Abstract
We report on the use of optical techniques to monitor and treat Pseudomonas aeruginosa wound infections in mice. Bioluminescent bacteria transduced with a plasmid containing a bacterial lux gene operon allow the infection in excisional mouse wounds to be imaged by use of a sensitive charge-coupled device camera. Photodynamic therapy (PDT) targeted bacteria, by use of a polycationic photosensitizer conjugate, which is designed to penetrate the gram-negative cell wall and was topically applied to the wound and was followed by red-light illumination. There was a rapid light dose–dependent loss of luminescence, as measured by image analysis, in the wounds treated with conjugate and light, a loss that was not seen in untreated wounds, wounds treated with light alone, or wounds treated with conjugate alone. P. aeruginosa was invasive in our mouse model, and all 3 groups of control mice died within 5 days; in contrast, 90% of PDT-treated mice survived. PDT-treated wounds healed significantly faster than did silver nitrate–treated wounds, and this was not due to either inhibition of healing by silver nitrate or stimulation of healing by PDT.
Photodynamic therapy (PDT) uses a combination of harmless dyes and visible light that, in the presence of oxygen, produces reactive oxygen species that damage biomolecules and kill cells [1]. Despite a century of use of PDT to kill bacteria in vitro [2], its use to treat infections in vivo has not been developed [3]. We have discovered a method to target bacteria by use of polycationic photosensitizer conjugates, and subsequent illumination with modest levels of red light produces ≤6 logs of bacterial killing in vitro [4]. The method is based on the covalent attachment of photosensitizer peptides to polycationic peptides, such as poly-L-lysine, that can bind to and penetrate both gram-positive and gram-negative bacteria. We recently reported on the use of these conjugates and red-light illumination to destroy Escherichia coli infections in mouse wounds [5]. Because these macromolecular conjugates were administered topically into sites of infection, and because the time needed to bind to bacteria was relatively short, the treatment showed good selectivity for bacteria, compared with that of host tissue. We used genetically engineered bacteria that emit bioluminescence and that can be detected in vivo by use of an intensified charge-coupled device (CCD) camera [6]. Quantification of the luminescence images can determine, in real time, the extent of infection in living animals and thereby can provide both temporal and spatial information about the labeled bacteria [7, 8].
Pseudomonas aeruginosa, an increasingly prevalent opportunistic human pathogen, is the most common gram-negative bacterium found in nosocomial infections. P. aeruginosa is responsible for 8% of surgical-wound infections [9] and for 10% of bloodstream infections [10]. Immunocompromised patients, such as neutropenic cancer and bone marrow–transplant patients, are particularly susceptible to opportunistic infections. In this group of patients, P. aeruginosa is responsible for pneumonia and septicemia, with attributable deaths reaching 30% [11, 12]. P. aeruginosa outbreaks in hospital burn units are still associated with high (60%) death rates [13]. To initiate infection, P. aeruginosa usually requires a substantial break in first-line defenses (e.g., trauma, surgery, serious burns, or indwelling devices) or alteration of the immunologic defense mechanisms (e.g., chemotherapy-induced neutropenia, mucosal clearance defects from cystic fibrosis, AIDS, and diabetes mellitus). P. aeruginosa produces several extracellular virulence factors that, after infection, can cause extensive tissue damage, bloodstream invasion, and dissemination [9]. Considering the intrinsic antibiotic resistance of P. aeruginosa, the worldwide rise in acquired antibiotic resistance, and the likelihood of P. aeruginosa infecting poorly perfused traumatized tissue, alternative topical antimicrobial treatments are needed.
Our other report [5] is a proof-of-principle study using a relatively nonpathogenic strain of E. coli (DH5a), which lacks virulence factors necessary to cause invasive infections [14]. We report here on the use of optical techniques (bioluminescence imaging and targeted PDT) to monitor and cure mice with otherwise fatal P. aeruginosa wound infections and on the beneficial effect of the treatment on infected wounds.
MATERIALS AND METHODS
Preparation and characterization of poly-L-lysine–ce6 conjugate
The preparation and characterization of poly-L-lysine–ce6 conjugate has been elsewhere described in detail [5]. In brief, poly-L-lysine–HBr (average molecular weight, 22,000; degree of polymerization, 110; Sigma) was reacted with ce6 (Porphyrin Products) and-1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma) in dry dimethyl sulfoxide containing triethylamine, for 24 h in the dark. The crude conjugate was purified on a column of Sephadex G25 and was eluted by use of sodium acetate buffer (10 mM; pH 5.5). The substitution ratio was calculated, from the absorption spectrum of the conjugate, to be an average of 7.4 ce6/poly-L-lysine chain, assuming that the absorption coefficient of conjugated ce6 was the same as that of free ce6 (ε400nm p 150,000 M–1cm–1).
Bacteria
We used P. aeruginosa strain 180 (ATCC 19660), which causes septicemia after intraperitoneal infection [15] and has been shown to be invasive in mice with skin burns [16]. The bioluminescent variant of this strain was constructed by transformation with plasmid pCGLS1, an expression vector that contains a complete bacterial luciferase operon, as described elsewhere [7]. Bacteria were grown in brain-heart infusion medium containing ampicillin (100 μg/mL) (to select for resistance encoded by the plasmid), in an orbital incubator (37°C; 100 rpm) to an optical density of 0.6 at 650 nm that corresponds to 108 organisms/mL (mid-log phase). This suspension was centrifuged, was washed with PBS, and was resuspended in PBS at the same density. Luminescence was routinely measured on 100-μL aliquots of bacterial suspensions in 96-well black-sided plates, by use of a Victor-2 1420 Multilabel Plate Reader (EG&G Wallac).
In vitro studies
In vitro studies were performed as described elsewhere [5] for E. coli. In brief, suspensions of the bacteria (108 cells/mL), in triplicate, were incubated in the dark, for 30 min at room temperature, with varying concentrations of conjugate measured as μM ce6 equivalent (final concentration in PBS). After centrifugation (9000 g; 1 min) and washing with PBS, the cell pellet was dissolved by digesting it in 1.5 mL of 0.1 M NaOH and 1% SDS, for at least 24 h, and the cellular uptake of ce6 was measured by fluorescence assay, as described elsewhere [4]. The protein content of the entire cell extract was then determined by a modified Lowry method [17], and results were expressed as moles of ce6 per milligram of cell protein.
Phototoxicity was measured as follows. Suspensions of bacteria (108/mL) were incubated with 1–18 μM ce6 equivalent of the conjugate in PBS, as described above. After centrifugation, washing, and resuspension in sterile PBS at the same density, 1-mL aliquots of suspensions were placed in 24-well plates. The wells were illuminated from below, by use of a 660-nm, 300-mW diode laser (SDL), an optical fiber, and a lens (to form a 2-cm-diameter spot on the base of the 24-well plates). Fluences ranged from 0 to 40 J/cm2, at an irradiance of 100 mW/cm2. At times during the illumination when the requisite fluences (10, 20, and 40 J/cm2) had been delivered, 2 aliquots (100 μL each) were taken from each well; care was taken to ensure thorough mixing before sampling, because bacteria can settle. For determination of colony-forming units, 1 aliquot was serially diluted on nutrient agar plates containing ampicillin, as described by Jett et al. [18]. Plates were streaked in triplicate and were incubated in the dark, for 24 h at 37°C. The remaining aliquot was used for luminescence measurement, as described above. Plates were inserted into the luminometer 15 min after completion of the illumination.
Mice
Male BALB/c mice (20–25 g) were used. Their backs were shaved and were depilated by use of Nair (Carter-Wallace). Mice were anesthetized by an intraperitoneal injection of ketamine-xylazine cocktail (90 mg/kg ketamine and 10 mg/kg xylazine) for surgery and for subsequent PDT and imaging. Surgical scissors and forceps were used to create either 1 or 2 full-thickness excisional wounds down to, but not through, the panniculus carnosus. There was no visible bleeding within the wounds. Mice were euthanized when their conditions were assessed to be moribund.
Bioluminescence imaging
The low-light imaging system (Hamamatsu Photonics) has been elsewhere described in detail [5]. It consists of an ICCD camera mounted in a light-tight specimen chamber, fitted with a light-emitting diode, a set-up that allows a background gray-scale image of the entire mouse to be captured. In the photon-counting mode, an image of the emitted light was captured by use of an integration time of 2 min, at a maximum setting on the image-intensifier control module. By use of ARGUS software, the luminescence image was presented as a false-color image superimposed on top of the gray-scale reference image. The image-processing component of the software provided mean pixel values from the luminescence images, on defined areas within each wound, on a 256 gray scale. The same analysis area (1200 pixels) was used for all wounds at all times.
In vivo studies
In the excisional wound model, initial studies were carried out to determine the degree of pathogenicity of this strain of P. aeruginosa. Mice with single wounds (area, 100 mm2 [8 mm × 12.5 mm]) received increasing inocula of mid-log phase P. aeruginosa, from 104 to 5 × 106 bacteria suspended in 50 μL of PBS, which were applied with a 200-μL pipette tip. These mice underwent imaging in the luminescence camera immediately afterward and then daily, until death or until the luminescence signal disappeared.
All experiments with mice whose wounds contained photosensitizer were carried out under subdued room lighting or in the dark, except when illumination was taking place. Forty mice were divided into 4 groups of 10, and all mice received a single excisional wound (8 mm × 12.5 mm) on the lower back, as described above. The 4 groups were as follows: (1) untreated controls (bacteria alone), (2) bacteria plus light (240 J/cm2), (3) bacteria plus conjugate, kept in the dark, and (4) the PDT group (bacteria, conjugate, and increasing light doses). The bacterial inoculum was chosen to be 5 × 106 (25 times the LD50). To ensure equal bacterial loading into all wounds, mice were subjected to imaging immediately after infection. After 30 min, to allow the bacteria to bind to the tissue, the mice underwent imaging again. Conjugate (50 μL; 200 μM ce6 equivalent) was added to the wounds of 20 mice and was retained by the edges of the wound. They were subjected to imaging immediately and then again after a 30 min interval, to allow the conjugate to both bind to and penetrate the bacteria. Control mice (untreated and conjugate-treated kept in the dark) were then subjected to imaging, after a further 30 min (approximate time needed for delivery of the highest light dose [240 J/cm2]). Mice in the PDT and light-alone groups were then illuminated with 665-nm light, delivered by a 1-W diode laser (BWF-665-1; B&W Tek), which was coupled into a 200-μm fiber to produce a circular spot on the mouse with a diameter of 2 cm, which completely covered the wound and ~5 mm of unbroken skin surrounding the wound. The power density was 100 mW/cm2. These animals underwent imaging after 40, 80, 160, and 240 J/cm2 light had been delivered. There were no visible differences between any of the wounds at the completion of illumination. Mice were kept in their cages, in the dark; on each succeeding day, the mice were anesthetized and were subjected to imaging, under the same conditions, for as long as they lived or until all luminescence had disappeared from the wound. Luminescence intensities were calculated for all wounds by use of the ARGUS software, as described above.
Wound healing
The dimensions of the wounds (width and length) were measured daily, by use of vernier calipers, and the areas were calculated. Because P. aeruginosa was invasive in this model, only the PDT-treated wounds could be monitored until healing had occurred, because all mice in the control groups died. We investigated an alternative topical antibacterial treatment (0.5% AgNO3 aqueous solution) to compare its effects on bacterial infection and wound healing with those of PDT. This treatment was added to the infected wounds of 10 mice, 30 min after introduction of the bacteria, as a 50-μL application into the wound. These mice survived, and their wounds were measured as described above. Experiments were then performed to investigate the mechanism responsible for the difference in wound healing observed between PDT and AgNO3 antibacterial treatment. Three groups of mice (n = 6/group) had 2 side-by-side wounds (area, 100-mm2) created on their lower backs (to the left and right sides of the spine). In 2 groups, these wounds remained uninfected: in the first group, we compared the healing rate of untreated wounds versus that of either PDT-treated wounds or AgNO3-treated wounds (3 mice treated on the right side and 3 mice treated on the left side); in the second group we compared the healing rate of untreated wounds versus that of AgNO3-treated wounds (3 mice treated on the right side and 3 mice treated on the left side); and, in the third group, both wounds were infected with a dose of 2 × 106 cfu luminescent P. aeruginosa. The infective dose was reduced to avoid the danger of mice dying from endotoxemia (despite successful killing of viable bacteria). Death from endotoxemia was occasionally observed in initial studies when mice received 2 wounds each with 5 × 106 cfu. These 6 mice had their pairs of wounds treated, on 1 side, with PDT and, on the other side, with AgNO3 (3 mice treated on the right side and 3 mice treated on the left side), as described above. All mice survived, and the dimensions of their wounds were measured daily.
Statistics
Differences between the means of wound areas were analyzed for statistical significance by the unpaired 2-tailed Student's t test. P < .05 was considered significant.
RESULTS
In vitro studies
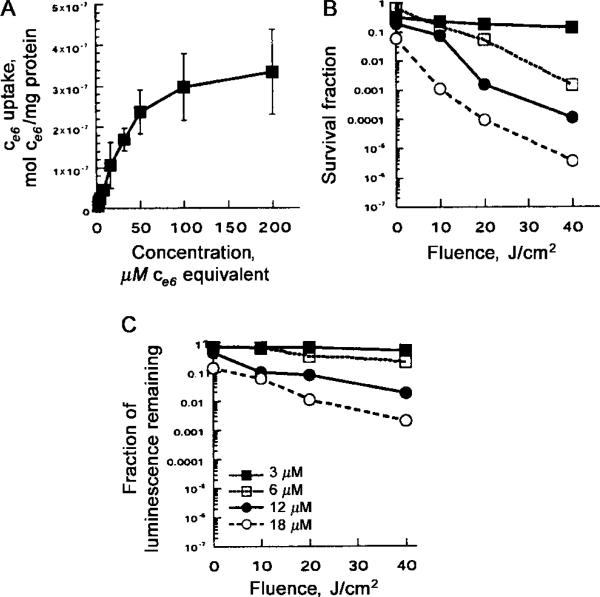
The luminescence signal measured in the luminometer was linearly proportional to bacterial colony-forming units (as determined by serial dilution and plating) from 103 to 107 organisms (data not shown). The uptake of the conjugate by the bacteria is shown in figure 1A. A diminution in the rate of increase of uptake was apparent starting at 50 μM ce6 equivalent and became more marked at higher concentrations tending towards saturation. The loss of viability curves, as measured by colony-forming units and by loss of luminescence as a function of light dose, for bacteria incubated with 3, 6, 12, and 18 μM conjugate, are shown in figure 1B and 1C. The colony-forming unit assay had a limit of sensitivity of 6 orders of magnitude, in reduction of viability, whereas the bioluminescence assay had a limit of 3 orders of magnitude [5]. Loss of luminescence showed the same dose-response curves as loss of colony-forming units, but the absolute reductions were 1–3 logs less. The reasons for this are 2-fold. First, the limit of sensitivity of the luminescence assay using the plate reader is a 3-log reduction in signal, whereas the colony-forming unit assay can measure a 6-log reduction in viability. Second, it appears that the cytotoxic insult to the bacteria causes loss of viability more readily than it causes loss of luminescence. The mechanism by which luminescence decreases after photoinactivation is not known, but this decrease may be due to exhaustion of ATP supplies from the bacteria (needed for the luciferase enzyme to make luminescence), which cannot be replenished if the cells are fatally damaged.
Figure 1.
A, Uptake of ce6 by Pseudomonas aeruginosa, after 30 min of incubation in PBS with stated concentrations of poly-L-lysine–ce6, followed by dissolution of cell pellet in NaOH–SDS and fluorescence and protein assays. Data points are means of triplicate determinations and 2 separate experiments, and bars indicate SDs. B and C, Phototoxicity, as determined by either colony-forming unit assay (B) or luminescence assay (C). Bacteria were incubated with conjugate and then were washed and were illuminated by fluences (0, 10, 20, and 40 J/cm2) of 665-nm light, by removing aliquots of bacterial suspension at intervals at 0, 100, 200, and 400 s, respectively, followed by serial dilution and plating or luminescence measurement in 96-well plates. Data points are means of triplicate determinations and 2 separate experiments, and bars indicate SDs.
In vivo studies
Mice with wounds infected with 5 × 106 cfu of P. aeruginosa quickly developed an illness consistent with systemic sepsis. They lost weight, had ruffled coats, and developed progressive inactivity, leading to a moribund condition, and death occurred between 24 h and 60 h after infection. When the effect of varying the initial bacterial inoculum was studied, we found that the LD50 was ~200,000 cfu. Mice that were infected with the lower numbers of bacteria and did not die had the same infection as the mice that did die, but in a less severe form, and they recovered between days 6 and 8. To nevertheless provide a robust test of the ability of the technology to prevent death from a fatal wound infection, we decided to use a bacterial challenge for the PDT experiments that was 25 times higher than the LD50.
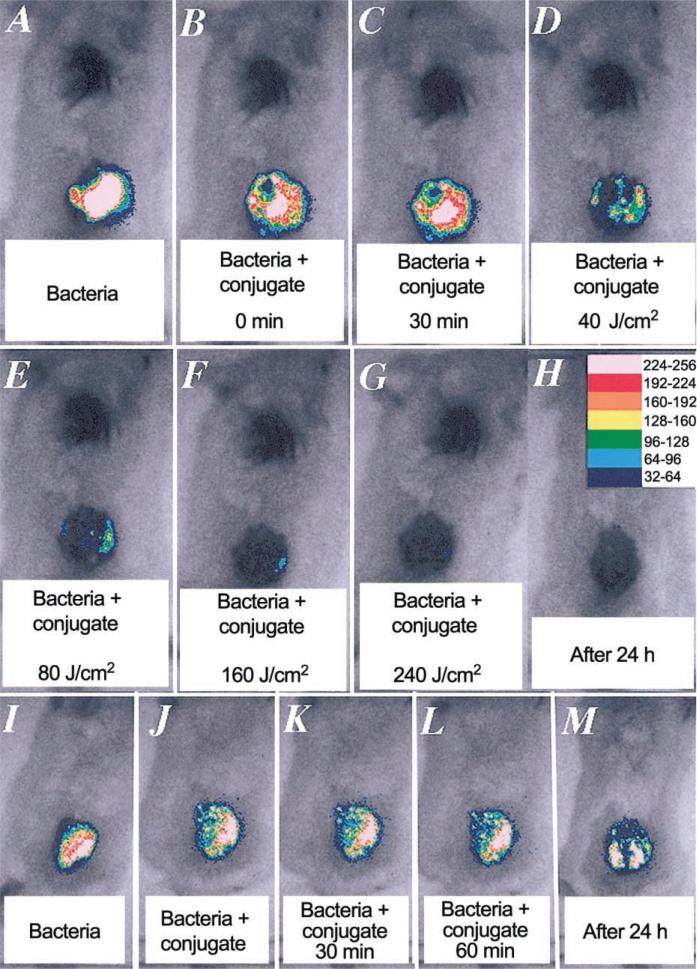
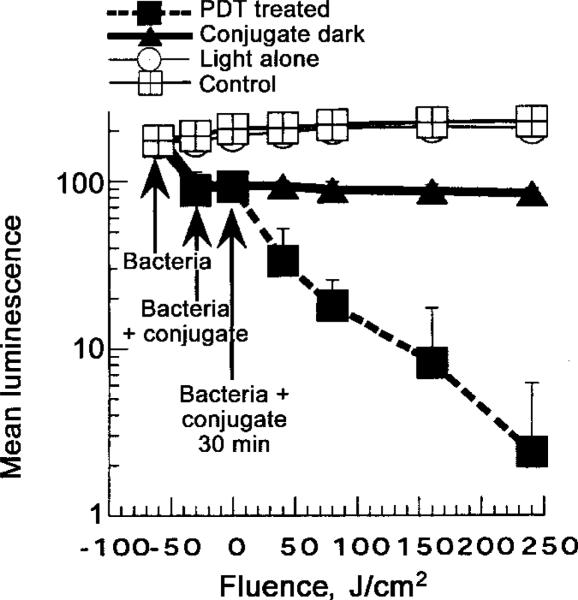
Mice were given a single dorsal excisional wound (area, 100 mm2) and were infected with 5 × 106 cfu of P. aeruginosa suspended in 50 μL of PBS. This inoculum gave a sufficiently bright luminescence signal from the wound to allow ≥2 logs of signal reduction to be accurately monitored. The bacteria quickly attached to the tissue surface of the panniculus carnosus, as evidenced by the failure to wash them off by irrigation with saline, 30 min after infection, and as quantitated by luminescence imaging. The conjugate was added as 50 μL of a 200-μM ce6 equivalent concentration, because preliminary experiments had shown lower concentrations to be less effective. This volume was sufficient to spread evenly throughout the surface of the wound and was retained by the edges of the wound. It was necessary to wait ≥30 min before commencing illumination, to allow the conjugate to bind to and penetrate the bacteria, so that effective loss of luminescence after illumination could be observed. As can be seen from a set of luminescence images from a representative mouse, shown in figure 2A–2G, PDT produced a fluence-dependent loss of luminescence, until only a trace remained, after 240 J/cm2 had been delivered. The next day, when the mouse underwent imaging, all traces of luminescence were gone (figure 2H). A drop in luminescence was seen shortly after application, in the dark, of the conjugate (figure 2B and 2J), but luminescence did not decrease further, either after 30 min of incubation (figure 2C and 2K) or, indeed, after 60 min of incubation (approximately equal to the time for illumination of the PDT wounds) (figure 2L). Infected wounds left untreated or treated with illumination alone showed a rise in luminescence signal (up to 2-fold) (figure 3), presumably due to growth of the bacteria in the nutrient-rich medium of the wound. Significant luminescence was present in control wounds, until death occurred 2–4 days later (figure 2M). The mean luminescence values determined from the infections in the wounds of all mice in the 4 groups was calculated by ARGUS software. The resulting curves are plotted in figure 3. The PDT-treated group showed a semilogarithmic relationship between bacterial luminescence and delivered fluence, until 99% of the luminescence had disappeared after 240 J/cm2. There was a significant difference between the luminescence found in the group that, in the dark, received conjugate, compared with the luminescence found in the light-alone and untreated control groups. This difference is due to 2 factors: (1) a degree of dark toxicity of the conjugate toward P. aeruginosa and (2) the ability of the bacteria in the untreated and light-alone control wounds to continue to grow.
Figure 2.
Successive overlaid luminescence false-color images and monochrome light-emitting diode images of mice bearing excisional wounds infected with 5 × 106 luminescent Pseudomonas aeruginosa, treated with poly-L-lysine–ce6 conjugate and increasing doses of light (A–H), or kept in the dark (I–M).
Figure 3.
Mean pixel values of luminescence signals from defined areas (1200 pixels) covering infected wounds, determined by image analysis. Data points are means of values from wounds, on 10 mice/group, and bars indicate SDs.
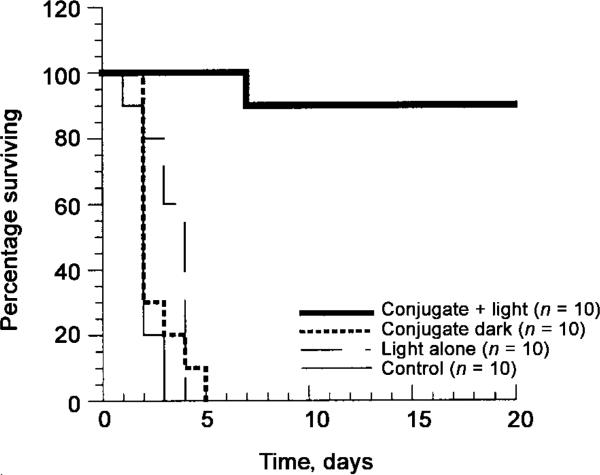
All mice in the 3 control groups (untreated, light alone, and conjugate-treated kept in the dark) died within 5 days of infection. In contrast, 90% of the mice treated with PDT survived, as seen in figure 4. These mice showed some symptoms of bacterial infection (weight loss, ruffled fur, and inactivity) similar to those seen in mice that received a sublethal dose of bacteria (described above). They recovered quickly, however, and, by 5 days after infection, were regaining weight and moving normally.
Figure 4.
Kaplan-Meier survival plot for photodynamic therapy–treated (conjugate + light), conjugate-treated control that were kept in the dark (conjugate dark), light-alone control (light alone), and untreated control (control) mice.
Wound healing
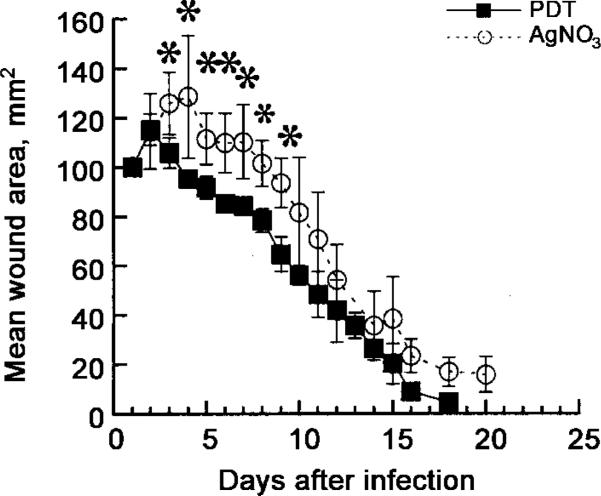
Among P. aeruginosa–infected mice, only those with PDT-treated wounds could be monitored for measurement of wound healing, because mice in the other groups did not survive. To compare the PDT treatment with an alternative topical antimicrobial treatment that would eradicate the bacteria from the wound and prevent the mice from dying from sepsis, we studied the application of AgNO3 solution. When 50 μL of a 0.5% AgNO3 solution was added to an infected wound, the bacterial luminescence started to decline after 5 min and was almost completely gone after 30 min (data not shown). Mice treated with AgNO3 survived as well as did those treated with PDT; that is, they recovered from some systemic illness. However, when, after wound healing, the wound area was measured daily, the wounds of those mice cured of their P. aeruginosa infections by PDT healed significantly faster than did those cured of their infections by AgNO3 solution (figure 5). This difference in healing rates was most marked between days 3 and 9 after infection (P < .05 to P < .001; 2-tailed un-paired Student's t test). AgNO3-treated mice had a greater amount of granulation tissue visible in the healing wounds than did PDT-treated mice.
Figure 5.
Mean areas of wounds of mice (n = 10/group) treated either with either photodynamic therapy (PDT) (50 μL of 200 μM ce6 equivalent poly-L-lysine–ce6 solution followed, after 30 min, by 240 J/cm2 red light) or topical AgNO3 (50 μL of 0.5% solution). Wounds were measured daily in 2 dimensions, and areas were calculated. Bars indicate SDs; *2-tailed P < .05 (unpaired Student's t test).
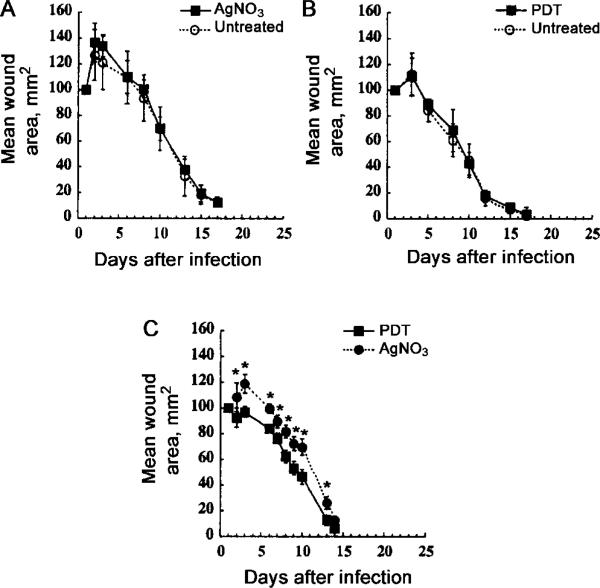
In an attempt to more closely define the mechanism responsible for this observed difference in wound healing, we asked (1) whether PDT can accelerate wound healing in uninfected wounds, (2) whether AgNO3 can slow wound healing in uninfected wounds, and (3) whether PDT accelerates wound healing, compared with the effect of AgNO3, in infected wounds. We therefore carried out 3 series of experiments in mice that had been given 2 wounds each, on the right and left sides of the lower back. In the first series, neither wound was infected, and the rate of healing after topical PDT was compared with that of untreated controls. In the second series, neither wound was infected, and the rate of healing after topical AgNO3 application was compared with that of untreated controls. In the third series, both wounds were infected with half the bacterial inoculum used in the single-wound model, and, on each mouse, 1 wound was treated with PDT and the other was treated with AgNO3. In the latter series, the bacterial luminescence decreased in both wounds by approximately the same extent, and all 6 mice survived (data not shown).
In uninfected wounds, AgNO3 did not significantly slow down wound healing, compared with that in untreated wounds, although there was a small increase in wound size at days 2–4 after infection (figure 6A) (P > .05). Topical PDT did not accelerate wound healing in uninfected wounds, compared with that in untreated wounds (figure 6B) (no significant differences at any time). A confirmation of our earlier findings, in infected wounds, PDT significantly accelerated wound healing, compared with that in wounds that received the alternative antimicrobial AgNO3 treatment (figure 6C). That this experiment was performed by use of 2 wounds/mouse eliminates any possible error caused by differences between mice in age, body weight, or other noncontrolled factors. The differences in the wound-healing curves of both PDT and AgNO3 that were observed in comparison of figures 5 and 6C are presumably due to the fact that, in the experiment represented by figure 6C, fewer bacteria were used in each wound. The uninfected wounds (whether PDT treated, AgNO3 treated, or untreated) and the infected PDT-treated wounds all had similar amounts of granulation tissue, and they healed at similar rates. The AgNO3-treated infected wounds had greater amounts of granulation tissue and showed delayed wound healing.
Figure 6.
Mean areas of wounds from mice (n = 6/group) bearing 2 wounds/mouse. Wounds were measured daily, in 2 dimensions, and areas were calculated. Bars indicate SD; *2-tailed P < .05 (unpaired Student's t test). A, Neither wound infected. In each mouse, 1 wound (3 left and 3 right) was treated with photodynamic therapy (PDT) (50 μL of 200 μM ce6 equivalent poly-L-lysine–ce6 solution followed after 30 min by 240 J/cm2 red light). B, Neither wound infected. In each mouse, 1 wound (3 left and 3 right) was treated with 50 μL of 0.5% solution of AgNO3. C, Both wounds infected. In each mouse, 1 wound was treated with AgNO3 and the other with PDT, as described above (3 left and 3 right).
DISCUSSION
This report has demonstrated the power of optical techniques to both monitor and treat potentially fatal wound infections. The method of optically monitoring bacterial numbers and viability, in real time, in living animals, by use of genetically engineered bacteria that emit luminescence, together with ultrasensitive photon-counting cameras, has been demonstrated in several models [5–8, 19]. Because the entire lux gene operon encoding both the bacterial luciferase and the biosynthetic enzymes for substrate synthesis is transfected, the resulting bacteria are bioluminescent without the need for exogenous administration of luciferin to animals in vivo [7]. Rocchetta et al. [7] studied the growth of bioluminescent E. coli in the neutropenic mouse–thigh abscess model of infection. They found that, at several times after inoculation and during the period of action of antibiotics, the number of colony-forming units extracted from the thighs of killed animals paralleled the luminescence signal. Although it was necessary to use antibiotic selection to maintain expression of the pCGLS1 plasmid when growing the bacteria in liquid culture, it was found unnecessary to give the mice antibiotics to preserve bacterial luminescence in vivo. This was due to the relative lack of selection pressure among the bacteria in a localized infection, because of their much slower growth rate.
To use luminescence imaging to monitor PDT of infections in vivo, it is necessary to show that the reduction in luminescence observed correlates with loss of viable bacteria in vitro. We previously showed that, for E. coli DH5 α, the loss of luminescence after photoinactivation in vitro actually underestimates the loss of colony-forming units determined by serial dilution [5]. Similar results were obtained in the present study by use of P. aeruginosa. The present study has shown that P. aeruginosa is significantly harder to kill in vitro than is E. coli (as described elsewhere), for which 12 mM conjugate and 30 J/cm2 gave 6 logs of bacterial killing [5]. This difference is probably due to the increased difficulty that polycationic molecules have in penetrating the gram-negative cell wall in Pseudomonas species [20].
As found elsewhere for E. coli, in the present study, it was necessary to use significantly higher amounts of both conjugate and light than was needed in vitro, to obtain an equivalent loss of luminescence from the bacteria in the mouse wounds. Preliminary experiments with lower concentrations of conjugate (10–100 μM) showed increasing effectiveness with increasing concentrations added to the wound (data not shown). Likewise, increased light doses produced a dose-dependent loss of luminescence. The uptake plot of ce6 bound to bacteria in vitro (figure 1A) demonstrated that, at high conjugate concentrations (100–200 μM), there was a tendency toward saturation. Therefore, we hypothesized that the need for much higher concentrations in vivo is due to large amounts of conjugate binding to the tissue in the wound, and, thus, large amounts of conjugate being unavailable for binding to bacteria. This would have the effect of dramatically reducing the effective conjugate concentration in the wound. Although the bacterial luminescence depends on the oxygen concentration being sufficiently high [6], and although PDT is known to consume oxygen [21], we believe that the relative superficiality of the infection in the wound allows free diffusion of atmospheric oxygen during treatment, ensuring that the observed reduction in luminescence was due to loss of bacterial viability.
In the literature of PDT, there are surprisingly few reports of its use to treat localized bacterial infections. Berthiaume et al. [22] used a monoclonal antibody that recognizes P. aeruginosa covalently conjugated to a ce6 derivative. To form a bubble into which the conjugate was subsequently injected, mice were injected subcutaneously with the bacteria; illumination was then performed. Bacteria extracted from skin removed from killed animals showed a 75% loss of colony-forming units, a loss not seen in conjugates formed from a nonspecific antibody. There is 1 report of topical PDT being used, with some success, to clinically treat brain abscesses, in 5 patients after surgery [23]. In recent studies, PDT has been used to treat oral Candida infections in mice [24], and a phase I clinical trial of aminolevulanic acid–mediated PDT has been used for eradication of Helicobacter pylori infection in the stomach [25].
Our other report [5] on the inactivation of E. coli in the infection of mouse wounds served to establish the principle that bacteria-targeted PDT could be used as a treatment for localized infections, without unacceptable host-tissue toxicity. That infection model used a nonpathogenic bacterial strain that was eliminated in untreated control wounds over a period of 1–2 days. The present study, however, has shown that PDT is equally effective in treating infections caused by an invasive pathogen that, if left untreated, will inevitably cause death. Further work should include sampling of blood from mice with untreated, light alone–treated, or conjugate alone–treated infected wounds, to check for transient or persistent bacteremia. Our approach of covalently conjugating a tetrapyrrole photosensitizer, such as ce6, with an overall anionic charge to a cationic poly-L-lysine chain produces a targeting vehicle that efficiently binds to gram-negative bacteria and allows the photosensitizer to penetrate the outer membrane in a short period of time [4]. The lack of host tissue phototoxicity we observed elsewhere and in the present study has been proposed to be due to the necessity for a macromolecular species, such as poly-L-lysine–ce6 (molecular weight, ~18,500), to be taken up into mammalian cells by the time-dependent process of endocytosis, thus allowing temporal selectivity for bacteria over host tissue after 30 min of incubation. That, until now, PDT of wound infections has not been widely explored may be due to inefficient photosensitizer, lack of selectivity for prokaryotic cells, compared with eukaryotic cells, or difficulties inherent in monitoring the response of localized infections in small rodents.
Because it expresses a large range of virulence factors, P. aeruginosa is known to be a highly invasive bacterium [9, 26]. It has been widely used to study determinants of pathogenicity and virulence in models involving rodents with skin burns [27, 28]. This research into mechanisms of pathogenicity is motivated by the widespread clinical problem of P. aeruginosa infections in patients with skin burns [29]. Because of the ubiquitous nature of the organism and its intrinsic antibiotic resistance, these infections still present a formidable therapeutic challenge. Our experiments with the series of mice with 2 wounds each were designed to explore the mechanism underlying the observation of faster wound healing in PDT-cured P. aeruginosa–infected wounds, compared with that in those cured with AgNO3. In uninfected wounds, AgNO3 was found to not slow wound healing and PDT was found to not accelerate wound healing; how can we explain that observation? We believe that the explanation lies in the ability of topical PDT to inactivate extracellular virulence factors (proteases, lipases, toxins, and siderophores), which are abundantly expressed by P. aeruginosa and have been proposed to aid in bacterial invasion and tissue damage [30]. We hypothesize that, at a relative early time after they have been applied to the wound, bacteria use their virulence factors to invade the tissue in the wound. This tissue invasion has 2 consequences: first, the bacteria can spread in the tissue and/or bloodstream to produce a fatal systemic infection, and, second, the proteases and other tissue-destructive virulence factors damage the tissue at an early time after infection, and this damage is responsible for delayed wound healing at days 3–9 after wounding. It is hypothesized that PDT-mediated destruction of these virulence factors abrogates both the invasion of the bacteria into the tissue, at the time of PDT, and also the tissue destruction leading to delayed wound healing. Komerik et al. [31] reported on the use of PDT mediated by toluidine blue O to inactivate the enzymatic activity of proteases in culture supernatants of P. aeruginosa in vitro. If confirmed, the hypothesis that PDT exerts an anti–virulence factor effect constitutes another reason for the preference of PDT over silver preparations as treatment for localized infections. An alternative explanation might be that AgNO3 somehow encourages release, from dead bacteria, of virulence factors that delay wound healing. Although AgNO3 and silver sulfadiazine are clinically effective in destruction of bacteria in wounds and burns, they do present several limitations: (1) they have limited penetration into tissue, (2) bacteria can develop resistance, (3) there have been reports of local toxicity manifested by delayed wound healing, and (4) silver may undergo systemic absorption, leading to argyria [32]. It should be noted that silver preparations are not the standard of care for surgical wound infections; rather, wound care and debridement and systemic antibiotics are used.
Further studies concerning our approach to use PDT for infections in vivo are underway. These studies will include the use of more realistic animal infection models, including full-thickness burns, acute soft-tissue infections, and chronic abscesses in which the bacteria have penetrated tissue and have been allowed to form established infections. In addition, studies will include in vivo photoinactivation of bacterial virulence factors and the correlation with tissue damage and wound healing.
Acknowledgments
We thank David A. O'Donnell for excellent technical assistance.
Financial support: National Institutes of Health (R01AI050879 to M.R.H);Department of Defense Medical Free Electron Laser Program (contract N00014-94-1-0927 toM.R.H. and T.H.; F49620-00-1-0349 to C.H.C.).
Footnotes
Presented in part: 13th International Congress on Photobiology, San Francisco, California, 3 July 2000 (abstract 393); 8th International Photodynamic Association Congress, Vancouver, Canada, 8 June 2001 (abstract 48-2); BioS 2002 Biomedical Optics, Photonics West, Optical Techniques for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XI, San Jose, California, 19 January 2002 (abstract 4612-07).
The animal experiments were approved by, and followed the guidelines of, the Massachusetts General Hospital Subcommittee on Research Animal Care.
M.R.H. and T.H. are inventors on US patent 6,462,070: photosensitizer conjugates for pathogen targeting.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniell MD, Hill JS. A history of photodynamic therapy. Aust N Z J Surg. 1991;61:340–8. doi: 10.1111/j.1445-2197.1991.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 3.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 4.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Che-mother. 1998;42:2595–601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. Photodynamic treatment of wound infections in vivo monitored by luminescence imaging. Photochem Photobiol. 2002;75:51–7. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA. Photonic detection of bacterial pathogens in living hosts. Mol Microbiol. 1995;18:593–603. doi: 10.1111/j.1365-2958.1995.mmi_18040593.x. [DOI] [PubMed] [Google Scholar]
- 7.Rocchetta HL, Boylan CJ, Foley JW, et al. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45:129–37. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–60. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon SM, Serkey JM, Keys TF, et al. Secular trends in nosocomial bloodstream infections in a 55-bed cardiothoracic intensive care unit. Ann Thorac Surg. 1998;65:95–100. doi: 10.1016/s0003-4975(97)01039-4. [DOI] [PubMed] [Google Scholar]
- 11.Bergen GA, Shelhamer JH. Pulmonary infiltrates in the cancer patient. New approaches to an old problem. Infect Dis Clin North Am. 1996;10:297–325. doi: 10.1016/s0891-5520(05)70300-7. [DOI] [PubMed] [Google Scholar]
- 12.Fergie JE, Shema SJ, Lott L, Crawford R, Patrick CC. Pseudomonas aeruginosa bacteremia in immunocompromised children: analysis of factors associated with a poor outcome. Clin Infect Dis. 1994;18:390–4. doi: 10.1093/clinids/18.3.390. [DOI] [PubMed] [Google Scholar]
- 13.Richard P, Le Floch R, Chamoux C, Pannier M, Espaze E, Richet H. Pseudomonas aeruginosa outbreak in a burn unit: role of antimicrobials in the emergence of multiply resistant strains. J Infect Dis. 1994;170:377–83. doi: 10.1093/infdis/170.2.377. [DOI] [PubMed] [Google Scholar]
- 14.Hirosue M, Maeda H, Miyamoto M, Takashiba S, Murayama Y, Chart H. An investigation into the pathogenic properties of Escherichia coli strains BLR, BL21, DH5alpha, and EQ1. Res Microbiol. 2000;151:721–5. doi: 10.1046/j.1365-2672.2000.01211.x. [DOI] [PubMed] [Google Scholar]
- 15.Milligan RC, Rust J, Rosenthal SM. Gamma globulin factors protective against infections from Pseudomonas and other organisms. Science. 1957;126:509–11. doi: 10.1126/science.126.3272.509-a. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal SM. Local and systemic therapy of pseudomonas septicemia in burned mice. Ann Surg. 1967;165:97–103. doi: 10.1097/00000658-196701000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–10. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 18.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–50. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 19.Siragusa GR, Nawotka K, Spilman SD, Contag PR, Contag CH. Real-time monitoring of Escherichia coli O157:H7 adherence to beef carcass surface tissues with a bioluminescent reporter. Appl Environ Microbiol. 1999;65:1738–45. doi: 10.1128/aem.65.4.1738-1745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock RE. The Pseudomonas aeruginosa outer membrane permeability barrier and how to overcome it. Antibiot Chemother. 1985;36:95–102. doi: 10.1159/000410475. [DOI] [PubMed] [Google Scholar]
- 21.Ochsner M. Photophysical and photobiological processes in the photo-dynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 22.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology. 1994;12:703–6. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 23.Lombard GF, Tealdi S, Lanotte MM. The treatment of neurosurgical infections by lasers and porphyrins. In: Jori G, Perria CA, editors. Photodynamic therapy of tumors and other diseases. Edizione Libreria Progetto; Padova, Italy: 1985. pp. 363–6. [Google Scholar]
- 24.Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue–mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:155–60. doi: 10.1067/moe.2002.120051. [DOI] [PubMed] [Google Scholar]
- 25.Wilder-Smith CH, Wilder-Smith P, Grosjean P, et al. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: preliminary human studies. Lasers Surg Med. 2002;31:18–22. doi: 10.1002/lsm.10066. [DOI] [PubMed] [Google Scholar]
- 26.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–60. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg JB, Coyne MJ, Jr, Neely AN, Holder IA. Avirulence of a Pseudomonas aeruginosa algC mutant in a burned-mouse model of infection. Infect Immun. 1995;63:4166–9. doi: 10.1128/iai.63.10.4166-4169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holder IA, Neely AN, Frank DW. PcrV immunization enhances survival of burned Pseudomonas aeruginosa–infected mice. Infect Immun. 2001;69:5908–10. doi: 10.1128/IAI.69.9.5908-5910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruitt BA, Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–45. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 30.Wretlind B, Pavlovskis OR. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev Infect Dis. 1983;5(suppl 5):S998–1004. doi: 10.1093/clinids/5.supplement_5.s998. [DOI] [PubMed] [Google Scholar]
- 31.Komerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol. 2000;72:676–80. doi: 10.1562/0031-8655(2000)072<0676:teopao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Monafo WW, West MA. Current treatment recommendations for topical burn therapy. Drugs. 1990;40:364–73. doi: 10.2165/00003495-199040030-00004. [DOI] [PubMed] [Google Scholar]