Abstract
Costs that individuals incur through mating can play an important role in understanding the evolution of life histories and senescence, particularly in promiscuous species. Copulation costs, ranging from energy expenditure to reduced longevity, are widely studied in insects but have received substantially less attention in other taxa. One cost of mating, the energetic cost, is poorly studied across all taxa despite its potential importance for the many species where copulation is physically demanding and/or frequent. Here, we investigated the energetic cost of mating in both male and female dumpling squid (Euprymna tasmanica). In this species, copulation can last up to 3 h and requires that the male physically restrains the female. We report that the act of copulation halves the swimming endurance of both sexes, and that they take up to 30 min to recover. Such a reduction in post-copulatory performance may have important implications for predator avoidance, foraging ability and energy allocation. Therefore, quantifying this cost is essential to understand the evolution of reproductive strategies and behaviours such as female receptivity and male and female mating frequency.
Keywords: resource allocation, copulation behaviour, sexual reproduction
1. Introduction
For many species, mating involves strenuous physical activity and inflicts significant costs, including physical injury [1], reduced lifespan [2] and even death from sexual cannibalism [3]. Such costs of mating have important implications for the evolution of life-history strategies and ageing [4,5], particularly for species with promiscuous mating systems. Despite the importance of copulation costs in evolutionary biology, some costs are substantially underrepresented in the literature.
One intuitive, though largely overlooked, consequence of mating is its energetic cost. Many taxa, including insects, crustaceans and reptiles, have prolonged, very active and/or frequent copulations [6–9]. Although traditionally assumed to be trivial, energetic costs are likely to be significant for these taxa. To date, energetic costs have only been confirmed in a millipede, in which oxygen consumption during copulation was elevated relative to resting levels [9]. Whether such metabolic costs affect an individual's physical capabilities post-copulation has never, to our knowledge, been demonstrated. However, a reduction in physical capabilities post-copulation may influence important behaviours such as predator avoidance and foraging which, in turn, may impact survival, growth and future reproduction.
Cephalopods (squid, octopus, cuttlefish and nautilus) are an excellent model system in which to investigate mating costs because all species are promiscuous and most live for less than one year. These traits are likely to compound the costs of mating and concentrate the costs within a short period, respectively. Furthermore, investigations into copulation costs in cephalopods are uncommon; thus such experiments can provide an insight into the evolution of reproductive strategies and behaviours in this group.
Here, we used wild-caught dumpling squid (Euprymna tasmanica) to investigate the energetic cost of copulation and subsequent recovery time. Mating is likely to be energetically costly in this species because both sexes mate multiply, copulation can last up to 3 h and the male must physically restrain the female (figure 1). We aimed to test this hypothesis and determine the recovery time for individuals of both sexes.
Figure 1.

Euprymna tasmanica mating, male on left and female on right (courtesy of Mark Norman).
2. Material and methods
We collected E. tasmanica through multiple shallow (less than 5 m) night SCUBA dives at St Leonards (38°10′13 S, 144°43′11 E) in southeastern Australia from March 2010 until June 2010. Upon capture, we transferred squid to facilities at the Victorian Marine Science Consortium in Queenscliff, where they were housed individually in glass tanks (24 cm3 volume). Each holding aquarium contained a layer of sand substrate and a short (6.5 × 5.5 cm diameter) length of PVC pipe for shelter. Aquarium lights provided a reverse 12 L : 12 D cycle and all aquaria received a continuous through-flow of aerated, ambient (14–20°C) seawater pumped directly from Port Phillip Bay. We fed squid Palaemon sp. shrimp ad libitum, checked the health of each squid and monitored water flow every second day. Squid had an acclimation period of at least 18 days prior to being used in experiments.
(a). Swimming endurance
We tested the energetic cost of mating by determining a squid's swimming endurance before and immediately after mating. This was measured by placing squid in a clear cylindrical swimming chamber in a flume and making them swim against a constant current (24 cm–1) until exhaustion. Methods used and flume design were adapted from Stobutzki & Bellwood [10], except that water velocity was kept constant at 24.4 cm−1 instead of steadily increasing.
Before treatment, sexually mature squid were randomly allocated into treatment (mated; n = 30) and control (not mated; n = 17) groups, then blotted to remove any water and weighed. On day 1, we tested baseline squid endurance. Squid were placed in the flume and once they had moved halfway down, the flow and timer were switched on. Squid would swim, then tire and touch the rear mesh. Squid were then gently touched three times at one second intervals and most would swim again. This was repeated until they would no longer swim following the three touches, at which time the flow and timer were switched off. On day 2, treatment squid were mated. Males were placed in a mating tank (volume 1.5 l) and left to acclimate for 10 min before the female was introduced. If they did not begin mating within 30 min, females were disturbed and generally the male would initiate copulation. Occasionally (n = 5), pairs would not mate for unknown reasons and different pairs were selected in these cases. During mating, we monitored squid from behind a curtain to avoid disturbance to the study animals and to determine when mating concluded. Immediately after the conclusion of mating, we tested their endurance following the earlier-mentioned method and alternating which sex was tested first. Control squid also had their endurance tested on day 2.
To investigate recovery time, we performed a second experiment using the same methods as earlier-mentioned except that endurance was tested 30 min after the conclusion of mating on day 2 (n = 23).
(b). Statistical analysis
We analysed the data (see electronic supplementary material, tables S1 and S2) via a repeated-measures linear mixed model (PROC MIXED, SAS v. 9.1) with swimming duration (seconds) as the response variable. Residual plots indicated that this variable did not require transformation to meet the assumptions of a linear model. The independent variables were sex (male, female), treatment (control, mated), time (before mating, after mating) and their interactions. Time was the repeated measure within individual squid (individual ID as the blocking factor). Initially, we included weight and copulation duration as random factors but they were found to have no influence on results (copulation duration: F1,40 = 0.02, p = 0.90; squid mass: F1,26 = 0.23, p = 0.63) and we therefore excluded them from the final model. For significant effects, we used post-hoc Tukey's tests to assess differences between group means.
3. Results
The endurance of both males and females was reduced by mating (F1,85 = 14.60, p < 0.01; figure 2a), and this reduction was not affected by squid mass or mating duration. There was also no difference in the size of this reduction between the sexes (F1,85 = 1.79, p = 0.18); however, males were observed to swim for longer durations than females (F1,85 = 13.31, p < 0.01). Squid recovered their endurance capacity within 30 min after mating (figure 2b). Swimming duration at this time was not significantly different from swimming duration recorded on day 1 (F1,21 = 1.85, p = 0.20). Males in this treatment group also swam for longer than females (F1,21 = 6.73, p = 0.02).
Figure 2.
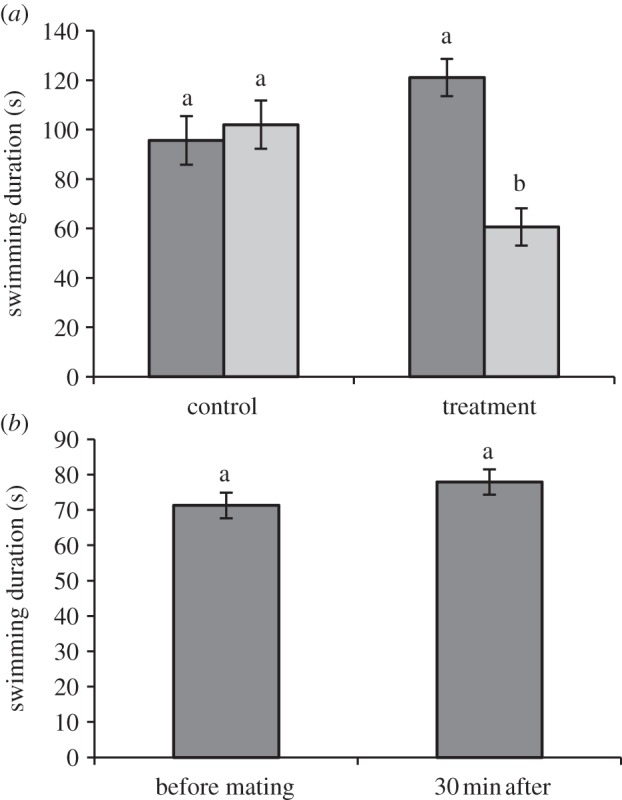
(a) The effect of copulation on swimming duration (mean ± s.e.m.) immediately after mating. Dark bars are swimming duration on day 1, light bars are swimming duration on day 2. (b) The effect of copulation on swimming duration (mean ± s.e.m.) 30 min after mating concluded.
4. Discussion
Swimming endurance was clearly reduced by mating and the magnitude of this effect was similar for both sexes. We expected males to be more strongly affected than females because males are more active during copulation. The males physically restrain the female, pump jets of water into her mantle and jet, change colour and ink more than females do. By contrast, the female appears relatively quiescent, only occasionally jetting or visibly elongating the mantle. The reduced endurance in males following copulation most likely arises because the level of activity that takes place during copulation cannot be sustained by aerobic metabolism alone; males may require anaerobic metabolism. This would impede swimming ability because anaerobic metabolism (glycolysis) increases lactate production, which is an important agent of muscle fatigue [11].
Reduced endurance in females, on the other hand, is unlikely to be due to female activity but rather a result of the copulatory position. The male's copulatory grip visibly constricts the female's mantle cavity throughout the duration of copulation (figure 1) and the hectocotylus will enlarge to twice its original size after insertion. These actions could cause an oxygen debt by decreasing the amount of oxygenated water that reaches her gills and inhibiting the circulation of oxygenated blood around her body. Squid can withstand hypoxic conditions by increasing anaerobic metabolism [12], which would result in lactate production [11]. Despite the different causes of anaerobic metabolism between the sexes, the result (muscle fatigue) is the same, which may explain why there was no difference in the reduction in swimming endurance between the sexes.
If copulation causes muscle fatigue, we might expect a correlation between swimming duration and copulation duration. However, individuals varied substantially in their motivation to swim and their style of swimming (jetting or finning). These factors would confound any correlation between swimming duration and copulation duration, which may be why there no correlation was observed.
The second experiment, which investigated time until recovery of endurance capacity, demonstrated that squid of both sexes recover from copulation within 30 min. A similarly short recovery from exercise or exhaustion is observed in other taxa [13,14], most likely because cellular changes that occur during exercise can be reversed quite quickly (generally within 1–60 min [11]). However, the energetic investment in mating still represents a significant cost. This cost is particularly relevant for species that are short-lived and promiscuous, like E. tasmanica. Frequent mating would compound energetic costs, an effect that may not be simply additive. Reduced locomotion ability is likely to be ecologically relevant as individuals may be more vulnerable to predation, have temporarily reduced foraging ability or be less competitive in seeking subsequent mating opportunities, thereby curtailing future reproduction. Squid, like many other species, lay several clutches over the reproductive period and the energy consumed in each copulation, together with lost foraging opportunities, could also affect investment into future clutches and growth [15,16].
Numerous taxa exhibit mating strategies with prolonged copulations, frequent mating and/or short lifespans [6,7,17]. To understand the evolution of reproductive strategies for these taxa, it is crucial to quantify the energetic costs. For instance, a significant energetic cost has the potential to influence diverse reproductive behaviours, such as female receptivity and mating frequency [18,19], and how such behaviours may change over an individual's lifetime and across different environmental conditions [20]. The new evidence presented here, showing that mating affects physical performance post-copulation, highlights the need to account for energetic costs when assessing the costs of mating.
Acknowledgments
We thank Rod Watson and Ben Wegener for their help with fieldwork, and Mark Elgar and Bob Wong for their comments about the manuscript. Funding was from the Hermon Slade Foundation. Research was carried out with approval from the University of Melbourne Animal Ethics Committee (ID: 0810874.3), and animals were collected under Fisheries Victoria collecting permit RP962.
References
- 1.Le Boeuf B. J., Mesnick S. 1991. Sexual behavior of male northern elephant seals. I. Lethal injuries to adult females. Behaviour 116, 143–162 (doi:10.1163/156853990×00400) [DOI] [Google Scholar]
- 2.Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 10.1038/373241a0 (doi:10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 3.Elgar M. A., Schneider J. M. 2004. Evolutionary significance of sexual cannibalism. In Advances in the study of behavior (ed. Slater P. J. B.), pp. 135–163 London, UK: Academic Press [Google Scholar]
- 4.Rose M. R. 1984. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38, 1004–1010 10.2307/2408434 (doi:10.2307/2408434) [DOI] [PubMed] [Google Scholar]
- 5.Partridge L. 1987. Is accelerated senescence a cost of reproduction? Funct. Ecol. 1, 317–320 10.2307/2389786 (doi:10.2307/2389786) [DOI] [Google Scholar]
- 6.Andrés J. A., Cordero Rivera A. 2000. Copulation duration and fertilization success in a damselfly: an example of cryptic female choice? Anim. Behav. 59, 695–703 10.1006/anbe (doi:10.1006/anbe) [DOI] [PubMed] [Google Scholar]
- 7.Burghard W., Maier G. 2000. The effect of temperature on mating duration in the freshwater cyclopoid copepod Cyclops vicinus (Uljanin, 1875). Crustaceana 73, 1259–1262 10.1163/156854000505236 (doi:10.1163/156854000505236) [DOI] [Google Scholar]
- 8.Olsson M., Madsen T. 1998. Sexual selection and sperm competition in reptiles. In Sexual selection and sperm competition (eds Birkhead T. R., Møller A. P.), pp. 503–577 London, UK: Academic Press [Google Scholar]
- 9.Telford S., Webb P. 1998. The energetic cost of copulation in a polygynandrous millipede. J. Exp. Biol. 201, 1847–1849 [DOI] [PubMed] [Google Scholar]
- 10.Stobutzki I. C., Bellwood D. R. 1994. An analysis of the sustained swimming abilities of pre- and post-settlement coral reef fishes. J. Exp. Mar. Biol. Ecol. 175, 275–286 10.1016/0022-0981(94)90031-0 (doi:10.1016/0022-0981(94)90031-0) [DOI] [Google Scholar]
- 11.Fitts R. H. 1994. Cellular mechanisms of muscle fatigue. Physiol. Rev. 74, 49–94 10.2466/pr0.1994.74.1.49 (doi:10.2466/pr0.1994.74.1.49) [DOI] [PubMed] [Google Scholar]
- 12.Zielinski S., Lee P. G., Portner H. O. 2000. Metabolic performance of the squid Lolliguncula brevis (Cephalopoda) during hypoxia: an analysis of critical PO2. J. Exp. Mar. Biol. Ecol. 243, 241–259 10.1016/S0022-0981(99)00117-3 (doi:10.1016/S0022-0981(99)00117-3) [DOI] [Google Scholar]
- 13.Farrell A. P., Gamperl A. K., Birtwell I. K. 1998. Prolonged swimming, recovery and repeat swimming performance of mature sockeye salmon Oncorhynchus nerka exposed to moderate hypoxia and pentachlorophenol. J. Exp. Biol. 201, 2183–2193 [DOI] [PubMed] [Google Scholar]
- 14.Wagner E. L., Gleeson T. T. 1997. The influence of thermoregulation on behavioural recovery from exercise in a lizard. Funct. Ecol. 11, 723–728 10.1046/j.1365-2435.1997.00146.x (doi:10.1046/j.1365-2435.1997.00146.x) [DOI] [Google Scholar]
- 15.Schwarzkopf L. 1993. Costs of reproduction in water skinks. Ecology 74, 1970–1981 10.2307/1940840 (doi:10.2307/1940840) [DOI] [Google Scholar]
- 16.Hanssen S. A., Hasselquist D., Folstad I., Erikstad K. E. 2005. Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. R. Soc. B 272, 1039–1046 10.1098/rspb.2005.3057 (doi:10.1098/rspb.2005.3057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher D. O., Blomberg S. P. 2011. Costs of reproduction and terminal investment by females in a semelparous marsupial. PLoS ONE 6, e15226. 10.1371/journal.pone.0015226 (doi:10.1371/journal.pone.0015226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mühlhäuser C., Blanckenhorn W. U. 2002. The costs of avoiding matings in the dung fly Sepsis cynipsea. Behav. Ecol. 13, 359–365 10.1093/beheco/13.3.359 (doi:10.1093/beheco/13.3.359) [DOI] [Google Scholar]
- 19.Watson P. J., Arnqvist G., Stallmann R. R. 1998. Sexual conflict and the energetic costs of mating and mate choice in water striders. Am. Nat. 151, 46–58 10.1086/286101 (doi:10.1086/286101) [DOI] [PubMed] [Google Scholar]
- 20.Garcia C., Huffman M., Shimizu K. 2010. Seasonal and reproductive variation in body condition in captive female Japanese macaques (Macaca fuscata). Am. J. Primatol. 72, 277–286 10.1002/ajp.20777 (doi:10.1002/ajp.20777) [DOI] [PubMed] [Google Scholar]


