Abstract
Pluripotent stem cells are regarded as a promising cell source to obtain human dopamine neurons in sufficient amounts and purity for cell replacement therapy. Importantly, the success of clinical applications depends on our ability to steer pluripotent stem cells towards the right neuronal identity. In Parkinson disease, the loss of dopamine neurons is more pronounced in the ventrolateral population that projects to the sensorimotor striatum. Because synapses are highly specific, only neurons with this precise identity will contribute, upon transplantation, to the synaptic reconstruction of the dorsal striatum. Thus, understanding the developmental cell program of the mesostriatal dopamine neurons is critical for the identification of the extrinsic signals and cell-intrinsic factors that instruct and, ultimately, determine cell identity. Here, we review how extrinsic signals and transcription factors act together during development to shape midbrain cell fates. Further, we discuss how these same factors can be applied in vitro to induce, select, and reprogram cells to the mesostriatal dopamine fate.
1. The Central Role of Ventral Midbrain Dopamine Neurons in Parkinson Disease
Parkinson disease is characterized by the progressive degeneration of dopamine (DA) neurons in the pars compacta of the substantia nigra (SNc) of the ventral midbrain (vm). Neuronal loss takes place also in other brainstem nuclei, such as the locus coeruleus and the dorsal motor nucleus of the vagus nerve [1]. In the adult human brain, these nuclei display a dark pigmentation due to the accumulation of neuromelanin that is lost in Parkinson disease. In addition, Lewy bodies, which are proteinaceous aggregates containing hyperphosphorylated alpha-synuclein [2], ubiquitin, and p62, among other proteins, are typically found in the brainstem of these patients. These aggregates appear also in other brain regions and outside the brain, for example, in the enteric plexus [3]. Although Lewy bodies are regarded as a pathological hallmark of Parkinson disease, there is no direct correlation between the presence of Lewy bodies and neuronal dysfunction [4]. Indeed, inherited forms of Parkinson disease display diverse brain pathology and often lack Lewy bodies [5–7] whilst, on the other hand, Lewy bodies can be found in asymptomatic individuals. Common to inherited and sporadic forms of the disease is the loss of DA neurons in the SNc. Neuronal loss is more pronounced in the ventrolateral subpopulation of vmDA neurons that project to the sensorimotor regions of the striatum [8], the mesostriatal group, and is accompanied by a corresponding somatotopic decrease of DA in these regions.
In order to generate in vitro an adequate cell type for replacement therapy, it is important to characterize the identity and properties of vmDA neurons. All DA neurons express tyrosine hydroxylase (TH), the enzyme that catalyzes the initial, rate-limiting step in the biosynthesis of catecholamines (including DA, noradrenaline, and adrenaline). The most vulnerable neurons, located in the ventrolateral SNc, are often large and heavily melanized and express high levels of the DA receptor D2 (DRD2) and the DA transporter protein (DAT, SLC6A3). In addition, these neurons have relatively low levels of TH and the vesicular monoamine transporter-2 (VMAT2, SLC18) [9], and the majority do not express calbindin-D28k [10]. Some of these features have been correlated with an enhanced susceptibility to oxidative stress and aging [11]. For instance, their high DA turnover combined with a lower intracellular storage capacity than the less vulnerable DA neurons located in the dorsal tier of the SNc, retrorubral field and ventral tegmental area (VTA) can contribute to an earlier and more severe loss of mesostriatal neurons. A greater dependency on calcium channels than the more resilient VTA neurons has also been implicated in the differential vulnerability of these vmDA subpopulations [12].
The mesostriatal vmDA subpopulation is often referred to as the A9 group, following the nomenclature of aldehyde fluorescent cell populations (i.e., containing monoamines) identified using the Falck-Hillarp technique, in the rodent brain [13]. However, the delineation of the equivalent human DA subpopulation is frequently inexact, because some subpopulations of the VTA (A10), mainly the parabrachial pigmented nucleus (PBP), are displaced dorsally and laterally [14]. The accuracy of the markers used to define specific vmDA subpopulations is especially relevant for neurons derived and grown in vitro, whose identification relies solely on the expression of those markers and electrophysiological features.
Expression of the G-protein inward rectifying potassium channel subunit 2 (Girk2, Kir3.2) is high in vmDA neurons, in which Girk channels are formed by homotetramers (i.e., four type-2 subunits), and has been considered a specific marker of vulnerable mesostriatal neurons [9, 15–17]. However, a detailed study has recently reported a similar expression level of Girk2 in the ventral and dorsal tiers of the human SNc [14], with 77% of SNc and 55% of VTA (62% in the PBP) neurons showing a strong Girk2 immunoreactivity. The proportion of TH neurons showing colocalization with Girk2 was similar in the mouse brain [18], where the majority of SNc and VTA neurons showed Girk2 expression [14]. At the ultrastructural level, the presence of this potassium channel had been previously described in all vmDA cells except in the interfascicular nucleus of the VTA [19]. Therefore, the most reliable criterion to separate mesostriatal (A9) and mesocorticolimbic (A10) neurons in vitro is not the presence of Girk2 but the absence of calbindin-D28k in the mesostriatal neurons [10, 14, 20, 21]. Notwithstanding, it should be noted that around 12% (20% in the mouse) of DA neurons in the pars medialis of SNc also coexpress calbindin-D28k [14].
Transplantation of fetal vm cells can restore function in Parkinson patients [22–24]. Because the symptoms appear late in the course of the disease, when a vast majority of the vmDA neurons are already lost, cell replacement approaches constitute an attractive alternative to drug replacement. However, clinical trials have shown a rather modest clinical success and, in some cases, worrying adverse effects [23, 25]. Both the limited benefit and the presence of graft-induced dyskinesias have been attributed to a suboptimal cellular composition of the fetal grafts, although other biological and technical factors are also important. The cells obtained from the fetal vm are heterogeneous; only ~5% are DA neurons [26, 27]. Serotonin neurons from the pontine raphe are usually included in the dissection area [28]. Thus, a substantial number of serotonin neurons as well as GABA neurons and glial cells are present in the fetal vmDA grafts [29]. At present, it is not known whether the presence of different neuronal and glial cell populations in the fetal grafts is detrimental, in terms of functional integration, or beneficial, for example, by providing trophic support to vmDA neurons (see Section 3.3). The presence of serotonin neurons in fetal grafts has been correlated with the development of graft-induced dyskinesias both in patients [30] and in experimental models [28, 31, 32]. Serotonin neurons have the capacity to decarboxylate L-dopa and store DA but cannot regulate DA release and reuptake, because they lack DRD2 autoreceptors and DAT. This imbalance has been proposed to underlie the appearance of graft-induced dyskinesia, based on PET studies and on the pharmacological improvement with buspirone (a 5HT1A partial agonist) [30]. However, the evidence supporting this mechanism in the transplanted patients has been questioned, as dyskinesias should then worsen with L-DOPA, which is not the case [33]. In addition, there is no direct correlation between serotonin hyperinnervation and the severity of the dyskinesias [33]. Finally, buspirone can also function as a partial antagonist on the DRD2 receptors in a model of graft-induced dyskinesia [34] and improve L-DOPA-induced dyskinesia very efficiently in nongrafted animal models [35, 36]. The proportion of mesoprefrontal and mesocorticolimbic DA subpopulations in the grafts has not been examined in detail but the presence of calbindin-D28k positive neurons does not appear to cause adverse effects (even if the mesoprefrontal DA neurons do not express DRD2 or DAT). However, these neurons would not contribute to the synaptic reconstruction of the dorsal striatum [37]. Synapses are highly specialized contacts between specific partners and require bidirectional recognition and communication [38]. Thus, only those cells that display a specific vmDA mesostriatal phenotype will be able to restore the physiological synaptic connections with the medium-size striatal spiny neurons and reestablish a regulated DA transmission leading to functional recovery. The limited availability of fetal tissue and ethical concerns regarding its use has led to an active search for alternative cell sources [39], and hopes are set on pluripotent stem cells to obtain human vmDA neurons in sufficient amounts and purity. Both for pluripotent stem cells and for reprogrammed cells, acquiring and maintaining the right identity will be a key determinant in the success of future clinical applications.
2. Dopamine Neurons: Lineage Specification and Cell Identity
For lineage specification, developmentally regulated morphogens activate transcriptional networks. Transcription factors control, in turn, the expression of receptors and downstream intracellular cascades that are necessary to transduce the extrinsic cues. Coordinated temporal and spatial integration of extrinsic signals and intrinsic determinants is thus required for proper specification of cell identity (Figure 1).
Figure 1.
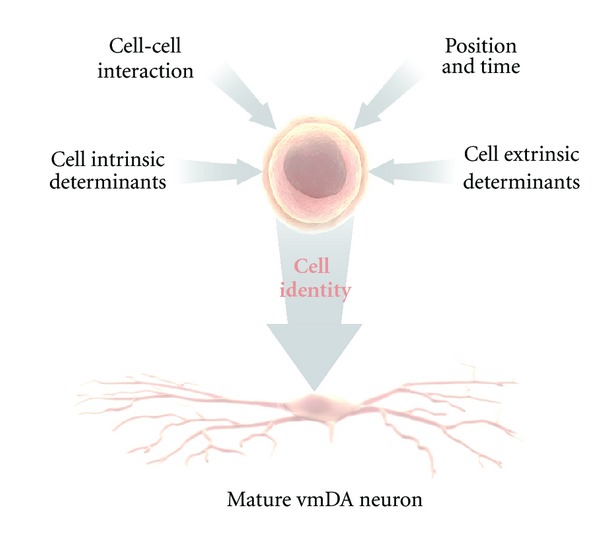
Cell identity is represented as the resultant of the integration of signals that the receptive, undifferentiated cell is exposed to, in a temporal and spatial coordinated fashion.
2.1. Extrinsic Signals
VmDA neurons are generated from ventral midline floor plate (FP) neuroepithelial cells of a nonneurogenic character [40, 41]. The FP is a specialized glial structure located in the most ventral midline of the neural tube from the midbrain to the tail region [42]. It controls neuronal subtype specification along the dorso-ventral (D-V) axis through secretion of the morphogen sonic hedgehog (Shh) [43]. The function of the FP as a ventral organizer of neuronal development is conserved from fish to mammals [44, 45]. The capacity of FP cells to generate neurons is spatially restricted along the rostrocaudal axis of the brain. FP cells in the midbrain acquire neuronal properties characteristic of mDA neurons, while FP cells located caudally to the mesencephalon normally do not give rise to neurons [41].
The isthmic organizer, which forms a boundary between the midbrain and the hindbrain, controls patterning of the midbrain and the anterior hindbrain. It is essential for the specification and normal development of DA neurons and serotonin neurons in the ventral midbrain and hindbrain, respectively [46]. Several signaling factors, including Shh, fibroblast growth factor (Fgf) 8, Fgf17, Fgf18, and Wnt1, are expressed by and around the isthmic organizer and are involved in this process (Figure 2). The combination of Shh and Fgf8 is necessary for the induction of DA neurons in the rostral forebrain and the lateral midbrain [47, 48]. However, Shh is no longer required after E10.5 in the mouse. At this developmental stage, Foxa2, a forkhead transcription factor, induced by Shh, is essential for the generation of vmDA neurons [49–51].
Figure 2.
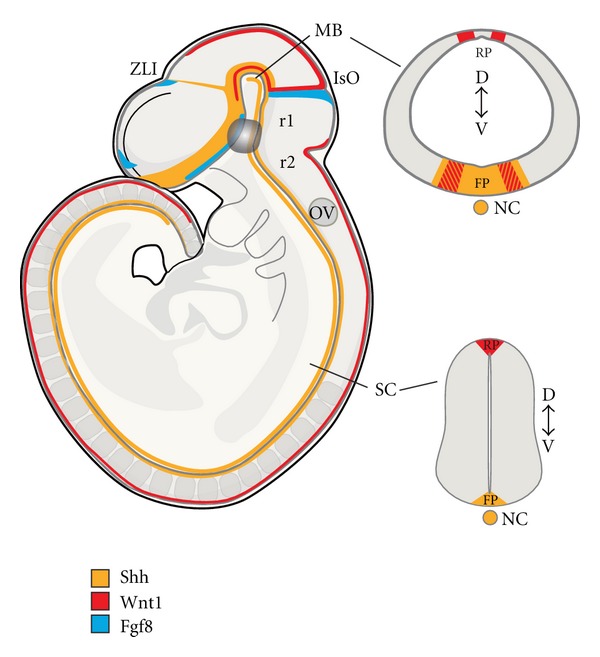
During embryogenesis ventral midbrain dopamine neurons are born at the intersection of three signaling molecules, Shh, Wnt1 and Fgf8, that pattern the neural tube along rostrocaudal, dorsoventral and mediolateral axes. Sagittal and coronal views at the midbrain and spinal cord levels of the mouse embryo showing the expression patterns of these morphogens at E9.5. FP: floor plate; IsO: isthmic organizer; MB: midbrain; NC: notochord; OV: otic vesicle; RP: roof plate; SC: spinal cord; ZLI: zona limitans intermedia.
During early development (starting at E9), Fgf8 is expressed by the isthmic organizer [52, 53] and can mimic the isthmic activity [54, 55]. Fgfs participate in the patterning of the midbrain and the induction of the cerebellum in rhombomere 1. Cerebellar development is induced by strong Fgf signaling mediated by Fgf8b through binding to its tyrosine kinase coupled receptor Fgfr1 and activation of the Ras-extracellular signal-regulated kinase (ERK) pathway. On the other hand, the induction of midbrain is mediated by a lower intensity of signaling, transduced by Fgf8a, Fgf17, and Fgf18 [56–58]. Inactivation of Fgf8 results in loss of midbrain and cerebellar tissues [59, 60]. The deletion of these anatomical structures appears to be due mainly to ectopic cell death, presumably caused by the dysregulation of a transcriptional network including Wnt1, Fgf17, Fgf18, Fgf8, and Gbx2 [61]. Furthermore, Fgf8 appears to maintain normal development of the midbrain and hindbrain by regulating transcription factors such as engrailed-1 (En1), engrailed-2 (En2), and Pax5 [62]. In addition to its function in vmDA neuron specification, Fgf8 directs the rostral growth of axons from vmDA neurons by inducing the repulsion factor semaphorin 3F [63].
Wnt signaling is required for early midbrain development. Wnt1 expression precedes Fgf8, starting at E8.0. During early somite stages, Wnt1 is broadly expressed in the presumptive mesencephalon (1-somite), but following neural tube closure, the expression gradually becomes refined to a narrow band of cells located immediately rostral to the isthmus, and the dorsal midline of the CNS (16 somites) [64] (Figure 2). Wnt1 does not have isthmic-like activity as Fgf8 does. However, Wnt1 is essential as its deletion results in loss of midbrain and cerebellar structures by E10 and in a substantial reduction in the number of vmDA neurons [65–68]. Moreover, Fgf8 and Shh fail to induce TH and Pitx3 expression in the Wnt1 knockout mouse, indicating that Wnt1 is necessary for the development of vmDA neurons [69]. Ectopic expression of Wnt1 in the rostral hindbrain can induce vmDA neurons through the activation of Otx2 expression and the subsequent repression of Gbx2 and Nkx2.2 and induction of mDA markers, including TH and Nurr1 [69]. If ectopic Wnt signaling is combined with restored Lmx1b levels, vmDA neurons appear to be generated also at more caudal levels of the hindbrain, although not in the spinal cord [70]. Interestingly, Otx2 appears to determine the anterior identity that confers neurogenic potential of FP cells. Consequently, ectopic expression of Otx2 in the ventral hindbrain induces vmDA neurons from FP cells, which normally do not give rise to neurons, partly by inducing Lmx1a [41].
Importantly, while Wnt1 expression is largely unaffected by Lmx1a loss-of-function, Lmx1b is a crucial regulator of Wnt1 expression in mDA progenitors at later developmental stages [71].
In addition to the role of canonical Wnt signaling in early specification, Wnt1 and Wnt3a increase neurogenesis and regulate the proliferation of Nurr1-positive vmDA precursor cells [72]. Likewise, disruption of canonical Wnt signaling leads to neurogenesis defects and perturbs the migration and segregation of vmDA neurons [73]. Wnt2 is also involved in vmDA neurogenesis through activation of the canonical pathway [74]. Wnt5a increases the number of vmDA neurons by promoting the acquisition of a fully mature vmDA phenotype through upregulation of Pitx3 expression [72]. Wnt5a is also thought to control morphogenesis, vmDA progenitor cell division, and cell cycle exit [75].
Retinoic acid (RA) also appears to play a role in vmDA neuronal differentiation. Retinal dehydrogenase 1 (Raldh1), which converts retinaldehyde into RA [76], is expressed in the vm already at E9.5 [77]. Pitx3 regulates RA levels in the midbrain by direct transcriptional activation of Raldh1 [78, 79]. Deficiency in Pitx3 results in the selective loss of SNc vmDA neurons [80]. Maternal supplementation of RA can partially rescue SNc degeneration in the Pitx3 knockout mice [79].
Other morphogens and growth factors are important for survival and maturation of vmDA neurons. Members of the transforming growth factors beta (TGFβ) superfamily, bone morphogenetic proteins (BMPs) 2, 6, and 7 are expressed in the developing vm and promote the survival of vmDA neurons in the rat [81–83]. Furthermore, TGFβ2-3, activin and glial cell line-derived neurotrophic factor (GDNF), are neurotrophic factors for vmDA neurons [84–89]. GDNF appears to act as a target-derived neurotrophic factor through its high expression in striatal neurons that are innervated by nigral vmDA neurons [81, 90]. In addition, GDNF is transiently expressed in the midbrain during vmDA neuron specification. Here, GDNF induces Pitx3 via NF-κB-mediated signaling [91]. Pitx3 is in turn required for activating the expression of brain-derived neurotrophic factor (BDNF) in a subpopulation of SNc DA neurons during embryogenesis. The loss of BDNF expression correlates with the increased apoptotic cell death of this vmDA subpopulation in the Pitx3 knockout mouse [91].
2.2. Intrinsic Determinants
Multiple cell-intrinsic factors are involved in the proliferation, specification, maturation, and maintenance of vmDA neurons. The homeobox transcription factor Otx2 controls the positioning of the isthmic organizer, which, in turn, defines the vmDA domain [46, 69, 92–96]. Furthermore, Otx2 participates in patterning the midbrain, regulating proneural gene expression and activating downstream factors of vmDA cell fate determinants, for example, Lmx1a and Msx1/2 [46, 69, 92–96]. Otx2 is thought to be a master regulator in the vmDA neuron developmental program by establishing the most ventral domains. Otx2 expression is maintained mostly in the VTA in the adult midbrain. Consequently, loss of Otx2 in adult shows reduced mesocortical and limbic innervation, but normal mesostriatal connectivity [46, 69, 92–96]. Otx2 appears to specify vmDA neuron subtype identity in the VTA by regulating the levels of Girk2 and DAT. Importantly, when Otx2 was ectopically expressed in SNc vmDA neurons, these vulnerable neurons were protected against MPTP-induced toxicity, presumably by limiting the number of SNc cells with efficient DA uptake and consequently also the uptake of the neurotoxic cation MPP+ [97].
Foxa1/2 expression is induced by Shh in the FP in the ventral midbrain. Specification of FP identity requires a Foxa2-dependent repression of determinants of ventrolateral midbrain fates, including Tle4, Otx1, Sox1, and Tal2, and reduction of Shh signaling [98]. Foxa1/2 is maintained in postmitotic vmDA neurons acting in a gene-dosage dependent manner to regulate the differentiation and phenotypic maturation of vmDA neurons by controlling the expression of Nurr1, En1, TH, and AADC [49, 99–101]. Foxa1/2 is also required for the maintenance of Lmx1a and Lmx1b expression and functions cooperatively with these transcription factors to regulate differentiation of vmDA neurons [96, 100, 101]. Moreover, a recent study has shown that Foxa2 positively regulates the transcription of most determinants of vmDA neuron fate in vm progenitors, including Lmx1a, Lmx1b, Msx1, and Ferd3l, while repressing components of Shh signaling pathway including the Shh receptor Patched-1, the transducers Gli1-3 and the transcription factors Nkx2.2 and Nkx2.9 [98]. Interestingly, maintaining appropriate gene dose levels of Foxa2 appears crucial for long-term survival of vmDA neurons in the adult, since aging Foxa2+/− heterozygous mice develop parkinsonian-like symptoms, correlated with a selective loss of SNc vmDA neurons [99].
Engrailed 1 and 2 (En1 and En2) are initially broadly expressed in the midbrain while at later stages their expression becomes restricted to postmitotic vmDA neurons [102–104]. En1/2 are required, in a gene-dose dependent manner, for the survival and maturation of vmDA neurons, but not for their specification [105, 106]. The vmDA neurons in the En1/2 knockout mice undergo apoptosis due to a cell-autonomous requirement for En1/2 and not due to the loss of mid/hindbrain structures [105, 107]. Furthermore, exogenous En1/2 can protect vmDA neurons from MPTP, 6-OHDA, and α-synuclein toxicity, presumably by increasing mitochondrial complex I activity [108].
The homeodomain proteins Lmx1a and Lmx1b are important for the specification of vmDA neurons and appear to have both specific and redundant functions [40, 41, 71, 109, 110]. VmDA progenitors can be subdivided into medial and lateral domains that are molecularly distinct in their expression of Wnt1, DRD2, and Corin expression. These subgroups show different sensitivity to the loss of Lmx1a and Lmx1b, with Lmx1a affecting the neurogenesis of medial progenitors and Lmx1b being necessary for the establishment of the lateral DA progenitor domain [71]. Lmx1a can induce a vmDA neuron phenotype in ventralized ES cells [40, 111, 112], but it is not absolutely required for the specification of these neurons [71]. Importantly, Lmx1a converts nonneuronal floor plate cells in the ventral midline into neuronal vmDA progenitors [40, 41]. This process includes a Lmx1a-triggered cell cycle exit, neuronal differentiation by activation of Ngn2 signaling, and the establishment of Notch signaling in ventral midlines cells, thereby providing neuronal potential to FP cells [40, 71]. The requirement for Lmx1a in midline cells is limited to early developmental stages and the deficient vmDA neurogenesis, (most evident along the midline), in the Lmx1a mutant mice recovers over time [41, 71]. Lmx1b controls the onset of Pitx3 expression relative to TH and is required for survival, as all vmDA neurons are lost after E16 in Lmx1b null mutants [109]. In addition, Lmx1b is required for the specification of lateral vmDA progenitors that do not appear to originate from the floor plate [71]. Furthermore, Lmx1b, and not Lmx1a, appears to be a crucial regulator of Wnt1 expression in vmDA progenitors at later developmental stages. While the function of Lmx1a appears devoted to the vmDA neuron lineage, Lmx1b has a broader function and influences the sequential specification of ocular motor neurons and red nucleus neurons from progenitors lateral to vmDA neurons in the midbrain [71].
Neurogenin 2 (Ngn2) is a key factor downstream of Lmx1a, Msx1/2, and Otx2 in the conversion of the glial-like FP into a neurogenic region in the vm [40, 41, 71]. Furthermore, Ngn2 is a regulator of mDA specification and neurogenesis, but its proneural function can be partially replaced by Mash1 (Ascl1) [40, 113].
The transcription factor Nurr1 (Nr4A2) is expressed in many neuronal populations in the brain, including all post-mitotic vmDA neurons. Nurr1 is required for the induction of TH and other proteins required for DA synthesis, storage and release, including VMAT2, DAT, aromatic L-amino acid decarboxylase (AADC), and also c-Ret [77, 114–116]. Furthermore, it appears that Nurr1 can physically interact with the cyclin-dependent kinase (CDK) p57 to promote maturation of vmDA neurons [117]. In Nurr1 knockout mice, vmDA neurons are born, but fail to acquire and/or maintain a proper phenotype [114, 118, 119].
The homeobox transcription factor Pitx3 shows a restricted expression in SNc and VTA DA neurons in the brain. Interestingly, loss of Pitx3 leads to a selective degeneration of SNc DA neurons, while VTA DA neurons remain intact [80, 120, 121]. The reasons for this selective dependence of SNc DA neurons on Pitx3 are not fully understood. As mentioned above, Raldh1 is a transcriptional target of Pitx3 [78, 79] and maternal supplementation of RA can partially rescue the SNc degeneration in the Pitx3 knockout mice [79]. Furthermore, Pitx3 is required to activate BDNF expression in a rostrocaudal population of SNc mDA neurons and loss of BDNF expression correlates with the increased apoptotic cell death of these mDA neurons in the Pitx3 knockout mouse [91]. In addition, Pitx3 regulates the level of TH in SNc mDA neurons [122].
In conclusion, a comprehensive understanding of the developmental pathways involved in vmDA specification and maturation facilitates their in vitro generation from different cell sources.
3. Dopamine Neurons from Pluripotent Stem Cells
Human pluripotent stem cells represent a good source of in vitro generated cells because they allow unlimited expansion (at least in theory) and derivation of any kind of cell type. However, their broad potential is also their main drawback, as it is difficult to restrict their differentiation into only one specific cellular phenotype. For cell-based therapies, cellular heterogeneity is problematic because of decreased safety, efficiency, and efficacy (which are the requisites for a biological agent to be approved as a therapy). Indeed, the presence of multiple cell phenotypes that are also at different developmental stages can cause several complications. Immature and proliferating cells pose a risk of teratoma formation [123, 124] and graft overgrowth [125–127]. The presence of contaminating cell phenotypes can interfere with the graft function in several ways, for example, by favoring graft self-innervation and decreasing graft-host integration [128] or through a direct interaction with host neurons, compromising function. In particular, while debatable (see Section 1), the presence of serotonin neurons in fetal vm grafts has been proposed to account for the development of graft-induced dyskinesia [30]. Finally, the presence of contaminating cells necessarily decreases the percentage of therapeutically relevant cells, leading to an increase in cell dose and injection volumes which is associated with higher surgical risks and adverse effects. Therefore, the challenge is to maximize the production of one (or several) therapeutically relevant cell type(s) and minimize the presence of other cell populations, in particular those which can cause direct damage or decrease the functional effect of the graft. With this goal, differentiation and selection protocols have been developed and optimized using the extrinsic signals and intrinsic markers discussed in Section 2, to guide pluripotent cells into the appropriate developmental program (Figure 3).
Figure 3.
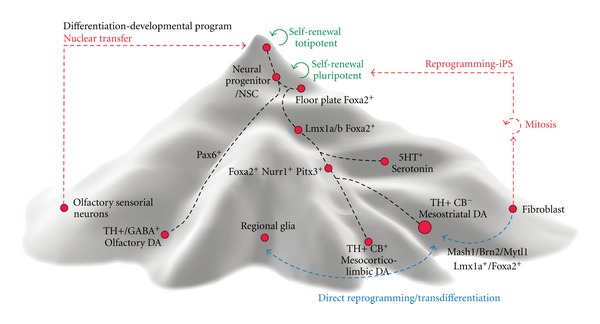
Customized rendering of the epigenetic landscape for ventral midbrain dopamine neurons representing the developmental program (downhill, black lines) and the reprogramming pathways back to pluripotency (red lines) and across mature fates (blue lines).
3.1. Inductive Cell Culture Protocols
Based on the information gathered from developmental studies, inductive culture protocols have been developed, using a sequential exposure to morphogens, in an effort to reproduce in vitro the convergence of signaling factors (described in Section 2.1) that takes place during vmDA neurogenesis in the embryo (Figure 2). Combinations of Shh and Fgf8 have been successful to induce DA neurons from pluripotent embryonic stem cells from mouse [129], primate [130–132] and human origins [133, 134]. For neural induction, coculture systems take advantage of the inductive properties of murine stromal cell lines like MS5 [135] or PA6 [130, 136]. The stromal-derived inductive activity has been related to the secretion of cytokines, growth factors, and axonal guidance molecules like CXCL12, pleiotrophin, insulin growth factor-2 (IGF2), and ephrinB1 [137]. Subsequent modifications of the basic protocols have sought to enhance the proportion of pluripotent cells committed to neural fates by blocking mesendodermal fates, using BMP inhibitors, such as noggin, and the activin and TGFβ inhibitor, SB431542 [124, 133, 138, 139]. Inhibitors of glycogen synthase kinase (GSK)3-β also favor neural induction and vmDA neuron differentiation, through enhancement of canonical Wnt signaling activity [140]. Other strategies include a transient inhibition of Fgf/Erk signaling at early stages of neural induction to ventralize neural progenitors and maintain Otx2 expression while repressing forebrain and hindbrain fates [141].
Differential expression of miRNAs has been correlated with the propensity of pluripotent cell lines to generate vmDA neurons using these inductive protocols [142]. Thus, the expression of miRNAs could be manipulated in order to enhance the differentiation process and, importantly, it can be used to choose the most efficient cell lines for differentiation, for example from patient derived iPS cell lines if several clones are available.
Long in vitro culture periods in the presence of BDNF, GDNF, Wnt5a and other factors, discussed in Section 2, stabilize the transcriptional network and enhance neuronal maturation [143], leading to a progressive enrichment by positive selection. However, in contrast to other cellular populations like the oligodendrocytes derived form human embryonic stem cells [144, 145], long culture periods may not be optimal for purification of vmDA neurons for transplantation due to their dense neuritic arborization, which increases their vulnerability during harvesting. To further increase the proportion of vmDA neurons from pluripotent stem cell-derived populations, over-expression of transcription factors and selection strategies have been evaluated.
3.2. Over-Expression
Several transcription factors, such as Nurr1, Lmx1a and Pitx3, have been used to enhance vmDA differentiation from pluripotent and neural stem cells (Table 1).
Table 1.
Summary of the studies that have used transcription factors and other markers to obtain and enhance the production of vmDA neurons in vitro, through overexpression and selection strategies.
| TF and lineage markers | Overexpression | Selection | Direct reprogramming | Comments |
|---|---|---|---|---|
| Pitx3 | ▴ mRNA levels of phenotypic markers of vmDA neurons after in vitro differentiation and the percentage of Pitx3/TH neurons after grafting [78] | ▴ Enrichment for vmDA neurons [27], which restored motor function in PD models [159, 179] | iDA neurons from human and mouse fibroblasts and mouse astrocytes (in combination) iDA from Pitx3-eGFP ki mouse cells sorted for Pitx3 showed some motor improvement after transplantation in 6-OHDA mice [142, 149, 151, 180] |
Specific marker for all postmitotic vmDA neurons |
|
| ||||
| Nurr1 | ▴ mRNA levels of phenotypic markers of vmDA neurons after in vitro differentiation and the percentage of TH+ neurons after transplantation leading to behavioural recovery with no signs of teratoma [78, 146, 147, 153, 181–183] | iDA neurons from human and mouse fibroblasts and mouse astrocytes (in combination) [142, 148–151, 180] | Regulates terminal acquisition of the DA phenotype but is expressed in many cell populations. Strong context dependency. | |
|
| ||||
| Lmx1a/b | Lmx1a/b proteins can increase the percentage of vmDA neurons with typical electrophysiological properties [40, 111, 157, 184] | iDA neurons from human and mouse fibroblasts and mouse astrocytes (in combination) [142, 148–150, 180] | Induce specification and maintenance of vmDA neurons. | |
|
| ||||
| Foxa2 | ▴ mRNA levels of phenotypic markers and TF of vmDA neurons after in vitro differentiation. Enhanced the resistance to neurotoxins and improved motor asymmetry after transplantation [183, 184] | iDA neurons from human and mouse fibroblasts and mouse astrocytes (in combination) [142, 149, 150, 180] | Required for specification, differentiation, and survival of vmDA neurons | |
|
| ||||
| Otx2 | ▴ mRNA levels of phenotypic markers and TF of mDA neurons after in vitro differentiation in combination with FoxA2 and Lmx1a [184] | ▴ Enriched the DA progenitor pool (in combination with Corin) and induced behavioural recovery after transplantation into PD models [185] | iDA neurons from mouse astrocytes (in combination) [180] | Important in midbrain regionalization, persists only in most medial vmDA (less vulnerable) populations |
|
| ||||
| Ngn1/2 | ▾ Number of TH+ cells in combination with Nurr1 [153] | Ngn2+ progenitors isolated at E12.5 from VM led to behavioural recovery in 6-OHDA lesioned rats [179, 186] | iDA neurons from human fibroblasts and mouse astrocytes (in combination) [151, 180] | Can be substituted by other proneural genes like Mash1 |
|
| ||||
| Mash1 (Ascl1) | ▴ In combination with Nurr1 increased the number of surviving TH+ cells after grafting and improved motor function [153] | iDA neurons from human and mouse fibroblasts and mouse astrocytes (in combination) [148, 151, 180] | Essential for direct reprogramming of fibroblast and astrocytes into iDA cells. | |
|
| ||||
| Engrailed | iDA neurons from human and mouse fibroblasts and mouse astrocytes (in combination) [142, 149, 150, 180] | Required for survival of mature vmDA neurons. | ||
|
| ||||
| Sox1 | ▾ Sox1+ neural progenitors avoid tumor formation after transplantation but few DA neurons [125, 158, 187] | ▾ Efficiency of direct reprogramming [142, 149] | Fail to produce vmDA neurons from human ESC [188]. | |
|
| ||||
| Sox2 | ▾ Broadly expressed in all VM domains [179] | iDA neurons from human fibroblasts (in combination) [151] | ||
|
| ||||
| TH | ▴ TH promoter: highly enriches for DA neurons, which improved motor behavior in animal models of PD upon transplantation [127, 165, 166, 168] | Regulatory sequences are valuable for vmDA neuron enrichment mostly from primary cells. | ||
|
| ||||
| DAT | ▴ DAT promoter: highly enriches for DA neurons, which survived in vitro when cocultured with glia [189] | Restricted expression to more mature populations. | ||
|
| ||||
| Nestin | ▾ Expressed in all VM domains [179] | Allows selection of neural progenitors but dynamic expression may exclude target cells at different developmental stages. | ||
|
| ||||
| Corin |
▾ Selection from primary cells resulted in low numbers of TH neurons and no behavioral recovery of grafted animals. ▴ When combined with Otx2, the DA progenitor pool was enriched and cells induced behavioural recovery after transplantation [41, 179, 185] |
Broad expression in the midline. Selection for this surface molecule is insufficient for DA enrichment. | ||
|
| ||||
| SSEA-1 (CD15) | ▴ To exclude stem cells (proliferating/undifferentiated) preventing tumor formation in grafts from mouse ES cells [127, 159, 160] | Negative selection of populations derived from mouse ES cells reduces the risk of teratoma formation. | ||
|
| ||||
| NCAM (CD56) | ▴ To isolate and/or evaluate percentage of post-mitotic neurons and prevent tumor formation in grafts [159, 171] | Positive selection of populations derived from mouse and human ES cells reduces the risk of teratoma formation. | ||
|
| ||||
| PSA-NCAM | ▴ To isolate and/or analyze percentage of progenitors or post- mitotic neurons [111, 159] | Positive selection of neural populations may result in exclusion of target neurons at different developmental stages. | ||
The role of Nurr1 as a terminal selector has been highlighted by over-expression studies that have demonstrated its capacity to upregulate the DA neurotransmitter phenotype by increasing expression of TH, DAT, AADC and c-ret in neurons derived from ES cells [146, 147]. In vivo, Nurr1-overexpressing neurons induced a faster and more complete behavioral recovery in hemi-parkinsonian rats, including spontaneous motor behaviors [147]. More recently, Nurr1 has been used in direct reprogramming experiments [148–151] (see below, Section 4). The effect of Nurr1 is highly context-dependent, failing to induce a vmDA neuronal phenotype in forebrain neural stem cells [152, 153] without the addition of other patterning factors. Likewise, Nurr1 can upregulate DA markers without inducing a neuronal phenotype in mouse ES cells [154].
Lmx1a can induce a vmDA neuron phenotype in previously ventralized mouse ES cells [40, 111, 112], but it is not absolutely required for the specification of these neurons [71]. Indeed, although over-expression in mouse ES cells improved the differentiation into vmDA neurons, the results in human ES cells did not meet the expectations [111, 155]. In another study, using vm progenitors from rodents, just a few Lmx1a-transduced cells matured into neurons but a more robust increase was found in human neural progenitors [156]. More recently, lentiviral vectors were used to stably transduce hES cells that expressed Lmx1a upon differentiation (driven by a nestin enhancer) and resulted in an increase of 40% in the TH positive neurons, with 75% of these coexpressing Girk2 [157].
3.3. Selection Approaches
Induction of the vmDA neuron fate is restricted temporally and spatially in the developing midbrain. Such restrictions are difficult to accomplish in vitro in stem cell derived cultures. While the addition of a specific set of morphogens (e.g., Fg8, Shh, and Wnt) to a stem cell culture can restrict the fates of the cells generated, multiple neuronal populations will still be formed, including serotonin neurons and motor neurons [112, 129, 134, 135]. This is not surprising since these neuronal populations are generated in a close temporal window within very proximal domains during embryonic development [71, 112], and in vitro culture systems cannot achieve the level of definition required to separate these domains (in time and space). However, exclusion of these neighboring populations may be desirable or even necessary, as discussed above. Furthermore, stem cells and actively dividing cells [125, 127, 158–160] could result in the generation of tumors or teratomas [123, 125, 126] and be detrimental to the host.
Target populations, such as vmDA neurons and/or their progenitors, can be enriched for during or after in vitro differentiation using fluorescent activated cell sorting (FACS) or magnetic activated cell sorting (MACS). Cells of interest can be positively selected for by using labeled antibodies that stain for specific cell surface markers with a restricted presence on the desired cellular population. Positive selection can also entail using a genetic internal selection marker (from transgenic cell lines, animal strains, or using viral vectors). In addition, the enrichment strategies can be combined with negative selection procedures to remove unwanted cellular populations, for example, proliferating cells that express markers, such as the stage specific embryonic antigens, SSEAs [161, 162] (e.g., SSEA-1 on mouse ESCs and SSEA-3 on human ESCs). Several strategies have been utilized so far, seeking to enrich for progenitors or postmitotic vmDA neurons (Table 1). Ideally, a combination of cell-surface markers that define a subpopulation, as for blood cells [163], would allow us to select the vmDA neurons at different stages. However, such a cell-surface fingerprint has yet to be defined for vmDA neurons. In addition to the choice of markers, the time of selection is also critical, as survival of post-mitotic neurons is compromised after sorting.
The initial proof-of-principle studies, demonstrated that primary, post-mitotic vmDA neurons could be enriched by FACS, using either dye labeling or TH-based fluorescence expression [164–166]. Furthermore, such cells survived in the striatum of adult 6-OHDA lesioned parkinsonian rats after transplantation and induced partial functional recovery [165, 166]. From selection studies it has become evident that highly enriched mDA neuronal populations need additional trophic support, which can be accomplished by coculture with astrocytes [159, 166]. Neuronal populations usually require target- (axonal or dendritic) derived trophic factor support for survival. Therefore, coculturing purified mDA neurons with their striatal target cells would likely promote survival. Furthermore, it is also possible that purified vmDA neuronal cultures would survive better if they were plated at a high enough density, to ensure increased cell-to-cell contact and exposure to trophic factors, for example, BDNF secreted by neighboring cell populations.
Isolation of stem cell-derived vmDA neurons has proven to be more complicated since the cells are not confined in a temporal or spatial manner, as in the embryo, (see above). For example, using TH as a selection marker poses challenges since TH is expressed in multiple cell types during development, including cells with proliferative capacity [167]. We, and others, have previously utilized TH driven eGFP expression in ES cells to enrich for vmDA neurons [127, 168]. However, due to the expression of eGFP in cells of nonneuronal morphology, the resulting grafts were composed of a majority of non-mDA neurons and most vmDA were generated after grafting, rather than prior to the sorting procedure [127, 168]. Combining the positive selection for TH-eGFP with a negative selection for immature cells using the cell surface marker SSEA-1 resulted in an enriched neuronal population [127].
A more restricted marker for vmDA neurons is the homeodomain transcription factor Pitx3, which is constitutively and selectively expressed in mDA neurons in the brain. Pitx3 is also transiently expressed in skeletal muscle and the lens of the eye [27, 121], but generation of those cellular populations can be avoided during in vitro differentiation using inductive protocols targeted towards a mesencephalic fate [129, 147, 159, 169]. In our study transplantation of an ESC-derived population enriched for Pitx3-eGFP expression could efficiently reverse amphetamine-induced rotational behavior and significantly reduced apomorphine-induced rotational behavior [159]. However, cellular populations that contained ~80% of Pitx3-eGFP cells could still occasionally give rise to teratoma formation. While this positive selection procedure resulted in a ten-fold decrease in the number of SSEA-1 positive cells, some undifferentiated cells with proliferative capacity remained. A second round of FACS for eGFP expression could remove such unwanted cells and enriched for up to 98% mDA neurons, which survived in vitro. Rather than putting the cells through a second round of FACS, a negative selection for SSEA-1 can be performed simultaneously with the positive selection for Pitx3-eGFP. Such negative selection has been previously successful in reducing the amount of proliferating cells [127] and avoiding tumor formation after grafting [160].
Sox1-GFP transgenic expression has been successfully used as a positive selection marker of neuronal progenitors from stem cells derived cultures to avoid tumor formation [125, 158]. However, while this strategy appears to diminish the risk of overgrowth from grafted cells, very few dopamine neurons are generated from an enriched Sox1 positive population [125, 158]. This result is not entirely surprising since the progenitor domain for vmDA neurons is devoid of Sox1 expression and a recent study found that removal of Sox1 from the reprogramming cocktail improved the generation of Pitx3 positive neurons from mouse fibroblasts [149].
Multiple studies have used the expression of the cell-surface membrane protein NCAM (neural cell adhesion molecule) and its polysialylated form, PSA-NCAM, to analyze or enrich for post-mitotic neurons [111, 112, 159, 170, 171]. Selection for PSA-NCAM and subsequent transplantation has shown that tumor formation can be averted [111, 171]. However, the resulting grafts were either very small due to poor survival [111] or lacking vmDA neurons of a proper identity [171].
4. Direct Reprogramming to vmDA Neurons
All cells in an individual have essentially the same genes and the distinct cellular phenotypes are determined by their unique gene expression profiles, which are controlled by transcription factors. Thus, manipulating the expression of certain key transcription factors allows for the modification of the cell transcriptional profile and, ultimately, the reprogramming of its phenotype [172]. Using reprogramming technology, it has been possible to generate induced-pluripotent stem (iPS) cell lines and also mature phenotypes, such as induced neurons (iNs) [173], from accessible cells, like dermal fibroblasts. Reprogramming techniques are particularly valuable to obtain human neurons carrying mutations associated with neurological diseases. An advantage of the direct reprogramming approach is to circumvent the pluripotent stage (Figure 3), which shortens the experimental procedures and avoids the hurdles associated with the redifferentiation process. On the other hand, there is no possibility to expand the resulting cell population, which entails the need to reprogram each cell. This inconvenience has been successfully overcome by direct reprogramming mouse and human fibroblasts to a neural stem cell stage by Sox2 over-expression [174]. Notwithstanding, the most critical issue associated with this approach is to determine whether the reprogramming process fully resets the cell identity and whether these cells become authentic functional neurons. In the initial report [173], a combination of Mash1, Brn2 and Mytl1 produced iN cells that did not have a clearly defined regional phenotype [175]. Since then, TH positive iN have been generated through direct reprogramming [148–151] by the addition of one or more transcription factors that are important during midbrain development, including Foxa2, Lmx1a/b, Nurr1, En1, and Pitx3, and in different combinations (Table 1). The interplay between intrinsic determinants and extrinsic signals is again underscored in a study using mouse Pitx3-eGFP transgenic fibroblasts [149]. In this study exposure to Shh and Fgf8 of reprogrammed cells partially overcame their lack of maturation and made the iN more similar to vmDA neurons. However, in spite of some evidence of in vivo function, those DA iNs were still different from primary neurons both in molecular and functional assays. Interestingly, overexpression of Sox1, Pax6, and, intriguingly, Lmx1b, had either an inhibitory effect or no effect on the reprogramming efficiency [149]. Thus, these studies are helping to establish the hierarchy of lineage determinants and the relative contribution of these transcription factors in crafting the vmDA neuronal identity.
So far, the emerging picture from these transdifferentiation studies (and previous over-expression assays) underscores the need to overcome context dependency, which appears to be the dictated by chromatin modifications. In this regard, it is rather puzzling that the exact same factors were sufficient to reprogram cells from different germ layers, that is, dermal fibroblasts and hepatocytes, into neurons [176], as, in principle, different endogenous programs need to be repressed in the starting cell population. This suggests that perhaps some of the proneural genes, most likely Mash1, are able to switch on and off whole transcriptional networks. A combination of the so-called master regulators, such as Mash1 (Ascl1) for ventral neurons and Foxa2 for the floor-plate neural progeny, and terminal selectors, like Nurr1 (Nr4a2), and Pitx3, together with extrinsic inductive signals [177] and chromatin modifiers [149, 178] may be required to generate vmDA neurons that have a correct molecular and functional identity, directly from unrelated somatic cells.
In summary, a precise temporal and spatial integration of extrinsic and intrinsic factors is required to establish the transcriptional network that confers cell identity. Only neurons with the appropriate mesostriatal vmDA identity will be able to replace the neurons lost in Parkinson disease and restore synaptic connectivity and function. Understanding the complex interplay of signals during embryonic development will help recognize the critical factors required to refine the production of these neurons in vitro from pluripotent stem cells and from somatic cells. Likewise, the capacity of individual transcription factors and extrinsic signals to induce and stabilize the vmDA phenotype will help determine their role in lineage specification, and further our understanding of human midbrain development.
Acknowledgments
J. C. Aguila is supported by an FPI fellowship from the Spanish Ministry of Economy and Competitiveness. Grant support from the Swedish Medical Research Council (no. 2011–2651) to E. Hedlund, from the Spanish Ministry of Science and Innovation (SAF2008-04615) to R. Sanchez-Pernaute, and from the Departments of Industry (PE10IB04) and Education (EC2010-28) of the Basque Government to R. Sanchez-Pernaute, is gratefully acknowledged. The authors thank Mattias Karlen for his excellent work generating the figures for this paper.
References
- 1.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Baba M, Nakajo S, Tu PH, et al. Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. American Journal of Pathology. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- 3.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathologica. 1990;79(6):581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 4.Burke RE, Dauer WT, Vonsattel JPG. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Annals of Neurology. 2008;64(5):485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant Parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Pramstaller PP, Schlossmacher MG, Jacques TS, et al. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Annals of Neurology. 2005;58(3):411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 7.Schiesling C, Kieper N, Seidel K, Krüger R. Review: Familial Parkinson’s disease—genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathology and Applied Neurobiology. 2008;34(3):255–271. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 8.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122(8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 9.Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain: I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122(8):1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- 11.Double DL, Reyes R, Werry WL, Halliday GM. Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Progress in Neurobiology. 2010;92(3):316–329. doi: 10.1016/j.pneurobio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. The Lancet Neurology. 2007;6(10):933–938. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- 13.Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20(7):398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- 14.Reyes S, Fu Y, Double K, et al. GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. The Journal of Comparative Neurology. 2012;520(12):2591–2607. doi: 10.1002/cne.23051. [DOI] [PubMed] [Google Scholar]
- 15.Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Human Molecular Genetics. 2005;14(13):1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128(7):1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson L, Barraud P, Andersson E, Kirik D, Björklund A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. The Journal of Neuroscience. 2005;25(27):6467–6477. doi: 10.1523/JNEUROSCI.1676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Yuan Y, Halliday G, Rusznak Z, Watson C, Paxinos G. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Structure and Function. 2012;217(2):591–612. doi: 10.1007/s00429-011-0349-2. [DOI] [PubMed] [Google Scholar]
- 19.Eulitz D, Prüss H, Derst C, Veh RW. Heterogeneous distribution of Kir3 potassium channel proteins within dopaminergic neurons in the mesencephalon of the rat brain. Cellular and Molecular Neurobiology. 2007;27(3):285–302. doi: 10.1007/s10571-006-9118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced Parkinsonism: sparing of calbindin-D(28k)-containing cells. Annals of the New York Academy of Sciences. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 21.Verney C. Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microscopy Research and Technique. 1999;46(1):24–47. doi: 10.1002/(SICI)1097-0029(19990701)46:1<24::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22.Björklund A, Dunnett SB, Brundin P, et al. Neural transplantation for the treatment of Parkinson’s disease. The Lancet Neurology. 2003;2(7):437–445. doi: 10.1016/s1474-4422(03)00442-3. [DOI] [PubMed] [Google Scholar]
- 23.Brundin P, Barker RA, Parmar M. Neural grafting in Parkinson’s disease. Problems and possibilities. Progress in Brain Research. 2010;184:265–294. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- 24.Lindvall O, Bjorklund A. Cell therapeutics in Parkinson’s disease. Neurotherapeutics. 2011;8(4):539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson’s disease. Journal of Internal Medicine. 2009;266(4):358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 26.Silani V, Mariani D, Donato FM, et al. Development of dopaminergic neurons in the human mesencephalon and in vitro effects of basic fibroblast growth factor treatment. Experimental Neurology. 1994;128(1):59–76. doi: 10.1006/exnr.1994.1113. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Maxwell S, Jimenez-Beristain A, et al. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. The European Journal of Neuroscience. 2004;19(5):1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson T, Carta M, Winkler C, Björklund A, Kirik D. Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. The Journal of Neuroscience. 2007;27(30):8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez I, Vĩuela A, Astradsson A, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nature Medicine. 2008;14(5):507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Politis M, Wu K, Loane C, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Science Translational Medicine. 2010;2(38) doi: 10.1126/scitranslmed.3000976.38ra46 [DOI] [PubMed] [Google Scholar]
- 31.Carlsson T, Carta M, Muñoz A, et al. Impact of grafted serotonin and dopamine neurons on development of L-DOPA-induced dyskinesias in Parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain. 2009;132(2):319–335. doi: 10.1093/brain/awn305. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz A, Carlsson T, Tronci E, Kirik D, Björklund A, Carta M. Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Experimental Neurology. 2009;219(1):298–307. doi: 10.1016/j.expneurol.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 33.Barker RA, Kuan WL. Graft-induced dyskinesias in Parkinson’s disease: what is it all about? Cell Stem Cell. 2010;7(2):148–149. doi: 10.1016/j.stem.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Shin E, Garcia J, Winkler C, Bjorklund A, Carta M. Serotonergic and dopaminergic mechanisms in graft-induced dyskinesia in a rat model of Parkinson’s disease. Neurobiology of Disease. 2012;47(3):393–406. doi: 10.1016/j.nbd.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. Buspirone in levodopa-induced dyskinesias. Clinical Neuropharmacology. 1994;17(1):73–82. doi: 10.1097/00002826-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behavioural Brain Research. 2007;179(1):76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1(4):382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen-Cory S, Lom B. Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses. International Journal of Developmental Biology. 2004;48(8-9):947–956. doi: 10.1387/ijdb.041883sc. [DOI] [PubMed] [Google Scholar]
- 39.Sonntag KC, Simunovic F, Sanchez-Pernaute R. Stem cells and cell replacement therapy for Parkinson’s disease. Journal of Neural Transmission, Supplementa. 2009;(73):287–299. doi: 10.1007/978-3-211-92660-4_24. [DOI] [PubMed] [Google Scholar]
- 40.Andersson E, Tryggvason U, Deng Q, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124(2):393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 41.Ono Y, Nakatani T, Sakamoto Y, et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134(17):3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 42.Strähle U, Lam CS, Ertzer R, Rastegar S. Vertebrate floor-plate specification: variations on common themes. Trends in Genetics. 2004;20(3):155–162. doi: 10.1016/j.tig.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews Genetics. 2000;1(1):20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 44.Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annual Review of Neuroscience. 1995;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. [DOI] [PubMed] [Google Scholar]
- 45.Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274(5290):1115–1122. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- 46.Brodski C, Vogt Weisenhorn DM, Signore M, et al. Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. The Journal of Neuroscience. 2003;23(10):4199–4207. doi: 10.1523/JNEUROSCI.23-10-04199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang MZ, Jin P, Bumcrot DA, et al. Induction of dopaminergic neuron phenotype in the midbrain by Sonic hedgehog protein. Nature Medicine. 1995;1(11):1184–1188. doi: 10.1038/nm1195-1184. [DOI] [PubMed] [Google Scholar]
- 48.Ye W, Shimamura K, Rubenstein JLR, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93(5):755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 49.Ferri ALM, Lin W, Mavromatakis YE, et al. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134(15):2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- 50.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez-Balaguer A, Puelles E, Wurst W, Martinez S. Shh dependent and independent maintenance of basal midbrain. Mechanisms of Development. 2009;126(5-6):301–313. doi: 10.1016/j.mod.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Heikinheimo M, Lawshe A, Shackford GM, Wilson DB, MacArthur CA. Fgf-8 expression in the post-gastrulation mouse suggests roles in the development of the face, limbs and central nervous system. Mechanisms of Development. 1994;48(2):129–138. doi: 10.1016/0925-4773(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 53.Ohuchi H, Yoshioka H, Tanaka A, Kawakami Y, Nohno T, Noji S. Involvement of androgen-induced growth factor (FGF-8) gene in mouse embryogenesis and morphogenesis. Biochemical and Biophysical Research Communications. 1994;204(2):882–888. doi: 10.1006/bbrc.1994.2542. [DOI] [PubMed] [Google Scholar]
- 54.Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380(6569):66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 55.Lee SMK, Danielian PS, Fritzsch B, McMahon AP. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124(5):959–969. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- 56.Liu A, Li JYH, Bromleigh C, Lao Z, Niswander LA, Joyner AL. FGF17b and FGF18 have different midbrain regulatory properties from FGF8b or activated FGF receptors. Development. 2003;130(25):6175–6185. doi: 10.1242/dev.00845. [DOI] [PubMed] [Google Scholar]
- 57.Sato T, Nakamura H. The Fgf8 signal causes cerebellar differentiation by activating the Ras-ERK signaling pathway. Development. 2004;131(17):4275–4285. doi: 10.1242/dev.01281. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki-Hirano A, Sato T, Nakamura H. Regulation of isthmic Fgf8 signal by sprouty2. Development. 2005;132(2):257–265. doi: 10.1242/dev.01581. [DOI] [PubMed] [Google Scholar]
- 59.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre-and Flp-mediated recombination. Nature Genetics. 1998;18(2):136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 60.Reifers F, Böhli H, Walsh EC, Crossley PH, Stainier DYR, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125(13):2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- 61.Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130(12):2633–2644. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- 62.Liu A, Losos K, Joyner AL. FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development. 1999;126(21):4827–4838. doi: 10.1242/dev.126.21.4827. [DOI] [PubMed] [Google Scholar]
- 63.Yamauchi K, Mizushima S, Tamada A, Yamamoto N, Takashima S, Murakami F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. The Journal of Neuroscience. 2009;29(13):4044–4055. doi: 10.1523/JNEUROSCI.4794-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119(1):247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 65.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 66.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 67.McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69(4):581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- 68.Panhuysen M, Vogt Weisenhorn DM, Blanquet V, et al. Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Molecular and Cellular Neuroscience. 2004;26(1):101–111. doi: 10.1016/j.mcn.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Prakash N, Brodski C, Naserke T, et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo . Development. 2006;133(1):89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- 70.Joksimovic M, Patel M, Taketo MM, Johnson R, Awatramani R. Ectopic Wnt/β-catenin signaling induces neurogenesis in the spinal cord and hindbrain floor plate. PloS ONE. 2012;7(1) doi: 10.1371/journal.pone.0030266.e30266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng Q, Andersson E, Hedlund E, et al. Specific and integrated roles of Lmx1a, Lmx1b and Phox2a in ventral midbrain development. Development. 2011;138(16):3399–3408. doi: 10.1242/dev.065482. [DOI] [PubMed] [Google Scholar]
- 72.Castelo-Branco G, Wagner J, Rodriguez FJ, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang M, Miyamoto Y, Huang EJ. Multiple roles of β-catenin in controlling the neurogenic niche for midbrain dopamine neurons. Development. 2009;136(12):2027–2038. doi: 10.1242/dev.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sousa KM, Carlos Villaescusa J, Cajanek L, et al. Wnt2 regulates progenitor proliferation in the developing ventral midbrain. The Journal of Biological Chemistry. 2010;285(10):7246–7253. doi: 10.1074/jbc.M109.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersson ER, Prakash N, Cajanek L, et al. Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo . PLoS ONE. 2008;3(10) doi: 10.1371/journal.pone.0003517.e3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindahl R, Evces S. Rat liver aldehyde dehydrogenase. II. Isolation and characterization of four inducible isozymes. The Journal of Biological Chemistry. 1984;259(19):11991–11996. [PubMed] [Google Scholar]
- 77.Wallén A, Zetterström RH, Solomin L, Arvidsson M, Olson L, Perlmann T. Fate of mesencephalic AHD2-expressing dopamine progenitor cells in Nurr1 mutant mice. Experimental Cell Research. 1999;253(2):737–746. doi: 10.1006/excr.1999.4691. [DOI] [PubMed] [Google Scholar]
- 78.Chung S, Hedlund E, Hwang M, et al. The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Molecular and Cellular Neuroscience. 2005;28(2):241–252. doi: 10.1016/j.mcn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Jacobs FMJ, Smits SM, Noorlander CW, et al. Retinoic acid counteracts developmental defects in the substantia nigra caused by Pitx3 deficiency. Development. 2007;134(14):2673–2684. doi: 10.1242/dev.02865. [DOI] [PubMed] [Google Scholar]
- 80.Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Molecular Brain Research. 2003;114(2):123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 81.Jordan J, Böttner M, Schluesener HJ, Unsicker K, Krieglstein K. Bone morphogenetic proteins: neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. The European Journal of Neuroscience. 1997;9(8):1699–1710. doi: 10.1111/j.1460-9568.1997.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 82.Chou J, Harvey BK, Ebendal T, Hoffer B, Wang Y. Nigrostriatal alterations in bone morphogenetic protein receptor II dominant negative mice. Acta Neurochirurgica, Supplementum. 2008;(101):93–98. doi: 10.1007/978-3-211-78205-7_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou J, Luo Y, Kuo CC, et al. Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience. 2008;151(1):92–103. doi: 10.1016/j.neuroscience.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin LFH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 85.Poulsen KT, Armanini MP, Klein RD, Hynes MA, Phillips HS, Rosenthal A. TGFβ2 and TGFβ3 are potent survival factors for midbrain dopaminergic neurons. Neuron. 1994;13(5):1245–1252. doi: 10.1016/0896-6273(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 86.Krieglstein K, Suter-Crazzolara C, Fischer WH, Unsicker K. TGF-β superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. The EMBO Journal. 1995;14(4):736–742. doi: 10.1002/j.1460-2075.1995.tb07052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krieglstein K, Suter-Crazzolara C, Hotten G, Pohl J, Unsicker K. Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-β superfamily, on midbrain dopaminergic neurons. The Journal of Neuroscience Research. 1995;42(5):724–732. doi: 10.1002/jnr.490420516. [DOI] [PubMed] [Google Scholar]
- 88.Farkas LM, Dünker N, Roussa E, Unsicker K, Krieglstein K. Transforming growth factor-βs are essential for the development of midbrain dopaminergic neurons in vitro and in vivo . The Journal of Neuroscience. 2003;23(12):5178–5186. doi: 10.1523/JNEUROSCI.23-12-05178.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roussa E, Wiehle M, Dünker N, Becker-Katins S, Oehlke O, Krieglstein K. Transforming growth factor β is required for differentiation of mouse mesencephalic progenitors into dopaminergic neurons in vitro and in vivo: ectopic induction in dorsal mesencephalon. Stem Cells. 2006;24(9):2120–2129. doi: 10.1634/stemcells.2005-0514. [DOI] [PubMed] [Google Scholar]
- 90.Oo TF, Ries V, Cho J, Kholodilov N, Burke RE. Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat. Journal of Comparative Neurology. 2005;484(1):57–67. doi: 10.1002/cne.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng C, Aron L, Klein R, et al. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. The Journal of Neuroscience. 2011;31(36):12802–12815. doi: 10.1523/JNEUROSCI.0898-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puelles E, Acampora D, Lacroix E, et al. Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nature Neuroscience. 2003;6(5):453–460. doi: 10.1038/nn1037. [DOI] [PubMed] [Google Scholar]
- 93.Puelles E, Annino A, Tuorto F, et al. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development. 2004;131(9):2037–2048. doi: 10.1242/dev.01107. [DOI] [PubMed] [Google Scholar]
- 94.Vernay B, Koch M, Vaccarino F, et al. Otx2 regulates subtype specification and neurogenesis in the midbrain. The Journal of Neuroscience. 2005;25(19):4856–4867. doi: 10.1523/JNEUROSCI.5158-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borgkvist A, Puelles E, Carta M, et al. Altered dopaminergic innervation and amphetamine response in adult Otx2 conditional mutant mice. Molecular and Cellular Neuroscience. 2006;31(2):293–302. doi: 10.1016/j.mcn.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 96.Omodei D, Acampora D, Mancuso P, et al. Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development. 2008;135(20):3459–3470. doi: 10.1242/dev.027003. [DOI] [PubMed] [Google Scholar]
- 97.di Salvio M, di Giovannantonio LG, Acampora D, et al. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nature Neuroscience. 2010;13(12):1481–1488. doi: 10.1038/nn.2661. [DOI] [PubMed] [Google Scholar]
- 98.Metzakopian E, Lin W, Salmon-Divon M, et al. Genome-wide characterization of Foxa2 targets reveals upregulation of floor plate genes and repression of ventrolateral genes in midbrain dopaminergic progenitors. Development. 2012;139(14):2625–2634. doi: 10.1242/dev.081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kittappa R, Chang WW, Awatramani RB, McKay RD. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biology. 2007;5(12, article e325) doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin W, Metzakopian E, Mavromatakis YE, et al. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Developmental Biology. 2009;333(2):386–396. doi: 10.1016/j.ydbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 101.Nakatani T, Kumai M, Mizuhara E, Minaki Y, Ono Y. Lmx1a and Lmx1b cooperate with Foxa2 to coordinate the specification of dopaminergic neurons and control of floor plate cell differentiation in the developing mesencephalon. Developmental Biology. 2010;339(1):101–113. doi: 10.1016/j.ydbio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 102.Davis CA, Joyner AL. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes & Development. 1988;2(12):1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- 103.Davis CA, Noble-Topham SE, Rossant J, Joyner AL. Expression of the homeo box-containing gene En-2 delineates a specific region of the developing mouse brain. Genes & Development. 1988;2(3):361–371. doi: 10.1101/gad.2.3.361. [DOI] [PubMed] [Google Scholar]
- 104.Millen KJ, Wurst W, Herrup K, Joyner AL. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development. 1994;120(3):695–706. doi: 10.1242/dev.120.3.695. [DOI] [PubMed] [Google Scholar]
- 105.Simon HH, Saueressig H, Wurst W, Goulding MD, O’Leary DDM. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. The Journal of Neuroscience. 2001;21(9):3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simon HH, Thuret S, Alberi L. Midbrain dopaminergic neurons: control of their cell fate by the engrailed transcription factors. Cell and Tissue Research. 2004;318(1):53–61. doi: 10.1007/s00441-004-0973-8. [DOI] [PubMed] [Google Scholar]
- 107.Albéri L, Sgadò P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131(13):3229–3236. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez-Fischer D, Fuchs J, Castagner F, et al. Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nature Neuroscience. 2011;14:1260–1266. doi: 10.1038/nn.2916. [DOI] [PubMed] [Google Scholar]
- 109.Smidt MP, Asbreuk CHJ, Cox JJ, Chen H, Johnson RL, Burbach JPH. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nature Neuroscience. 2000;3(4):337–341. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- 110.Yan CH, Levesque M, Claxton S, Johnson RL, Ang SL. Lmx1a and lmx1b function cooperatively to regulate proliferation, specification, and differentiation of midbrain dopaminergic progenitors. The Journal of Neuroscience. 2011;31(35):12413–12425. doi: 10.1523/JNEUROSCI.1077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friling S, Andersson E, Thompson LH, et al. Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7613–7618. doi: 10.1073/pnas.0902396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panman L, Andersson E, Alekseenko Z, et al. Transcription factor-induced lineage selection of stem-cell-derived neural progenitor cells. Cell Stem Cell. 2011;8(6):663–675. doi: 10.1016/j.stem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Kele J, Simplicio N, Ferri ALM, et al. Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development. 2006;133(3):495–505. doi: 10.1242/dev.02223. [DOI] [PubMed] [Google Scholar]
- 114.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hermanson E, Joseph B, Castro D, et al. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Experimental Cell Research. 2003;288(2):324–334. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 116.Smits SM, Ponnio T, Conneely OM, Burbach JPH, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. The European Journal of Neuroscience. 2003;18(7):1731–1738. doi: 10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 117.Joseph B, Wallén-Mackenzie A, Benoit G, et al. p57Kip2 cooperates with Nurr1 in developing dopamine cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15619–15624. doi: 10.1073/pnas.2635658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castillo SO, Baffi JS, Palkovits M, et al. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Molecular and Cellular Neurosciences. 1998;11(1-2):36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- 119.Kadkhodaei B, Ito T, Joodmardi E, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. The Journal of Neuroscience. 2009;29(50):15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van den Munckhof P, Luk KC, Ste-Marie L, et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130(11):2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 121.Smidt MP, Smits SM, Burbach JPH. Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell and Tissue Research. 2004;318(1):35–43. doi: 10.1007/s00441-004-0943-1. [DOI] [PubMed] [Google Scholar]
- 122.Maxwell SL, Ho HY, Kuehner E, Zhao S, Li M. Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Developmental Biology. 2005;282(2):467–479. doi: 10.1016/j.ydbio.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 123.Björklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25(2):411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung S, Shin BS, Hedlund E, et al. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. Journal of Neurochemistry. 2006;97(5):1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature Medicine. 2006;12(11):1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 127.Hedlund E, Pruszak J, Ferree A, et al. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells. 2007;25(5):1126–1135. doi: 10.1634/stemcells.2006-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferrari D, Sanchez-Pernaute R, Lee H, Studer L, Isacson O. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. The European Journal of Neuroscience. 2006;24(7):1885–1896. doi: 10.1111/j.1460-9568.2006.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nature Biotechnology. 2000;18(6):675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 130.Kawasaki H, Suemori H, Mizuseki K, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sanchez-Pernaute R, Studer L, Ferrari D, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (Cyno-1) after transplantation. Stem Cells. 2005;23(7):914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. Journal of Clinical Investigation. 2005;115(1):102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ben-Hur T, Idelson M, Khaner H, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22(7):1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 134.Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(34):12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barberi T, Klivenyi P, Calingasan NY, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nature Biotechnology. 2003;21(10):1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 136.Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28(1):31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 137.Vazin T, Becker KG, Chen J, et al. A novel combination of factors, termed SPIE, which promotes dopaminergic neuron differentiation from human embryonic stem cells. PLoS ONE. 2009;4(8) doi: 10.1371/journal.pone.0006606.e6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6(4):336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Li W, Sun W, Zhang Y, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(20):8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaeger I, Arber C, Risner-Janiczek J, et al. Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development. 2011;138:4363–4437. doi: 10.1242/dev.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim H, Lee G, Ganat Y, et al. miR-371-3 expression predicts neural differentiation propensity in human pluripotent stem cells. Cell Stem Cell. 2011;8(6):695–706. doi: 10.1016/j.stem.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 143.Sanchez-Pernaute R, Lee H, Patterson M, et al. Parthenogenetic dopamine neurons from primate embryonic stem cells restore function in experimental Parkinson’s disease. Brain. 2008;131(8):2127–2139. doi: 10.1093/brain/awn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49(3):385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 145.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28(1):152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chung S, Sonntag KC, Andersson T, et al. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. The European Journal of Neuroscience. 2002;16(10):1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim JH, Auerbach JM, Rodríguez-Gómez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 148.Caiazzo M, Dell’Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 149.Kim J, Su SC, Wang H, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9(5):413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pfisterer U, Kirkeby A, Torper O, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu X, Li F, Stubblefield EA, et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Research. 2012;22:321–332. doi: 10.1038/cr.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wagner J, Åkerud P, Castro DS, et al. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nature Biotechnology. 1999;17(7):653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 153.Park CH, Kang JS, Shin YH, et al. Acquisition of in vitro and in vivo functionality of Nurr1-induced dopamine neurons. The FASEB Journal. 2006;20(14):2553–2555. doi: 10.1096/fj.06-6159fje. [DOI] [PubMed] [Google Scholar]
- 154.Sonntag KC, Simantov R, Kim KS, Isacson O. Temporally induced Nurr1 can induce a non-neuronal dopaminergic cell type in embryonic stem cell differentiation. The European Journal of Neuroscience. 2004;19(5):1141–1152. doi: 10.1111/j.1460-9568.2004.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cai J, Donaldson A, Yang M, German MS, Enikolopov G, Iacovitti L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson’s disease model. Stem Cells. 2009;27(1):220–229. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- 156.Roybon L, Hjalt T, Christophersen NS, Li JY, Brundin P. Effects on differentiation of embryonic ventral midbrain progenitors by Lmx1a, Msx1, Ngn2, and Pitx3. The Journal of Neuroscience. 2008;28(14):3644–3656. doi: 10.1523/JNEUROSCI.0311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanchez-Danes A, Consiglio A, Richaud Y, et al. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Human Gene Therapy. 2012;23(1):56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fukuda H, Takahashi J, Watanabe K, et al. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24(3):763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- 159.Hedlund E, Pruszak J, Lardaro T, et al. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson’s disease. Stem Cells. 2008;26(6):1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proceedings of the National Academy of Sciences of the United States of America. 1978;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35(5):865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 163.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125(9):1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 164.di Porzio U, Rougon G, Novotny EA, Barker JL. Dopaminergic neurons from embryonic mouse mesencephalon are enriched in culture through immunoreaction with monoclonal antibody to neural specific protein 4 and flow cytometry. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(20):7334–7338. doi: 10.1073/pnas.84.20.7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sawamoto K, Nakao N, Kobayashi K, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(11):6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Donaldson AE, Marshall CE, Yang M, Suon S, Iacovitti L. Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture. Molecular and Cellular Neurosciences. 2005;30(4):601–610. [PubMed] [Google Scholar]
- 167.Lindeberg J, Usoskin D, Bengtsson H, et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40(2):67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 168.Yoshizaki T, Inaji M, Kouike H, et al. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neuroscience Letters. 2004;363(1):33–37. doi: 10.1016/j.neulet.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 169.Rodriguez-Gomez JA, Lu JQ, Velasco I, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25(4):918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells. 2007;25(9):2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hargus G, Cooper O, Deleidi M, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nature Reviews Molecular Cell Biology. 2008;9(7):505–516. doi: 10.1038/nrm2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ring KL, Tong LM, Balestra ME, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11(1):100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nicholas CR, Kriegstein AR. Regenerative medicine: cell reprogramming gets direct. Nature. 2010;463(7284):1031–1032. doi: 10.1038/4631031a. [DOI] [PubMed] [Google Scholar]
- 176.Marro S, Pang ZP, Yang N, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9(4):374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ladewig J, Mertens J, Kesavan J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nature Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 178.Tursun B, Patel T, Kratsios P, Hobert O. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011;331(6015):304–308. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jönsson ME, Ono Y, Björklund A, Thompson LH. Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Experimental Neurology. 2009;219(1):341–354. doi: 10.1016/j.expneurol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 180.Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PloS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028719.e28719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim JY, Koh HC, Lee JY, et al. Dopaminergic neuronal differentiation from rat embryonic neural precursors by Nurr1 overexpression. Journal of Neurochemistry. 2003;85(6):1443–1454. doi: 10.1046/j.1471-4159.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 182.Kim DW, Chung S, Hwang M, et al. Stromal cell-derived inducing activity, Nurr1, and signaling molecules synergistically induce dopaminergic neurons from mouse embryonic stem cells. Stem Cells. 2006;24(3):557–567. doi: 10.1634/stemcells.2005-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee HS, Bae EJ, Yi SH, et al. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28(3):501–512. doi: 10.1002/stem.294. [DOI] [PubMed] [Google Scholar]
- 184.Chung S, Leung A, Han BS, et al. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5(6):646–658. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chung S, Moon JI, Leung A, et al. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(23):9703–9708. doi: 10.1073/pnas.1016443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thompson LH, Andersson E, Jensen JB, et al. Neurogenin2 identifies a transplantable dopamine neuron precursor in the developing ventral mesencephalon. Experimental Neurology. 2006;198(1):183–198. doi: 10.1016/j.expneurol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 187.Parmar M, Li M. Early specification of dopaminergic phenotype during ES cell differentiation. BMC Developmental Biology. 2007;7, article 86 doi: 10.1186/1471-213X-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan Y, Yang D, Zarnowska ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhou W, Lee YM, Guy VC, Freed CR. Embryonic stem cells with GFP knocked into the dopamine transporter yield purified dopamine neurons in vitro and from knock-in mice. Stem Cells. 2009;27(12):2952–2961. doi: 10.1002/stem.216. [DOI] [PubMed] [Google Scholar]


