Abstract
Climate change is driving adaptive shifts within species, but research on plants has been focused on phenology. Leaf morphology has demonstrated links with climate and varies within species along climate gradients. We predicted that, given within-species variation along a climate gradient, a morphological shift should have occurred over time due to climate change. We tested this prediction, taking advantage of latitudinal and altitudinal variations within the Adelaide Geosyncline region, South Australia, historical herbarium specimens (n = 255) and field sampling (n = 274). Leaf width in the study taxon, Dodonaea viscosa subsp. angustissima, was negatively correlated with latitude regionally, and leaf area was negatively correlated with altitude locally. Analysis of herbarium specimens revealed a 2 mm decrease in leaf width (total range 1–9 mm) over 127 years across the region. The results are consistent with a morphological response to contemporary climate change. We conclude that leaf width is linked to maximum temperature regionally (latitude gradient) and leaf area to minimum temperature locally (altitude gradient). These data indicate a morphological shift consistent with a direct response to climate change and could inform provenance selection for restoration with further investigation of the genetic basis and adaptive significance of observed variation.
Keywords: Climate change, leaf morphology, adaptive shifts, latitude gradient, altitude gradient, Dodonaea viscosa
1. Introduction
Climate change is expected to drive adaptive shifts within species but work to date on plants has been focused almost exclusively on phenology [1]. A body of evidence suggests that leaf morphology has adaptive significance linked with climate. Fossil leaf assemblages are used to infer paleoclimate, particularly temperature [2]. Indeed, leaf morphology has been demonstrated to vary within species along spatial climate (latitude and altitude) gradients [3], and broader leaves are more susceptible to extremes of temperature [4]. In previous studies, leaf size within species decreased spatially with increasing mean temperature, decreasing rainfall or increasing altitude [3,5–8].
Analysis of morphological and genetic variation in animals has demonstrated correlation, adaptation or plasticity along climate gradients and shifts along gradients related to climate change [9–13]. Previous studies of leaf morphology within plant species along climate gradients have focused on localized altitude gradients [3,7,14], microhabitats [4], or changes along large-scale gradients [5,6,8,15,16].
Between 1950 and 2005, mean maximum temperatures in South Australia rose by 1.2°C, whereas mean rainfall changed little in the study region [17]. Accordingly, we predicted that leaf width should have decreased temporally within some species. Morphological shifts linked to contemporary climate change have been reported sparsely in animals [11] but not plants. We used herbarium and field-collected specimens to investigate change in leaf morphology of Dodonaea viscosa subsp. angustissima (DC.) J. G. West in the Adelaide Geosyncline region of South Australia over approximately 120 years in the context of a 5° latitude gradient (herbarium specimens) and a localized 500 m altitude gradient (field samples). We tested the hypothesis that leaf morphology is spatially structured along latitude and altitude gradients and that contemporary climate change has driven a narrowing of leaf width regionally due to increased summer maximum temperatures.
2. Material and methods
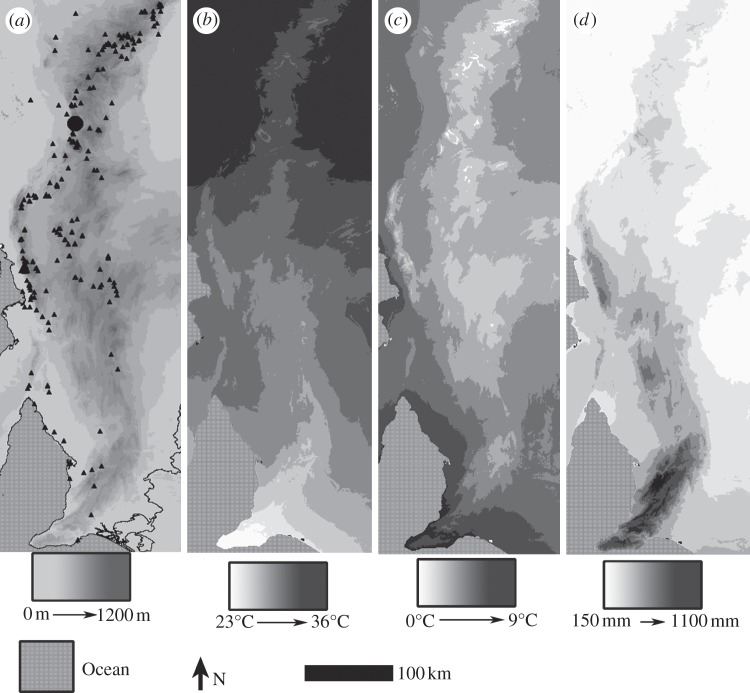
The study area was the Adelaide Geosyncline region, South Australia, bound by latitude −30 to −35° N and longitude 137 to 139° E. The region is dominated by north–south-orientated ranges and experiences a Mediterranean climate. Mean maximum summer temperatures are most strongly correlated with latitude, mean minimum winter temperatures (negatively) with altitude and mean annual rainfall (negatively) with latitude (figure 1).
Figure 1.
Maps of study region (Adelaide Geosyncline) and key climate gradients. (a) Field altitude sampling site (large filled circle) and collection locations of historical herbarium specimens of Dodonaea viscosa subsp. angustissima used in the study (triangles) over altitude base map; (b) mean maximum temperature of warmest month; (c) mean minimum temperature of coldest month and (d) mean annual rainfall.
Dodonaea viscosa (L.) Jacq. is a variable species with subspecies distributed across six continents [18–20]. Variation in leaf morphology within the species is putatively linked to climate [19]. Dodonaea viscosa subsp. angustissima has linear to narrowly oblong leaves, with length-to-width ratio typically more than 7.5.
Collections with sufficient data (n = 255) of D. viscosa subsp. angustissima held at the State Herbarium of South Australia were examined for herbarium regions FR (Flinders Ranges), NL (Northern Lofty) and SL (Southern Lofty). Scaled photographs of collections were taken with a standard digital camera for digital measurement.
Field population samples of D. viscosa subsp. angustissima were taken over an altitude gradient in the Heysen Range (G. R. Guerin 1196 & D. I. Jardine; −31.31455°, 138.56702°). Branchlets were taken from five individuals at 11 × 50 m altitude intervals estimated with a GPS between 300 and 800 m. Five (/four) mature leaves per branchlet were sampled (n = 274). Leaf samples were fully dried before scanning for measurement.
Herbarium collection data were sourced, including date and latitude. Leaf width (at widest point), length and area were measured (table 1). Pearson correlation coefficients (Pearson's r) were calculated, comparing leaf parameters, latitude, altitude and year. Statistically significant coefficients (adjusted p-values using Holm's method) were used to select response and explanatory variables for modelling. Fit and significance of models were evaluated with p and r2 values and visual evaluation of residuals plots for trends suggesting significant outliers or non-normality. Coefficients were bootstrapped with 1000 replicates and 95% CIs calculated. Statistical analyses were conducted in R. Data are available in the electronic supplementary material.
Table 1.
Leaf morphology statistics for samples of Dodonaea viscosa subsp. angustissima from the Adelaide Geosyncline region, South Australia.
| samples | n | leaf width (mm)a | leaf length (mm)a | leaf area (mm2)a | leaf length-to-width ratioa |
|---|---|---|---|---|---|
| herbarium samples (regional latitude gradient) | 255 | 3.2 ± 1.5 [0.46] (0.7–8.8) | 59.8 ± 12.6 [0.21] (29.9–103.2) | 121.4 ± 59.2 [0.49] (28.4–356.8) | 23.3 ± 14.0 [0.60] (6.4–129.4) |
| field samples (Heysen Range altitude gradient) | 274 | 1.9 ± 0.5 [0.27] (1.0–3.5) | 57.1 ± 11.2 [0.19] (18.7–90.8) | 77.7 ± 23.5 [0.30] (29.4–171.6) | 31.3 ± 8.4 [0.27] (12.4–58.8) |
aMean ± standard deviation [coefficient of variation] (range).
3. Results
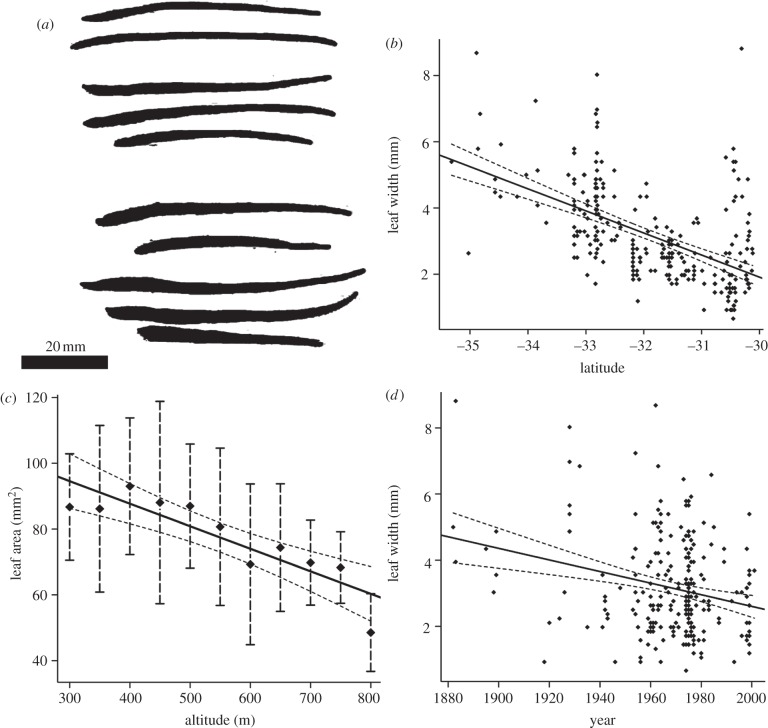
Leaf width was negatively correlated with latitude regionally and leaf area was negatively correlated with altitude locally (table 2, figure 2). A temporal shift towards narrower leaves was confirmed. Leaves have become narrower by 2 mm, equivalent to a 3° northward latitude shift. Variation was a linear function of latitude and year (r2 = 0.33400; p < 0.0001) plus within-population variance±local altitudinal variation (sampled population: cv = 0.27, width of widest leaves 3.5× narrowest, range covers variation in herbarium samples at most latitudes). Temporal variation was not confounded by correlation between latitude and year of collection (p = 0.539). Ninety-five per cent confidence intervals obtained from bootstrapping of coefficients in the bivariate model (latitude and year) confirmed the relationships (i.e. slopes remained negative within confidence intervals).
Table 2.
Statistics for leaf width and area in terms of explanatory variables for Dodonaea viscosa subsp. angustissima.
| explanatory variable | slope | intercept | adjusted r2 | p-value | Pearson correlation coefficient (adjusted p-value) |
|---|---|---|---|---|---|
| herbarium samples (leaf width) | |||||
| latitude | −0.66753 | −18.11617 | 0.28740 | <0.0001 | −0.54 (<0.0001) |
| year | −0.01756 | 37.72309 | 0.05723 | <0.0001 | −0.25 (0.0017) |
| latitude and year covariates | see below | 13.94 | 0.33400 | <0.0001 | — |
| latitude (covariate with year) | −0.65535 | — | — | <0.0001 | — |
| year (covariate with latitude) | −0.01609 | — | — | <0.0001 | — |
| field samples (leaf area) | |||||
| altitude | −0.067684 | 114.765793 | 0.2025 | <0.0001 | −0.45 (<0.0001) |
Figure 2.
Variation in leaf parameters in Dodonaea viscosa subsp. angustissima. (a) Scanned leaf examples (two individuals from altitude gradient). (b) Leaf width versus latitude (herbarium collections). (c) Leaf area versus altitude (field samples). (d) Leaf width versus year (herbarium collections) (dots, raw data; solid lines, linear models; dashed lines, 95% confidence; bars, one standard deviation).
4. Discussion
Morphological variation within species along gradients suggests adaptation which may allow local persistence and migration of adaptive potential [21], or at least response linked to climate. As such, spatial gradients are surrogates for temporal change and can link temporal changes to climate. Temporal change predicted by a latitudinal gradient in leaf width in the study taxon was confirmed through analysis of historical collections. We conclude that leaf width is closely linked to maximum temperature at a regional level (latitude gradient) and leaf area to minimum temperature locally (altitude gradient).
There was no correlation between latitude and year of collection that might generate bias. The influence of latitude across the temporal dataset was accounted for by including latitude as a covariate with year. The width of leaves from the earliest collections was not inflated by a southerly bias in latitude of those collections, in fact many were collected at more northern latitudes.
The relative influence of genetic and plastic response in this dataset is unknown but could be tested formally, for example, through common garden experiments. The adaptive significance of observed spatial and temporal shifts could be tested in terms of the physiology or fitness of individuals with different morphologies.
Within-population variation measured from field altitude sampling was significant. Observed spatial, temporal and population variations suggests that there is potential for further shifts in leaf morphology to occur within populations. Greater shifts could potentially occur through gene flow, the potential for which is currently unknown.
Restoration practices are needed that pre-empt environmental change and choose species and propagule sources to ensure sufficient adaptive potential [22,23]. The study taxon has potential for morphological shifts in response to climate and may be suitable for restoration in a changing climate. If sufficiently sampled, within-population variation and/or plasticity should allow such shifts. At exposed sites, consideration could be given to sourcing seed from further north where individuals may be pre-adapted to warmer temperatures.
This study was only possible with herbarium collections throughout the region spanning three centuries and their institutional maintenance. Biological collections have proved to be useful in tracking changes in the distribution, phenology and morphology of species due to climate change [24]. They have also been used in studies of morphological variation along spatial gradients [9], although less frequently than field sampling. Although not systematically sampled, such collections allow wider spatial and temporal sampling than possible with de novo field sampling and one can supplement them with field sampling of populations. Datasets with less spatial and temporal coverage have less potential for detecting temporal shifts.
This study adds to a growing number that not only predict temporal shifts in morphology within species through spatial gradient analysis [5–9,11,12,14–16] but also attest to real, recent, temporal change [11,25].
Acknowledgements
Research was supported by Premier's Science and Research Fund, Terrestrial Ecosystems Research Network, Australian Research Council (LP110100721), State Herbarium of South Australia, Duncan Jardine.
References
- 1.Walther G., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J., Hoegh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 2.Peppe D. J., et al. 2011. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol. 190, 724–739 10.1111/j.1469-8137.2010.03615.x (doi:10.1111/j.1469-8137.2010.03615.x) [DOI] [PubMed] [Google Scholar]
- 3.Byars S. G., Papst W., Hoffmann A. A. 2007. Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution 61, 2925–2941 10.1111/j.1558-5646.2007.00248.x (doi:10.1111/j.1558-5646.2007.00248.x) [DOI] [PubMed] [Google Scholar]
- 4.Ackerly D. D., Knight C. A., Weiss S. B., Barton K., Starmer K. P. 2002. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130, 449–457 10.1007/s004420100805 (doi:10.1007/s004420100805) [DOI] [PubMed] [Google Scholar]
- 5.Wu L., Kang H., Zhuang H., Liu C. 2010. Variations of Quercus variabilis leaf traits in relation to climatic factors at regional scale. Chin. J. Ecol. 29, 2309–2316 [Google Scholar]
- 6.Danquah J. A., Appia M., Ari P. 2011. Eco-geographic variation in leaf morphology of African Mahogany (Khaya anthotheca and Khaya ivorensis) provenances in Ghana. Eur. J. Sci. Res. 51, 18–28 [Google Scholar]
- 7.Hoffmann A. A., Griffin P. C., Macraild R. D. 2009. Morphological variation and floral abnormalities in a trigger plant across a narrow altitudinal gradient. Aust. Ecol. 34, 780–792 10.1111/j.1442-9993.2009.01984.x (doi:10.1111/j.1442-9993.2009.01984.x) [DOI] [Google Scholar]
- 8.Mediavilla S., Gallardo-López V., González-Zurdo P., Escudero A. 2011. Patterns of leaf morphology and leaf N content in relation to winter temperatures in three evergreen tree species. Int. J. Biometeorol. (doi:10.1007/s00484-011-0498-2) [DOI] [PubMed] [Google Scholar]
- 9.Schäuble C. S. 2004. Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biol. J. Linn. Soc. 82, 39–56 10.1111/j.1095-8312.2004.00315.x (doi:10.1111/j.1095-8312.2004.00315.x) [DOI] [Google Scholar]
- 10.Umina P. A., Weeks A. R., Kearney M. R., McKechnie S. W., Hoffmann A. A. 2005. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308, 691–693 10.1126/science.1109523 (doi:10.1126/science.1109523) [DOI] [PubMed] [Google Scholar]
- 11.Gardner J. L., Heinsohn R., Joseph L. 2009. Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines. Proc. R. Soc. B 276, 3845–3852 10.1098/rspb.2009.1011 (doi:10.1098/rspb.2009.1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf M., Friggens M., Salazar-Bravo J. 2009. Does weather shape rodents? Climate related changes in morphology of two heteromyid species. Naturwissenschaften 96, 93–101 10.1007/s00114-008-0456-y (doi:10.1007/s00114-008-0456-y) [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann A. A., Sgrò C. M. 2011. Climate change and evolutionary adaptation. Nature 470, 479–483 10.1038/nature09670 (doi:10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 14.Montesinos-Navarro A., Wig J., Pico F. X., Tonsor S. J. 2011. Arabidopsis thaliana populations show clinal variation in a climatic gradient associated with altitude. New Phytol. 189, 282–294 10.1111/j.1469-8137.2010.03479.x (doi:10.1111/j.1469-8137.2010.03479.x) [DOI] [PubMed] [Google Scholar]
- 15.Castro-Díez P., Villar-Salvador P., Pérez-Rontomé C., Maestro-Martínez M., Montserrat-Martí G. 1997. Leaf morphology and leaf chemical composition in three Quercus (Fagaceae) species along a rainfall gradient in NE Spain. Tree 11, 127–134 [Google Scholar]
- 16.Castro-Díez P., Villar-Salvador P., Peérez-Rontomé C., Maestro-Martínez M., Montserrat-Martí G. 1998. Leaf morphology, leaf chemical composition and stem xylem characteristics in two Pistacia (Anacardiaceae) species along a climatic gradient. Flora 193, 195–202 [Google Scholar]
- 17.Suppiah R., Preston B., Whetton P. H., McInnes K. L., Jones R. N., Macadam I., Bathols J., Kirono D. 2006. Climate change under enhanced greenhouse conditions in South Australia. Aspendale, Victoria, Australia: Climate Impacts and Risk Group, CSIRO Marine and Atmospheric Research; See http://www.climatechange.sa.gov.au/uploads/pdf/SA_CMAR_report_High_resolution.pdf [Google Scholar]
- 18.West J. G. 1984. A revision of Dodonaea Miller (Sapindaceae) in Australia. Brunonia 7, 1–194 10.1071/BRU9840001 (doi:10.1071/BRU9840001) [DOI] [Google Scholar]
- 19.West J. G., Noble I. R. 1984. Analyses of digitised leaf images of the Dodonaea viscosa complex in Australia. Taxon 33, 595–613 10.2307/1220777 (doi:10.2307/1220777) [DOI] [Google Scholar]
- 20.Harrington M. G., Gadek P. A. 2009. A species well travelled—the Dodonaea viscosa (Sapindaceae) complex based on phylogenetic analyses of nuclear ribosomal ITS and ETS sequences. J. Biogeogr. 36, 2313–2323 10.1111/j.1365-2699.2009.02176.x (doi:10.1111/j.1365-2699.2009.02176.x) [DOI] [Google Scholar]
- 21.Davis M. B., Shaw R. G., Etterson J. R. 2006. Evolutionary responses to changing climate. Ecology 86, 1704–1714 10.1890/03-0788 (doi:10.1890/03-0788) [DOI] [Google Scholar]
- 22.Rice K. J., Emery N. C. 2003. Managing microevolution: restoration in the face of global change. Front. Ecol. Environ. 1, 469–478 10.1890/1540-9295(2003)001[0469:MMRITF]2.0.CO;2 (doi:10.1890/1540-9295(2003)001[0469:MMRITF]2.0.CO;2) [DOI] [Google Scholar]
- 23.Harris J. A., Hobbs R. J., Higgs E., Aronson J. 2006. Ecological restoration and global climate change. Restoration Ecol. 14, 170–176 10.1111/j.1526-100X.2006.00136.x (doi:10.1111/j.1526-100X.2006.00136.x) [DOI] [Google Scholar]
- 24.Suarez A. V., Tsutsui N. D. 2004. The value of museum collections for research and society. BioScience 54, 66–74 10.1641/0006-3568(2004)054[0066:TVOMCF]2.0.CO;2 (doi:10.1641/0006-3568(2004)054[0066:TVOMCF]2.0.CO;2) [DOI] [Google Scholar]
- 25.Millien V., Lyons S. K., Olson L., Smith F. A., Wilson A. B., Yom-Tov Y. 2006. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol. Lett. 9, 853–869 10.1111/j.1461-0248.2006.00928.x (doi:10.1111/j.1461-0248.2006.00928.x) [DOI] [PubMed] [Google Scholar]