Abstract
Objective:
To report the prevalence of myopia and its association with diabetic retinopathy in subjects with type II diabetes mellitus and compare the diabetic retinopathy status in the myopic group vs the emmetropic group.
Design:
Population-based study.
Materials and Methods:
The population-based study estimated the prevalence of myopia from 1058 subjects, who were more than 40 years old and had type II diabetes mellitus; the patients were enrolled from a cross-sectional study. Participants answered a detailed questionnaire and underwent biochemical, physical and comprehensive ocular examination which included grading of nuclear sclerosis by lens opacities classification system III (LOCS III), seven field fundus photography and ultrasonography. Diabetic retinopathy and diabetic maculopathy were graded using the Klein's classification and early treatment diabetic retinopathy study (ETDRS) criteria respectively.
Results:
The prevalence of mild, moderate and high myopia in type 2 diabetes was 15.9, 2.1 and 1.9% respectively. The prevalence of any myopia was found to be 19.9% in our study population. After adjusting the age, gender, duration of diabetes, hemoglobin A1c and other factors, increasing age was associated with mild and moderate myopia [OR 1.11 (95% CI 1.05 – 1.18)]. Compared to emmetropia, complete posterior vitreous detachment (CPVD) was associated with high myopia (50% Vs 12.2%, P < 0.0001). Myopia had no association with diabetic retinopathy.
Conclusion:
The prevalence of myopia and high myopia was found to be 19.9 and 1.9% respectively among subjects with type II diabetes. Myopia was not associated with diabetic retinopathy, thereby, suggesting the need for a longitudinal study.
Keywords: Diabetes, high myopia, myopia
Introduction
Diabetic retinopathy (DR) is a major cause of visual loss worldwide — the presence of this sight-threatening disease is seen in approximately 10% of persons with type II diabetes.[1] We have previously reported the prevalence of diabetic retinopathy, in a population with diabetes mellitus, to be 18.0%.[2] Myopia, especially high myopia, has been suggested to have a protective effect against DR.[3]
There are few population-based studies which have studied the association of myopia and DR.[1,4] Wisconsin epidemiologic study of diabetic retinopathy (WESDR)[4] did not find any association between myopia (spherical equivalent ≤ 2 diopters) and diabetic retinopathy incidence or progression on univariate analysis. However, the multivariate models showed a protective effect against progression to proliferative diabetic retinopathy (PDR) in subjects with younger onset diabetes. The Singapore Malay eye study (SiMES) reported that diabetic subjects with myopic refraction and a longer axial length had a lower risk of developing DR, particularly sight-threatening DR.[1]
We have also reported the prevalence of refractive errors in subjects with type II diabetes.[5] The prevalence of myopia (SE <- 0.50 DS), high myopia (SE <- 5.00 DS), hyperopia (SE >+ 0.50 DS), and astigmatism (SE <- 0.50 cyl) were 19.4, 1.6, 39.7, and 47.4%, respectively.[5] We also found that poor glycemic control was associated with myopia (OR [95% CI] 4.15 [1.44-11.92]).[5]
The purpose of this study was to elucidate the association of myopia with diabetic retinopathy and correlate the role of parameters like posterior vitreous detachment, axial length and spherical equivalent in diabetic retinopathy among myopic patients in population-based subjects with type II diabetes.
Materials and Methods
Sankara Nethralaya— Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN DREAMS 1) is a population-based, cross-sectional study of urban Chennai adults above 40 years with stratification based on the socio-economic status scoring: low (score, 0–14), middle (score, 15–28), and high (score, 29–42).[6] The details of the study design and research methodology of SN—DREAMS1 have been described elsewhere.[7] In brief, the study area was the Chennai metropolis, with a population of 4.3 million, out of which 5999 individuals ≥ 40 years, were enumerated from 155 divisions of 10 zones, using the multistage random sampling method. Subjects with diabetes, according to the World Health Organization criteria,[8] underwent biochemical examination (fasting blood sugar, HbA1C, Lipid profiles), comprehensive ocular examination like slit lamp evaluation, 45° four-field stereoscopic digital photography, corneal pachymetry and ultrasound B-scan. Figure 1 shows the enumeration details of the myopic subjects with type II diabetes. After all exclusions, 1058 subjects with diabetes were analyzed for this study.
Figure 1.
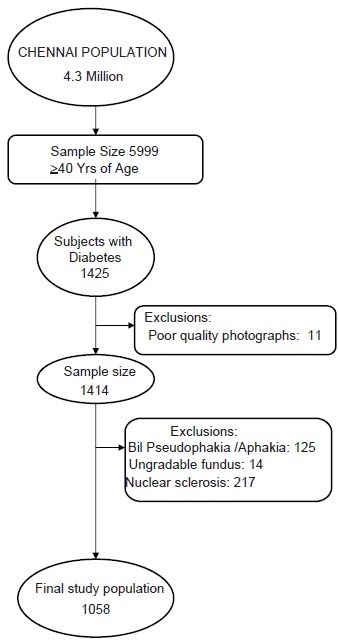
Enumeration details of myopic subjects with Type II diabetes mellitu
Body mass index (BMI) was calculated by using the formula weight (kg)/height (m2). The presence of hypertension was defined as systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg or if the patient was on an anti-hypertensive therapy.
Visual acuity estimation was done using, modified early treatment of diabetic retinopathy study chart (Light House Low Vision Products, New York, NY); Landolt's ring test was used for those who were found unfamiliar with English alphabets. Objective refraction (streak retinoscope, Beta 200, Heine, Germany) was always followed by a subjective one.
Grading of myopia
Mild myopia was defined as spherical equivalent from -0.50 DS to -3.0 DS, moderate myopia as - 3.0 DS to – 5.00 Ds and high myopia as less than -5.00 DS.
Grading of lens opacity
The grading was done as per the Lens Opacity Classification System (LOCS) III system by a trained ophthalmologist.[9] We considered LOCS III grade ≥ N2 as a cutoff point to prevent the confounding effect of nuclear sclerosis.[5] The severity of the lens opacities, according to the photographic standards, was separated into four major groups: nuclear opalescence, nuclear color, cortical, and posterior sub capsular.
Both graders (PKR, RR) were experienced ophthalmologists (8 years). For assessing the grading agreement, 50 patients with various grades of cataract were recruited from the pilot study and were assessed independently by both graders. The grading agreements were nuclear opalescence (k = 0.87), nuclear color (k = 0.83), cortical (k = 0.89), and posterior subcapsular (k = 0.81). Overall, the average grading agreement was high (k = 0.85).
Grading for diabetic retinopathy
Diabetic retinopathy was diagnosed based on the modified Klein classification of the Early Treatment Diabetic Retinopathy Study (ETDRS) scale.[10] as described in Table 1A and 1B.
Table 1A.
Definitions

Table 1B.
Grading of diabetic retinopathy

This grading was done by two independent observers in a masked fashion; the grading agreement was high (k = 0.83).[7] Corneal pachymetry (Alcon ultrasound pachymeter) was performed to measure the corneal thickness. An average of three readings was taken for each eye. Echography was done using UltraScan (Alcon) with 10 MHz B-Scan probe and 1) No Posterior vitreous detachment (PVD) 2) Incomplete PVD 3) Complete PVD. For the purpose of final analysis, only the Phakic right eye in bilaterally phakic subjects was considered, in subjects, who underwent cataract surgery, the other phakic eye was used. To prevent the confounding effect of nuclear sclerosis on myopia of those nuclear opalescence with score of ≥ 2 was considered as a cutoff point. This study was approved by the Institutional Review Board, and a written informed consent was obtained from the subjects according to the Helsinki Declaration.
Results
A total of 1058 subjects with type II diabetes were included in the analysis after excluding 11 subjects with bilateral poor quality photographs, 14 subjects with unilateral poor quality photographs and 125 subjects with a history of bilateral cataract surgery. Only the right eye, in case of bilaterally Phakic subjects, and the phakic other eye, in case of unilateral pseudophakia / aphakia subjects, were included in the analysis.
We found the prevalence of mild, moderate and high myopia in type 2 diabetes to be 15.9, 2.1 and 1.9% respectively. The prevalence of any myopia was found to be 19.9% in our study population. The mean age ± standard deviation was 53.1 ± 8.4 years, 578 (54.6%) were males, 169 subjects had mild myopia and their mean age was 52.9 ± 9.2 years, 22 subjects had moderate myopia and their mean age was 56.5 ± 10.2 years, 20 subjects had high myopia and their mean age was 54.0 ± 11.5 years.
As shown in Table 2, moderate myopics were significantly older when compared to others (mean age 56.5 ± 10.2 years, trend P = 0.016).
Table 2.
Univariate analysis of demographic, biochemical and ocular parameters in different grades of myopic subjects with Type II diabetes mellitus
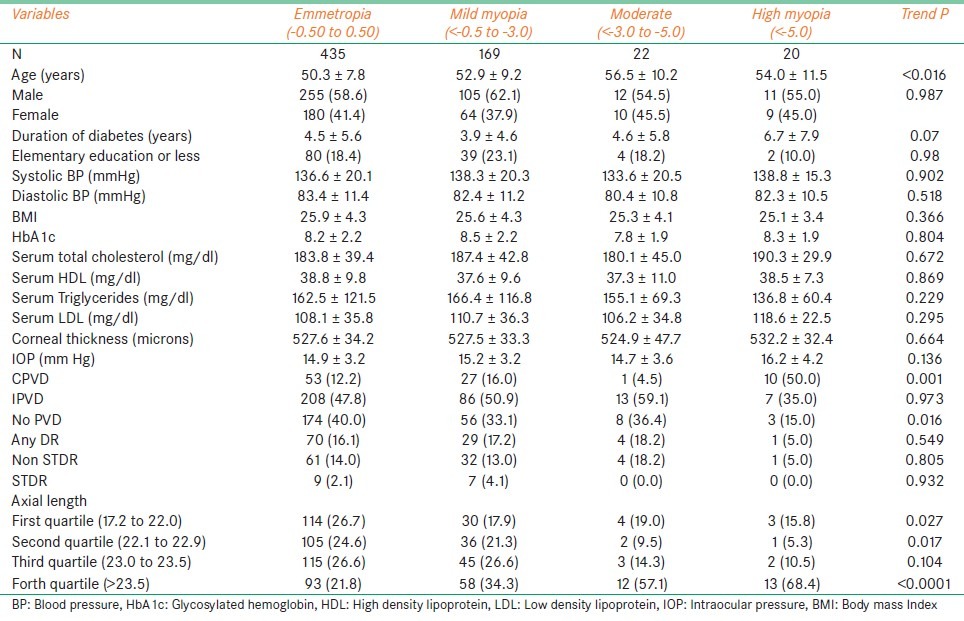
In the ultrasound parameters, high myopes had more subjects with complete PVD as compared to emmetropia (50% Vs 12.2%, trend P = 0.001). We also found no PVD to be less associated with high myopia (15.0%) than emmetropia (40%) (trend P = 0.016).
Subjects with high myopia had an increased axial length (>23.5 mm) compared to emmetropia, mild and moderate myopia (trend P < 0.0001).
Table 3 shows increasing age to be significantly associated with mild [OR 1.03 (95% CI 1.01 – 1.06)] and moderate myopia [OR 1.11 (95% CI 1.05 – 1.18)]. Females were found to be at significant risk of having high myopia [OR 3.67 (1.03 – 13.5)]. Subjects with complete PVD had a higher risk of developing high myopia when compared to incomplete PVD [OR 0.12 (95% CI 0.03 – 0.44), P = 0.001] and no PVD [OR 0.08 (95% CI 0.02 – 0.42), P = 0.003]. Subjects with a longer axial length (>23.5 mm) were found to be at risk of having all grades of myopia, mild myopia [OR 2.29 (95% CI1.34 –3.95), P = 0.003], moderate myopia [OR 4.58 (95% CI1.32 –15.91), P = 0.017] and high myopia [OR 5.10 (95% CI1.17 –22.22), P = 0.030].
Table 3.
Multivariate analysis of associated factors in myopic subjects with Type II diabetes mellitus
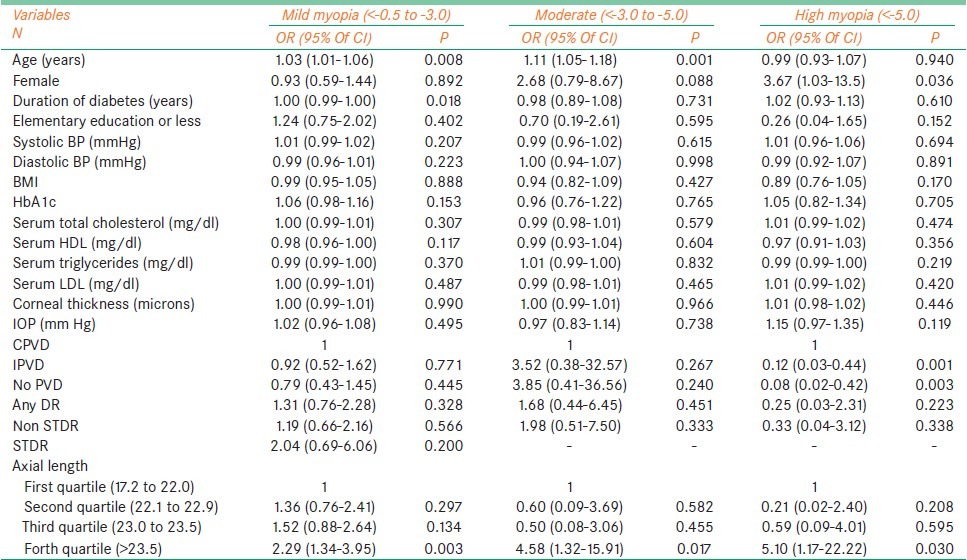
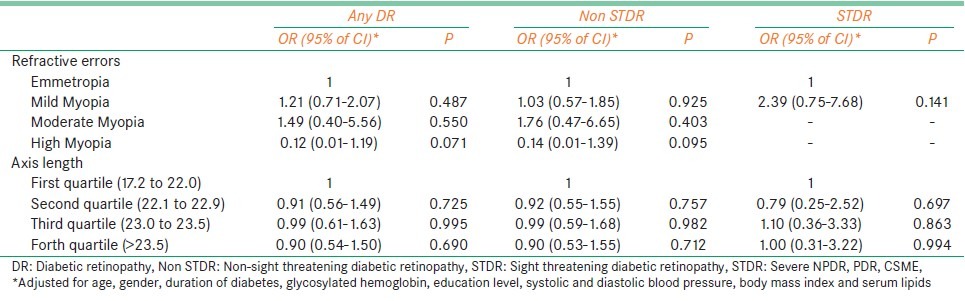
Table 4 shows the association of the different grades of myopia and axial length with severity of DR. It can be seen that subjects with different grades of myopia and with increased axial length were not at risk for developing DR.
Table 4.
Associations of grades of myopia and axial length with severity of diabetic retinopathy
Discussion
This article reports the relationship between ranges of myopia with DR in a population- based study in Chennai. The prevalence of mild, moderate and high myopia in type 2 diabetes was 15.9, 2.1 and 1.9% respectively. We also found women to be at risk of developing high myopia than emmetropia. This finding was similar to the study reported by the National Health and Nutrition Examination Survey (NHANES).[11]
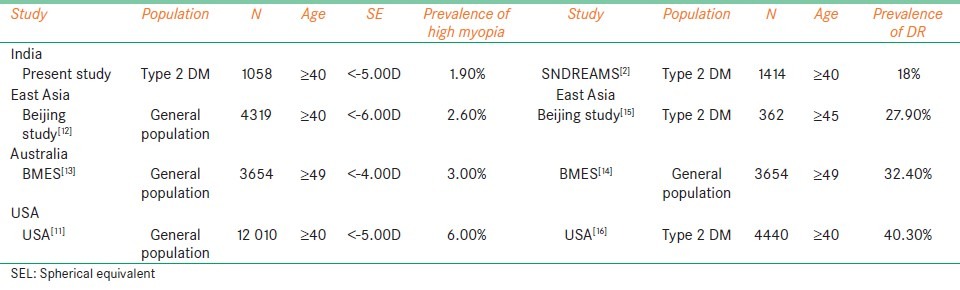
We also found the prevalence of high myopia to be 1.9% and the prevalence of diabetic retinopathy, as reported from our previous study, to be 18%.[2] Similarly, the population- based study from China, the Beijing eye study,[12] reported the prevalence of high myopia to be 2.6% and diabetic retinopathy to be 27.9%; the Blue mountain eye study, (Australia)[13,14] reported the prevalence of high myopia and diabetic retinopathy to be 3.0% and 32.40% respectively; the Eye Diseases Prevalence Research Group in the US,[11] reported the prevalence of high myopia to be 6.0% and diabetic retinopathy to be 40.30%, as summarized in Table 5. The population which had a higher prevalence of high myopia also had a higher prevalence of diabetic retinopathy. This supports the observation that myopia does not seem to protect against diabetic retinopathy. Although there may be various other variables like the duration of diabetes, age, glycemic control etc which might have a role in developing diabetic retinopathy.
Table 5.
Comparison of prevalence of high myopia and prevalence of diabetic retinopathy between various other population-based studies and present study
It is not possible to make close comparisons with other studies because of the different definitions used for myopia. The prevalence of myopia of −0.5 D or less in the current study was 19.9% for persons 40 years and older. This definition is comparable with most other studies like the Blue Mountains Eye Study (BMES)(15.4%)[13] and Beijing study (21.8%).[12] The USA study, however, reported a higher prevalence (41%).[11] The racial difference, probably, played a vital role in the distribution of myopia over different groups. We found increasing age to be one of the factors associated with myopia in our study (trend P < 0.016) unlike the Kinmen study,[17] The difference observed could be due to the fact that Kinmen study was based on a self-reported group and our study was an epidemiological one.
PVD was noted in 50% of high myopia with trend for P = 0.001. This may be one of the reasons for our population to have a lower prevalence of DR (18%)[2] compared to other reported studies like Beijing study (27.90%),[15] BMES (32.4%)[14] and USA (40.3%).[16] Several studies have demonstrated the protective effect of complete PVD being associated with a reduced likelihood of progression to neovascularization and PDR.[18–20] This has been attributed to the removal of the vitreous scaffold for neovascular proliferation, as well as to improved oxygen diffusion across the liquefied vitreous.[21,22] We also found no PVD to be more associated with hypermetropia (40%) than high myopia (15.0%) stressing the protective nature of myopia for DR.
After adjusting all the factors, we found that increasing age was associated with mild and moderate myopia [OR 1.11 (95% CI 1.05 – 1.18), P = 0.001]. This could be due to the biometric changes in the optical system of the lens. To prevent the confounding effect of nuclear sclerosis on the myopic status NO ≤ 2 were only included for this study. Women had a predilection for mild myopia, than emmetropia [OR 0.93 (95% CI 0.59 – 1.44), P = 0.018], and high myopia [OR 3.67 (95% CI 1.03 – 13.5), (P = 0.036 after adjusting for all factors). There are conflicting reports about refractive error and gender. The National Health and Nutrition Examination Survey (NHANES),[23] the Beaver Dam Eye Study,[24] and a study of refractive error in a Finnish rural population[25] showed higher prevalence rates of myopia among female subjects than in male subjects.
Subjects with longer axial lengths (>23.5 mm) were more likely to develop mild myopia [OR 2.29 95% CI (1.34 – 3.95), P = 0.003], moderate myopia [OR 4.58 95% CI (1.32 – 15.91), P = 0.017] and high myopia [OR 5.10 95% CI (1.17 – 22.22), P = 0.030]. A 1-mm elongation of axial length (AL) without other compensation is equivalent to a myopia shift of −2 or −2.5 dpt.[26] Since most agree that AL is the largest determinant of refractive error,[27] this factor was analyzed in the study.
We did not find refractive error, over differing ranges of myopia and increasing ranges of axial length, to be a risk factor for developing any form of DR. Our findings are similar to Wisconsion epidemiologic study of diabetic retinopathy (WESDR),[4] which found no association between myopia and the presence of DR. To summarize, we found that high myopes had longer axial length and CPVD and had no association with DR. It would be interesting to see the effect of myopia on the progression DR in the Indian population.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Lim LS, Lamoureux E, Saw SM, Tay WT, Mitchell P, Wong TY. Are myopic eyes less likely to have diabetic retinopathy? Ophthalmology. 2010;117:524–30. doi: 10.1016/j.ophtha.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 2.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India sankara nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Pierro L, Brancato R, Robino X, Lattanzio R, Jansen A, Calori G. Axial length in patients with diabetes. Retina. 1999;19:401–4. doi: 10.1097/00006982-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Moss SE, Klein R, Klein BE. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology. 1994;101:77–83. doi: 10.1016/s0161-6420(94)31353-4. [DOI] [PubMed] [Google Scholar]
- 5.Rani PK, Raman R, Rachapalli SR, Kulothungan V, Kumaramanickavel G, Sharma T. Prevalence of refractive errors and associated risk factors in subjects with type 2 diabetes mellitus SN-DREAMS, report 18. Ophthalmology. 2010;117:1155–62. doi: 10.1016/j.ophtha.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Oakes JM, Rossi PH. The measurement of SES in health research: Current practice and steps toward a new approach. Soc Sci Med. 2003;56:769–84. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal S, Raman R, Paul PG, Rani PK, Uthra S, Gayathree R, et al. Sankara nethralaya-diabetic retinopathy epidemiology and molecular genetic study (SN-DREAMS 1): Study design and research methodology. Ophthalmic Epidemiol. 2005;12:143–53. doi: 10.1080/09286580590932734. [DOI] [PubMed] [Google Scholar]
- 8.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 9.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93:1183–7. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 11.Vitale S, Ellwein L, Cotch MF, Ferris FL, 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111–9. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Li J, Cui T, Hu A, Fan G, Zhang R, et al. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005;112:1676–83. doi: 10.1016/j.ophtha.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: The blue mountains eye study. Ophthalmology. 1999;106:1066–72. doi: 10.1016/S0161-6420(99)90251-8. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P, Smith W, Wang JJ, Attebo K. Prevalence of diabetic retinopathy in an older community. The blue mountains eye study. Ophthalmology. 1998;105:406–11. doi: 10.1016/S0161-6420(98)93019-6. [DOI] [PubMed] [Google Scholar]
- 15.Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy.The Beijing eye study 2006. Graefes Arch Clin Exp Ophthalmol. 2008;246:1519–26. doi: 10.1007/s00417-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 16.Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, et al. Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 17.Chen SJ, Tung TH, Liu JH, Lee AF, Lee FL, Hsu WM, et al. Prevalence and associated factors of refractive errors among type 2 diabetics in Kinmen, Taiwan. Ophthalmic Epidemiol. 2008;15:2–9. doi: 10.1080/09286580701585736. [DOI] [PubMed] [Google Scholar]
- 18.Tagawa H, McMeel JW, Furukawa H, Quiroz H, Murakami K, Takahashi M, et al. Role of the vitreous in diabetic retinopathy. I. Vitreous changes in diabetic retinopathy and in physiologic aging. Ophthalmology. 1996;93:596–601. doi: 10.1016/s0161-6420(86)33690-x. [DOI] [PubMed] [Google Scholar]
- 19.Tagawa H, McMeel JW, Trempe CL. Role of the vitreous in diabetic retinopathy. II. Active and inactive vitreous changes. Ophthalmology. 1996;93:1188–92. doi: 10.1016/s0161-6420(86)33608-x. [DOI] [PubMed] [Google Scholar]
- 20.Akiba J, Arzabe CW, Trempe CL. Posterior vitreous detachment and neovascularization in diabetic retinopathy. Ophthalmology. 1990;97:889–91. doi: 10.1016/s0161-6420(90)32486-7. [DOI] [PubMed] [Google Scholar]
- 21.Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51:364–80. doi: 10.1016/j.survophthal.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Hendrikse F, Yeo KT. Role of the vitreous body in diabetic retinopathy. Klin Monbl Augenheilkd. 1993;203:319–23. doi: 10.1055/s-2008-1045684. [DOI] [PubMed] [Google Scholar]
- 23.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101:405–7. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam eye study. Invest Ophthalmol Vis Sci. 1994;35:4344–7. [PubMed] [Google Scholar]
- 25.Aine E. Refractive errors in a finnish rural population. Acta Ophthalmol. 1984;62:944–54. doi: 10.1111/j.1755-3768.1984.tb08447.x. [DOI] [PubMed] [Google Scholar]
- 26.Meng W, Butterworth J, Malecaze F, Calvas P. Axial length of myopia: A review of current research. Ophthalmologica. 2011;225:127–34. doi: 10.1159/000317072. [DOI] [PubMed] [Google Scholar]
- 27.Young TL, Metlapally R, Shay AE. Complex trait genetics of refractive error. Arch Ophthalmol. 2007;125:38–48. doi: 10.1001/archopht.125.1.38. [DOI] [PubMed] [Google Scholar]


