conflicting signals to conspecifics simultaneously.
Much of this debate on signal honesty has centred on the cost of producing the signal, but if the costs of signal production are low, then the potential for cheating greatly increases. Cuttlefish visual signalling systems have received substantial attention, not only because they are spectacular, but the rate at which signals can change is exceptional [8–10]. This rapid ability to change both the colour and texture of the skin is employed for camouflage and for communication [11]. Thus, the cuttlefish display seems to be a system in which cheating could potentially be rife. Observations of the giant cuttlefish (Sepia apama), for example, have shown that small males can mimic females as part of an alternative mating tactic that is successful in nearly half of all attempts [11].
Mourning cuttlefish (Sepia plangon) are found in social aggregations along the east coast of Australia. Similar to other cephalopods, they use dynamic visual displays for intraspecific communication. Males generally exhibit a pattern of pulsating stripes on the mantle during interspecific interactions, whereas females have characteristic mottled camouflage coloration [12]. Populations are male biased and males compete for receptive females, display mate guarding, displace rivals and interrupt courtship attempts (C. McBride & J. E. Williamson, unpublished data). In this context, natural selection should strongly favour any tactic that reduces the probability of courtship interruption and thereby maximizes male reproductive success.
Here, we describe a form of cheating used by male mourning cuttlefish wherein they mimic female displays towards rival males on one side of their mantle while simultaneously displaying typical male courtship patterns towards potential mates on the other side (figure 1 and the electronic supplementary material, video S1). We examined the mating displays used by males in a range of social contexts in the wild to determine how often male mourning cuttlefish employ this tactical deception strategy. We hypothesized that males would use this tactical deception only in social contexts where they are unlikely to be discovered and punished by rival males.
Figure 1.

Male mourning cuttlefish (M) displaying a male-specific pattern towards a female (F) while simultaneously displaying deceptive female coloration towards a rival male (A).
2. Material and methods
The frequency of deceptive visual displays employed by courting male mourning cuttlefish in a range of social groups was scored from photographs taken in situ on SCUBA during the breeding season (July–November) in various shallow subtidal sites (2–9 m depth) within Sydney Harbour, Australia, over 6 years. Divers proceeded until a focal individual was encountered, then all individuals grouping with the focal individual (i.e. those within two body lengths of each other) were photographed. For all groups encountered, we recorded the number of individuals within the group and their sex. The sex of the individuals can be readily determined because males have longer arms than females and the tips are modified to aid the transmission of spermatophores to females. Encounters with single focal individuals were included as a comparison. Only photographs where both sides of all individuals were visible were scored. The animals are very short-lived and show high site fidelity, with home ranges of less than 800 m2 (C. McBride & J. E. Williamson, unpublished data). By observing populations at multiple locations over multiple years, we can be confident that no individual was recorded more than once. In total, we obtained suitable quality photographs of 108 groups, including single males and females. Within each group, any one of the males could have been displaying the deceptive signal, thus the total number of males observed is obtained by multiplying the number of males in a given social group by the number of observations of that group (n = 138 males).
In addition to the observations of animals in the wild, we also made casual observations of groups housed in a large mesocosm. Mixed sex groups of up to eight individuals were introduced to an aquarium (2.5 m long × 1.5 m high × 1 m wide) that was furnished to simulate a sandy embayment. The groups were housed for several weeks before all the animals were returned to the wild and replaced with a similar number of new animals. Water was drawn directly from the sea and circulated through the aquarium. This allowed us to closely examine the success or otherwise of courtship attempts involving deception, but no attempt was made to systematically manipulate group sizes or sex ratios. Data are available in the electronic supplementary material.
3. Results
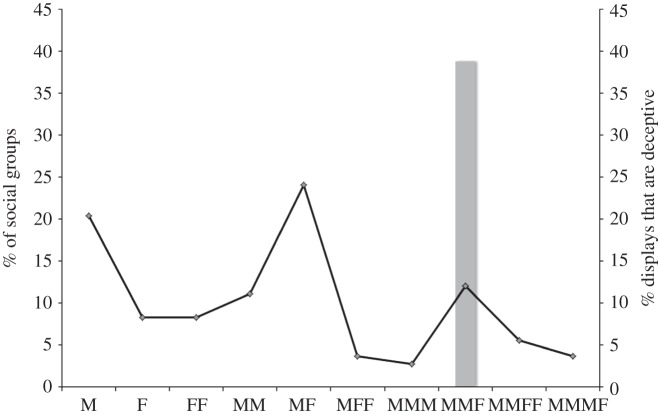
Deceptive displays, where males simultaneously produced male patterns on one side of the mantle and mimicked the female on the other (figure 1), were produced only by males when courting a single female in the presence of a single rival male, despite the wide range of social groups observed. While the deception was confined to a single social context, it was common within that context, with 39 per cent of groups containing males employing the tactic (figure 2). This result is compelling, because most males were observed either by themselves or in a mating pair; thus, if the behaviour occurred at random, it should have been observed more readily in the most common social contexts. Lone males, male–female pairs and groups containing two males and a female were the most common groups encountered in our surveys (figure 2).
Figure 2.
The percentage of observed social groups composed of different numbers of individuals in various combinations of males (M) and females (F) (line) and the percentage of male courtship displays that were tactically deceptive (column).
Our casual examination of courtship behaviour in the mesocosm revealed that the tactical deception technique, while rarely employed, was successful. On two occasions, males using the deceptive tactic were observed transferring spermatophores to the females.
4. Discussion
These observations suggest that cuttlefish cognition is sophisticated: they recognize when only one rival male is present and only then elect to employ a deceptive display. Courting males may refrain from such displays if they detect more than one rival male perhaps because there is a high probability that their deception will be discovered, the tactic will fail and they will be punished by larger males. Furthermore, even in the presence of a single rival, males must orient themselves accurately between the female and the rival male during mate guarding for the deception tactic to be effective. The strategy appears to work, because we observed two successful copulations by deceptive males housed in groups in a large mesocosm. On these occasions, deceptive males carried out uninterrupted courtship without drawing attention to the presence of a receptive female, thus enhancing their own reproductive success.
Males did not employ deception when group membership was composed of two females and a single rival. In this context, it seemed that the male attempted to court both females because it was impossible for him to orientate the deceptive dual display towards both females and the rival male in an appropriate manner. Perhaps the males suffer from divided attention, in this context, making it difficult for them to focus on just a single female.
Complete female mimicry has been previously described in giant cuttlefish as an alternative mating strategy in undersized males [11,12]. There are also observations of cephalopods using a laterally split body pattern to direct a different display to each half of their mantle as a form of defence against predation threat during courtship [13]. This study, however, is the first account of simultaneous dual gender signalling in cephalopods combining both female mimicry and laterally split displays.
The common use of deception in communication networks arguably represents the greatest paradox in the evolution of animal signals because signals should be inherently honest [5]. However, game theory approaches to communications networks suggest that such honest systems are open to exploitation by a small number of cheats [14]. While cheats can be controlled if retribution is sufficiently strong, dishonest individuals are always capable of invading honest populations in the absence of honest phenotypic limitation [15,16]. In the case of the mourning cuttlefish, males were capable of sending conflicting signals to two intended receivers simultaneously and could rapidly switch from honest to dishonest signals. Nearly 40 per cent of males used dual-gender signalling when courting a female in the presence of a single rival male, suggesting that tactical deception is common in this system just as we predicted. This is to be expected given that visual displays are not inherently costly to produce, the signal can be rapidly switched on or off and the (dis)honesty of the signal can be difficult for the receiver to determine from a distance. Our observations of the courtship displays in the wild and in captivity revealed that if the deception is discovered by rival males, then fights will typically ensue in which the dominant male will emerge the victor. Thus, there is an inherent cost to employing the tactic too often and in inappropriate contexts. It appears that the primary problem males face is having enough time to convince the female to mate while remaining undetected (and unmolested) by rival males. Thus, the use of tactical deception under specific social conditions may increase courtship time sufficiently for the male to mate successfully.
Tactical intraspecific deception in animals is commonly associated with higher vertebrates because of its link with sophisticated cognitive function [2]. For example, females and subordinate male gorillas move out of sight of the dominant male to mate surreptitiously. In primates, deception correlates with neocortex size [17] and tactical deception is considered one of many telltale signs of the cognitive demands associated with living in complex social groups [18]. Brain sizes of cephalopods are more comparable to those of vertebrates than of other molluscs [19] and, although brain size per se has yet to be correlated with deception in cephalopods, this trait is indicative of highly complex behaviour that includes mimicry, tool use and tactical deception [20–22]. We suggest that social complexity may be one of the driving forces behind the evolution and development of tactical deception and advanced cognitive behaviour in cephalopods in general, which parallels that of primates.
Acknowledgements
We thank M. Gillings, J. Havenhand, M. Herberstein, D. J. Kemp and N. Moltschaniwskyj for insightful discussions. C.B. was supported by the ARC. This work was conducted under licence from Macquarie University (ARA 2010 006).
References
- 1.Munn C. A. 1986. Birds that ‘cry wolf’. Nature 319, 143–145 10.1038/319143a0 (doi:10.1038/319143a0) [DOI] [Google Scholar]
- 2.Wheeler B. C. 2009. Monkeys cry wolf? Tufted capuchin monkeys use anti-predator calls to usurp resources from conspecifics. Proc. R. Soc. B 276, 3013–3018 10.1098/rspb.2009.0544 (doi:10.1098/rspb.2009.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahavi A. 1975. Mate selection: a selection for handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 4.Johnstone R. A., Grafen A. 1993. Dishonesty and the handicap principle. Anim. Behav. 46, 759–764 10.1006/anbe.1993.1253 (doi:10.1006/anbe.1993.1253) [DOI] [Google Scholar]
- 5.Dawkins M. S., Guilford T. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14 10.1016/S0003-3472(05)80600-1 (doi:10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 6.Ryan M. J., Tuttle M. D., Taft L. K. 1981. The costs and benefits of frog chorusing behavior . Behav. Ecol. Sociobiol. 8, 273–278 10.1007/BF00299526 (doi:10.1007/BF00299526) [DOI] [Google Scholar]
- 7.Dzieweczynski T. L., Earley R. L., Green T. M., Rowland W. J. 2005. Audience effect is context dependent in Siamese fighting fish, Betta splendens. Behav. Ecol. 16, 1025–1030 10.1093/beheco/ari088 (doi:10.1093/beheco/ari088) [DOI] [Google Scholar]
- 8.Packard A. 1972. Cephalopods and fish: the limits of convergence. Biol. Rev. 47, 241–307 10.1111/j.1469-185X.1972.tb00975.x (doi:10.1111/j.1469-185X.1972.tb00975.x) [DOI] [Google Scholar]
- 9.Messenger J. B. 2001. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 76, 473–528 10.1017/S1464793101005772 (doi:10.1017/S1464793101005772) [DOI] [PubMed] [Google Scholar]
- 10.Zylinski S., How M. J., Osorio D., Hanlon R. T., Marshall N. J. 2011. To be seen or to hide: visual characteristics of body patterns for camouflage and communication in the Australian giant cuttlefish Sepia apama. Am. Nat. 177, 681–690 10.1086/659626 (doi:10.1086/659626) [DOI] [PubMed] [Google Scholar]
- 11.Hanlon R. T., Naud M., Shaw M. J., Havenhand J. N. 2005. Transient sexual mimicry leads to fertilization. Nature 433, 212. 10.1038/433212a (doi:10.1038/433212a) [DOI] [PubMed] [Google Scholar]
- 12.Norman M. D., Finn J., Tregenza T. 1999. Female impersonation as an alternative reproductive strategy in giant cuttlefish. Proc. R. Soc. Lond. B 266, 1347–1349 10.1098/rspb.1999.0786 (doi:10.1098/rspb.1999.0786) [DOI] [Google Scholar]
- 13.Moynihan M., Rodaniche A. F. 1982. The behavior and natural history of the Caribbean reef squid Sepioteuthis sepioidea with a consideration of social, signal, and defensive patterns for difficult and dangerous environments. Adv. Ethol. 25, 1–151 [Google Scholar]
- 14.Johnstone R. A. 1988. Game theory and animal behavior. New York, NY: Oxford University Press [Google Scholar]
- 15.Owens I. P. F., Hartley I. R. 1991. ‘Trojan sparrows’: evolutionary consequences of dishonest invasion for the badge-of-status model. Am. Nat. 138, 1187–1205 10.1086/285277 (doi:10.1086/285277) [DOI] [Google Scholar]
- 16.Whiten A., Byrne R. W. 1988. Tactical deception in primates. Behav. Brain Sci. 11, 233–273 10.1017/S0140525X00049682 (doi:10.1017/S0140525X00049682) [DOI] [Google Scholar]
- 17.Byrne R. W., Corp N. 2004. Neocortex size predicts deception rates in primates. Proc. R. Soc. Lond. B 271, 1693–1699 10.1098/rspb.2004.2780 (doi:10.1098/rspb.2004.2780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiten A., Byrne R.W. 1997. Machiavellian intelligence. II. Extensions and evaluations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 19.Packard A. 1972. Cephlapods and fish: the limits of convergence. Biol. Rev. 47, 241–307 10.1111/j.1469-185X.1972.tb00975.x (doi:10.1111/j.1469-185X.1972.tb00975.x) [DOI] [Google Scholar]
- 20.Hanlon R. T., Messenger J. B. 1996. Cephalopod behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 21.Finn J. K., Tregenza T., Norman M. D. 2009. Defensive tool use in a coconut carrying octopus. Curr. Biol. 19, R1069–1070 10.1016/j.cub.2009.10.052 (doi:10.1016/j.cub.2009.10.052) [DOI] [PubMed] [Google Scholar]
- 22.Norman M. D., Finn J., Tregenza T. 2001. Dynamic mimicry in an Indo–Malayan octopus. Proc. R. Soc. Lond. B 268, 1755–1758 10.1098/rspb.2001.1708 (doi:10.1098/rspb.2001.1708) [DOI] [PMC free article] [PubMed] [Google Scholar]