Abstract
In mice, it has been demonstrated that at 7 days after burn injury, injection of LPS is more lethal than the same dose at one day after injury. In the present study, we examined the effect of LPS injection to mice burned seven days previously on glucose metabolism (18FDG uptake) in vivo. CD-1 male mice (25-28 grams, Charles River breeding laboratories) were anesthetized, backs shaven, and subjected to dorsal full thickness burn on 25% total body surface area. Sham treated animals were used as controls. Six days after burn injury all mice were fasted overnight. One half of the burned and sham controls were subsequently injected i.p. with LPS (10 mg/kg, E. Coli). The remaining animals were injected with saline i.p. Two hours later all mice were injected i.v. with 50 μCi of 18F FDG. One hour later the animals were euthanized and biodistribution was measured. Tissues were weighed and radioactivity was measured with a well-type gamma counter. Results were expressed as %dose/gram tissue, mean ± SEM. The combination of burn 7 days previously and LPS significantly increased mortality compared animals with burn alone, LPS alone or sham controls. Burn injury seven days previously caused a significant reduction in 18FDG uptake by the brain compared to sham controls. The combination of LPS and burn injury seven days previously produced a significant increase in 18FDG uptake by brown adipose tissue (BAT) and heart compared with either treatment separately. LPS produced a significant increase in 18FDG uptake by lung, spleen and GI tract of the sham animals, changes that were different in mice burned 7 days previously and injected with LPS. The present results suggest that burn injury seven days previously predisposes mice to alterations in 18FDG uptake produced by LPS. These changes may relate in part, to the increased lethality of LPS injection in previously burned mice.
INTRODUCTION
Sepsis and septic shock are the tenth most common causes of death in the United States (1,2). Infection is the most common and most serious complication of a major burn injury related to burn size (3). Despite improvements in antimicrobial therapies, sepsis still accounts for 50-60% of deaths in burn patients. Sepsis in burn patients is commonly due to bronchopneumonia, pyelonephritis, thrombophlebitis, or invasive wound infection. The burn wound is an ideal media for bacterial growth and provides a wide portal for invasion. Microbial colonization of open burn wounds, primarily from endogenous sources, is usually established by the end of the first week after injury. Infection is further promoted by loss of the epithelial barrier, malnutrition induced by the hypermetabolic response to burn injury, and by a generalized post-burn immunosuppression.
The major cause of morbidity and mortality in burned patients, is the development of sepsis and the multiple organ system failure (MOSF), which occur at some point after the initial injury (4). In these patients, the acute inflammatory insult of thermal injury is followed by infection, which represents a “second hit” to the patient (5). Under these circumstances, a normally well-tolerated insult, such as pneumonia, may lead to an exaggerated inflammatory response and progression to multiple organ failure with associated high morbidity and mortality. Burn patients appear to be especially susceptible to a “second hit.” The development of pneumonia is associated with increased mortality, and sepsis is associated with at least 50% mortality in this patient population (6,7). Despite intensive study, the etiology of susceptibility to the “second hit” remains incompletely understood.
One of the major pathophysiological disturbances after thermal injury and sepsis is insulin resistance, with the manifestation of hyperglycemia as the consequence of altered glucose homeostasis, which contributes to the mortality and morbidity of burn victims (8). Previous studies have reported that persistent hyperglycemia may occur in burn patient with sepsis from pan-drug-resistant bacterial strains (9) and there were differences between septic and post burn insulin resistance (10). Furthermore, clinical studies have demonstrated that hyperglycemia control in burn patient reduced infectious complications (11). All these findings suggested that there is a difference in the extent of altered glucose metabolism in burn patients before and after the development of the complications of sepsis. Understanding this difference would help the management of burn patients before and after the development of sepsis and possibly reduce the frequency of sepsis. In vivo glucose metabolism in multiple organs can be evaluated using the positron emitting tracer, 2-fluoro-2-deoxy-D-Glucose (18FDG) (12). Burn injury has been shown to alter 18FDG accumulation in mice (13), rats (14), and rabbits (15). The purpose of the present study was to examine the effects of burn injury seven days previously followed by LPS injection on survival and 18FDG utilization in a variety of organs.
METHODS
Mice
Male CD-1 mice (25-28 grams, Charles River Laboratories) were housed with food and water available ad libitum in a barrier facility kept under specific pathogen-free conditions. Temperature (70–72°F), humidity (45–55%), and a 12/12-hour light/ dark cycle were controlled. Animals were allowed to acclimatize for one week before experimentation. All animal studies described here were approved and performed in strict accordance to the guidelines established by the Massachusetts General Hospital Animal Care and Use Committee.
Burn Injury
CD-1 male mice (25-28 grams, Charles River breeding laboratories) were anesthetized, backs shaven, and then subjected to dorsal full thickness burn on 25% total body surface area as described previously (10). Sham treated animals were used as controls.
LPS Treatment
Six days after burn injury all mice were fasted overnight. One half of the sham and burn injured mice were subsequently injected i.p. with LPS (10 mg/kg, E.Coli). Sham animals were injected with saline i.p. One group of mice burned seven days previously and injected with LPS was evaluated for survival over 7 days. The results obtained with these animals were compared to the survival of sham treated mice injected with saline, sham treated mice injected with LPS and mice burned seven days previously and injected with saline. There were 12 mice in each group for these studies. The Log-Rank (Mantel-Cox) method was used for statistical analysis. P values of < 0.05 were considered to be statistically significant. For the survival studies there were 12 animals in each group.
Additional groups of 6 animals were studied with 18FDG: (a) burned seven days previously and injected with LPS, (b) sham controls injected with saline, (c) sham controls injected with LPS and (d) mice burned seven days previously and injected with saline.
18FDG Biodistribution
Two hours after i.p. injection of LPS or saline (see above) each mouse received 50 μCi of 18F FDG injected via tail vein without anesthesia. One hour later the animals were euthanized and biodistribution was measured. Tissues were weighed and radioactivity was measured with a Wizard gamma counter. Results were expressed as % injected dose/gram tissue, mean ± SEM.
Statistical Analysis
The survival data was analyzed by the Log-Rank (Mantel-Cox) method. Statistical analysis of the biodistribution data was performed by two-way analysis of variance (ANOVA) with the linear model: 18FDG uptake = State + Treatment + State × Treatment, where state (burn vs. sham) and treatment (LPS vs. saline) were the classification variables and State × Treatment was the state by treatment interaction. Individual means were compared by Duncan’s multiple range test. Differences with a p-value of less than 0.05 were considered to be statistically significant.
RESULTS
Survival after burn, LPS, or burn + LPS
There were no fatalities in the sham mice or mice burned seven days previously treated with saline injection (Figure 1). Similarly, injection of LPS (10 mg/kg) to sham mice produced no fatalities over the study period (Figure 1). In contrast, the combination of LPS (10 mg/kg) and burn injury seven days previously increased mortality to 30% on day 3 and 70% on day 4 (Figure 1). Statistical analysis by the Log-Rank (Mantel-Cox) method demonstrated significantly greater survival in the sham group injected with saline, or mice burned seven days previously and injected with saline, or sham group injected with LPS than in the mice burned seven days previously and injected with LPS, (p < 0.01).
Figure 1. Effect of LPS Injection on Survival of Mice Burned Seven Days Previously.
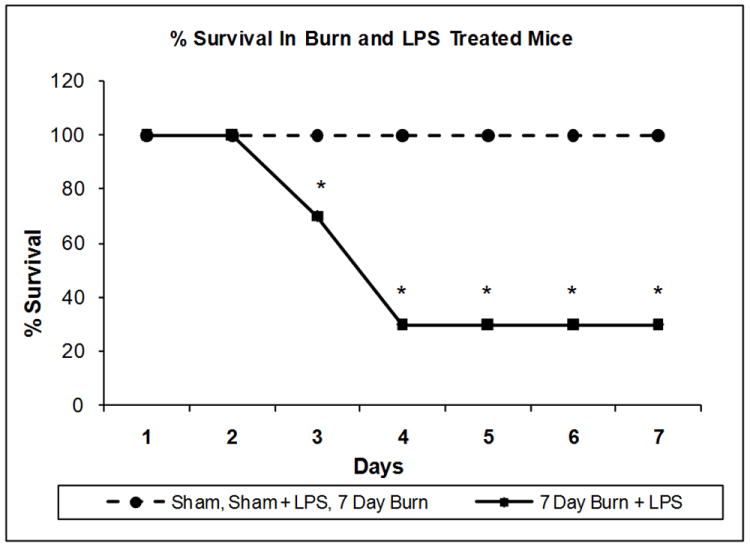
Groups of 12 mice were subjected to sham or burn injury or sham treatment as described in the methods section. Seven days after burn injury, the mice were injected with LPS as described in the methods. The survival was recorded for each group. *P < 0.01 compared to shams + saline, burns + saline, and shams + LPS.
Lower doses of LPS (1 or 5 mg/kg) did not result in increased morality in the mice burned 7 days previously (data not shown).
18FDG Accumulation by various tissues after burn, LPS or burn + LPS
Heart
The accumulation of 18FDG in the heart was examined 7 days after burn injury, 3 hours after LPS injection or after the combination of both treatments. Two-way ANOVA demonstrated highly significant main effects of state (burn vs. sham), treatment (LPS vs. saline) and state by treatment interaction on 18FDG accumulation; F1,20 = 34.32, P < 0.001), F1,20 = 34.07, P < 0.001) and F1,23 = 29.79, P < 0.001 respectively. In the heart, burn injury 7 days previously produced a small increase in 18FDG accumulation compared to the shams (36%, P < 0.01) (Figure 2) whereas LPS treatment alone did not have a significant effect on 18FDG uptake. In contrast, injection of LPS to mice burned 7 days previously produced a much greater increase (1,103%) in 18FDG accumulation than either treatment separately (p<0.0001) (Figure 2).
Figure 2. Effect of LPS Injection on 18FDG Accumulation in the heart of Mice burned Seven Days Previously.
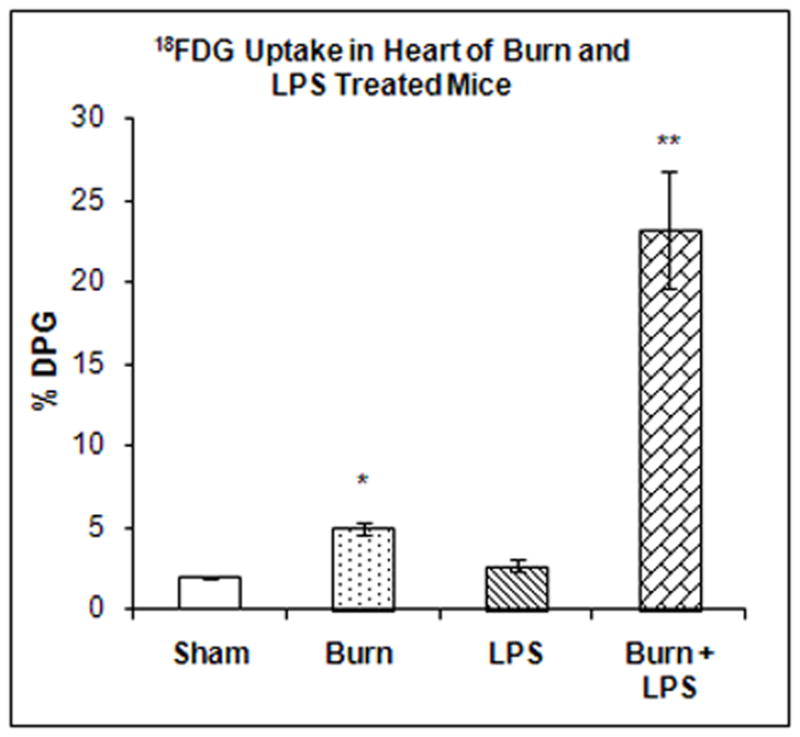
The mice were treated as described in the methods section. There were six mice in each group. Results are expressed as % Injected Dose per gram tissue, mean ± SEM. *P< 0.01 compared to shams.**P< 0.001 compared to shams
Brain
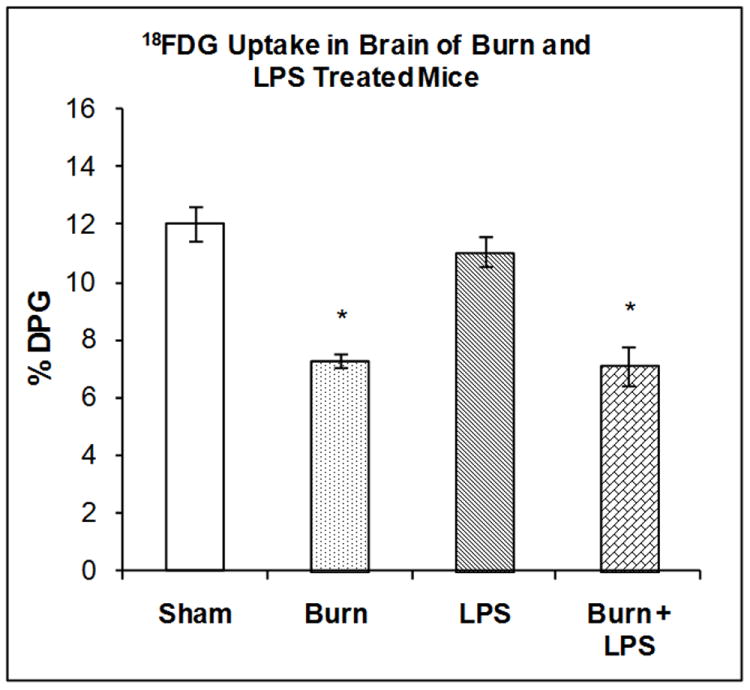
The accumulation of 18FDG in the brain was examined 7 days after burn injury, 3 hours after LPS injection or after the combination of both treatments. Two-way ANOVA demonstrated a highly significant main effect of state (burn vs. sham), on 18FDG accumulation (F1,20) = 64.46, P < 0.001). However the effects of treatment and state by treatment interaction were not statistically significant. 18FDG accumulation in the brain was reduced by burn injury (39%, P < 0.01) but not by LPS injection to sham animals (Figure 3). The combination of burn 7 days previously and LPS produced a reduction in brain 18FDG accumulation to the same degree as burn injury alone (Figure 3).
Figure 3. Effect of LPS Injection on 18FDG Accumulation in BAT of Mice burned Seven Days Previously.
The mice were treated as described in the methods section. There were six mice in each group. Results are expressed as % Injected Dose per gram tissue, mean ± SEM. *P< 0.01 compared to shams.
Brown Adipose Tissue (BAT)
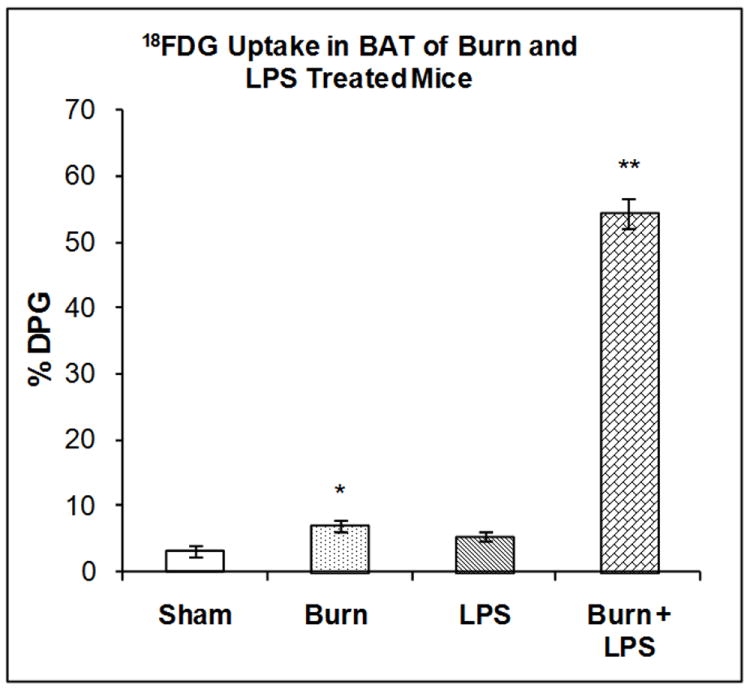
The accumulation of 18FDG in BAT was examined 7 days after burn injury, 3 hours after LPS injection or after the combination of both treatments. Two-way ANOVA demonstrated highly significant main effects of state (burn vs. sham), treatment (LPS vs. saline) and state by treatment interaction on 18FDG accumulation; F1,20 = 182.62, P < 0.001), F1,20 = 221.98, P<0.001 and F1,23 = 256.01, P<0.001 respectively. Burn injury 7 days previously increased 18FDG accumulation in BAT compared with sham treated animals (120%, P < 0.01) but LPS did not (Figure 4). The combination of burn 7 days previously and LPS produced a highly significant increase in 18FDG accumulation by BAT (1,591%, P < 0.0001); greater than either treatment separately (Figure 4).
Figure 4. Effect of LPS Injection on 18FDG Accumulation in the Brain of Mice burned Seven Days Previously.
The mice were treated as described in the methods section. There were six mice in each group. Results are expressed as % Injected Dose per gram tissue, mean ± SEM. *P< 0.01 compared to shams
Spleen
The accumulation of 18FDG in the spleen was examined 7 days after burn injury, 3 hours after LPS injection or after the combination of both treatments. Two-way ANOVA demonstrated highly significant main effects of state (burn vs. sham), treatment (LPS vs. saline) and state by treatment interaction on 18FDG accumulation; F1,20 = 193.04, P<0.001, F1,20 = 29.57, P < 0.001 and F1,23 =33.37 respectively. Burn 7 days previously did not produce significant changes in 18FDG accumulation (Figure 5). LPS injection in sham mice produced a 297% increase in 18FDG accumulation in the spleen (P < 0.001). LPS injection in the mice burned 7 days previously did stimulate 18FDG accumulation in the spleen (127.7%), but not to the same level as LPS injection in sham mice (296.7%); a difference that was statistically significant (P < 0.01).
Figure 5. Effect of LPS Injection on 18FDG Accumulation in the Spleen of Mice burned Seven Days Previously.
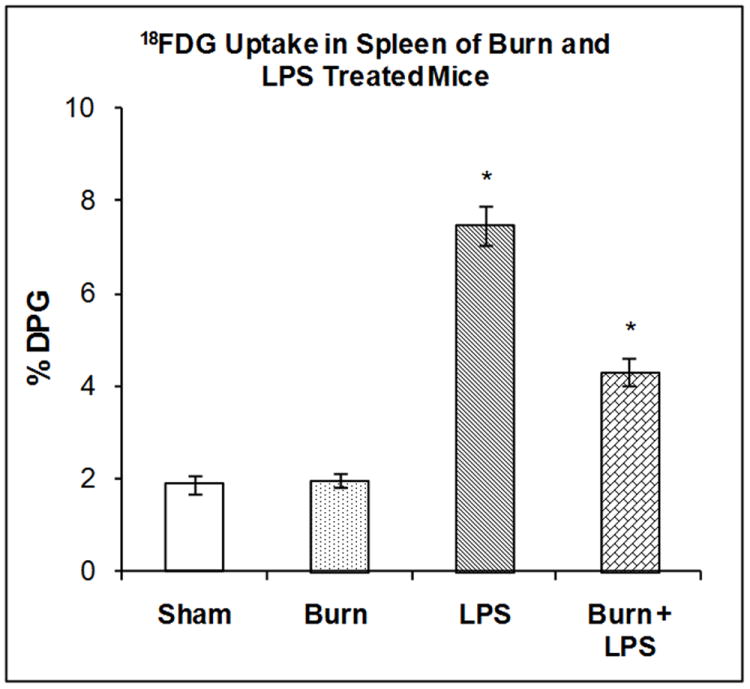
The mice were treated as described in the methods section. There were six mice in each group. Results are expressed as % Injected Dose per gram tissue, mean ± SEM. *P< 0.01 compared to shams
Lung
The accumulation of 18FDG in the lung was examined 7 days after burn injury, 3 hours after LPS injection or after the combination of both treatments. Two-way ANOVA demonstrated significant main effects of state and treatment on 18FDG accumulation; F1,20 = 5.45, P < 0.03) and F1,20= 78.9, P<0.001 respectively. In the lung burn 7 days previously produced no significant changes in 18FDG accumulation (Figure 6). LPS injection in sham mice produced a 67.56% increase in 18FDG accumulation in the lung compared to shams (P < 0.001). LPS injection to the mice burned 7 days previously stimulated 18FDG accumulation in the lung (104.6%) to a level, higher than LPS injection in the sham mice (67.56%); a difference that was statistically significant (P < 0.001).
Figure 6. Effect of LPS Injection on 18FDG Accumulation in the Lung of Mice burned Seven Days Previously.
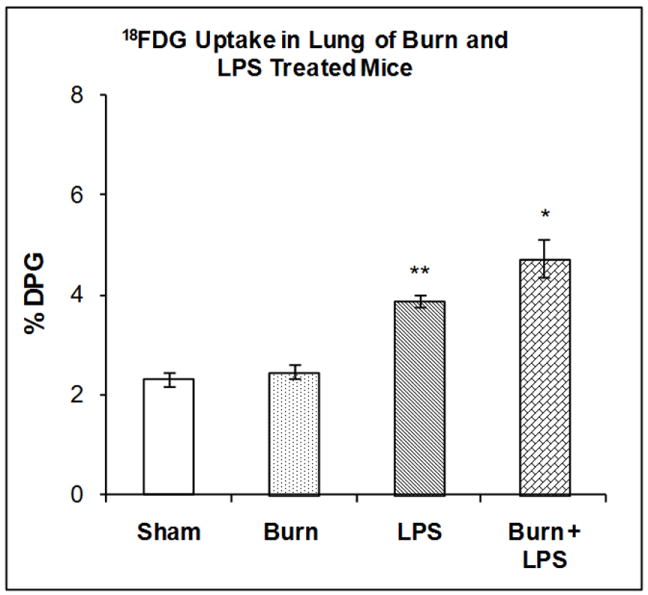
The mice were treated as described in the methods section. There were six mice in each group. Results are expressed as % Injected Dose per gram tissue, mean ± SEM. *P< 0.01 compared to shams, **P< 0.001 compared to shams
GI Tract
The accumulation of 18FDG in the GI tract was examined 7 days after burn injury, 3 hours after LPS injection or after the combination of both treatments. Two-way ANOVA demonstrated a highly significant main effect of state on 18FDG accumulation, F1,20 = 529.84, P<0.001 on 18FDG accumulation. However the effects of treatment and state by treatment interaction were not significant. In the GI tract, burn 7 days previously produced no significant changes in 18FDG accumulation (Figure 7)). LPS injection in sham mice produced a 155.52% increase in 18FDG accumulation in the G.I tract compared to shams (P < 0.001). LPS injection in mice burned 7 days previously stimulated 18FDG accumulation in the GI tract (88.13 %%) but to a level lower than LPS injection in sham mice (155.52%); a difference that was statistically significant (P <0.01).
Figure 7. Effect of LPS Injection on 18FDG Accumulation in the GI Tract of Mice burned Seven Days Previously.
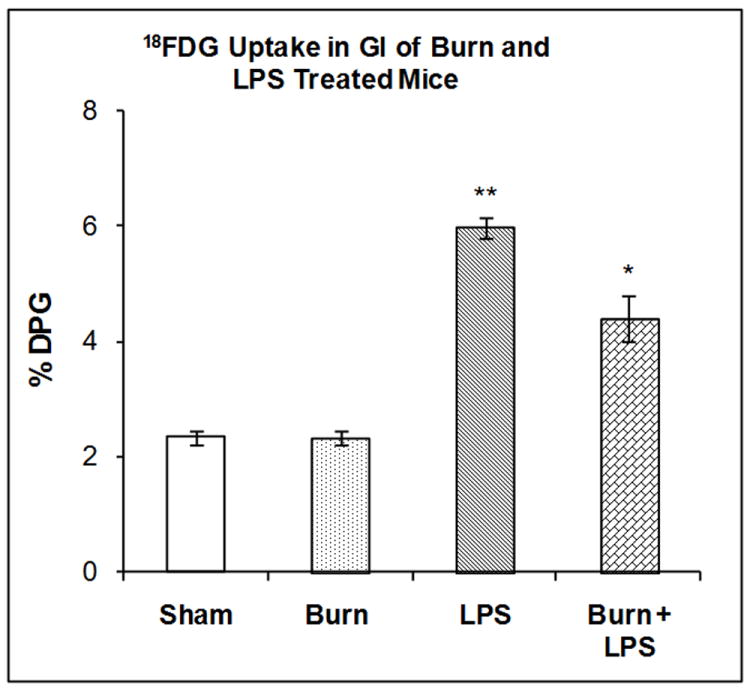
The mice were treated as described in the methods section. There were six mice in each group. Results are expressed as % Injected Dose per gram tissue, mean ± SEM. *P< 0.01 compared to shams, **P< 0.001 compared to shams
DISCUSSION
The data presented here represents the third study in which the effect of LPS injection (10 mg/kg) to mice burned 7 days previously was studied. All three studies used similar approaches to examine the effect of the LPS “second hit” at the same time points 2 hours after the injection, namely, thermal injury to the mice followed by either in vivo injection of LPS or exposure of cultured cells from the mice to LPS. It should be pointed out that the LPS injection model of burn induced sepsis is not the same as the burn plus cecal ligation and puncture model. With the bolus injection of LPS, the effects of bacterial overgrowth, namely increased bacterial coat, are produced immediately. Hence, the effects are produced as soon as the LPS binds to its receptors and initiates the associated cascades. With the cecal ligation and puncture model, the bacteria have to proliferate in the peritoneum to a level that is sufficient to initiate the inflammation cascades; presumably after overwhelming the host defense mechanisms. Despite these differences, both models have been shown to result in increased mortality in animals previously subjected to burn injury as demonstrated in the present study and in a previous report from our laboratory (16).
In a study by Murphy et al (17), spleen cells isolated from mice at seven days after burn injury were cultured and subsequently exposed to various concentration of LPS in vitro. The results demonstrated that LPS augmented proinflammatory cytokine responses in vitro. These authors also measured plasma and organ cytokine and chemokine levels in mice burned 7 days earlier followed by LPS injection in vivo. The response during the first 4 hours following LPS injection was much greater in the animals on day 7 with previous burn injury than that in sham controls or in animals that received LPS injection on day 1 post burn.
In another study, Huber et al (18) examined the effect of thermal injury on the inflammatory response of the intestine to a “second hit” of LPS. In this study, mice were exposed to either sham burn or thermal injury, allowed to recover for 7 days, treated with LPS in vivo and sacrificed 1 or 4 hrs after LPS injection. Prior burn injury (7 days) followed by LPS resulted in significantly increased IL-6 production (protein and mRNA levels) in jejunum and colon at 4 hours after LPS injection compared with mice that received sham injury followed by LPS injection; a point when increased mortality was not observed.
In the present investigation we studied the uptake of 18FDG in a variety of tissues in mice burned seven days previously and three hours after LPS injection at a time when there was no increase in mortality following LPS injection between the mice burned seven days previously and shams. We hypothesized that these 18FDG studies might provide an understanding of the changes that may have occurred in target tissues with respect to glucose metabolism before increased mortality occurs in this model system.
When 18FDG is injected, it is transported from plasma into cells according to the rate constant K1, transported back into plasma with the rate constant K2, phosphorylated with a rate constant k3 and dephosphorylated with a rate constant k4. The differential equations described by the model can be solved to yield the following relationship for the rate of glucose metabolism (MRGlc, μmole/min/g):
Where, Cp is the concentration 18FDG in plasma and LC is the “Lumped Constant’; a dimensionless parameter that corrects for differences in the kinetic behavior of 18FDG and endogenous glucose. Hence, the accumulation of 18FDG in tissues can be used as a marker of glucose utilization (9).
We confirmed that at a dose of 10 mg/kg, LPS decreased the survival of the mice burned 7 days previously. We also found that the combination of burn plus LPS produced alterations in 18FDG accumulation in six organs (heart, brain, BAT, spleen, lung, and GI tract). Overall, there was no consistent change (either increased or decreased) in 18FDG accumulation in the organs examined compared to the uptake produced in mice burned 7 days previously or shams injected with LPS, but rather differential responses depending on the tissue examined.
Greater accumulation in 18FDG by BAT was observed in mice burned 7 days previously and injected with LPS compared with either treatment (burn or LPS) separately. The mechanism(s) for activation of BAT thermogenesis are assumed to be through the sympathetic nervous system (19). The lateral hypothalamic area of the brain is involved in several aspects of autonomic regulation, including thermoregulation and energy expenditure. Burn injury has been shown to elevate epinephrine and norepinephrine levels (20), as does sepsis (21). Hence, the effect of LPS on the BAT of the mice burned 7 days previously may be mediated, in part, through catecholamines and other blood borne mediators.
Greater accumulation of 18FDG in the heart was observed in mice that received LPS injection on post burn day 7 compared with either treatment (burn or LPS) separately. This increased 18FDG uptake may reflect the response of the heart to the stress condition and a switch from aerobic to anaerobic glucose metabolism; possibly due to a greater stress response at the cardiac level with the combined injury of burn and LPS. Sepsis is associated with the development of a hyperdynamic cardiac response and a marked decrease in peripheral vascular tone (22). Excessive production of nitric oxide has been implicated in these vascular responses (23) and burn injury has been shown to produce significant increases in nitric oxide (24). Hence, nitric oxide production may play a role in the additive effect seen on 18FDG accumulation by the heart after burn injury and LPS injection.
Burn injury to the mice produced a reduction in 18FDG accumulation in the brain, which is in agreement with our previous findings (25). LPS injection did not change 18FDG accumulation in the brain in the sham animals. This confirms the work of Meszaros et al (26) which demonstrated similar results using 14C-2-deoxyglucose and E. Coli injection in rats. LPS injection to mice burned 7 days previously did not further reduce 18FDG accumulation in the brain to a greater degree than either treatment separately. Clearly, the mechanism(s) for the effect of burn injury on the reduction of 18FDG accumulation by the brain require further investigation.
Meszaros et al (26) observed increased 2-14C-deoxyglucose utilization by the spleen, lung and GI tract in a rat model of E. coli sepsis. Zhou et al (27) observed similar increases in the lung of mice after LPS injection using 18FDG. The mechanism(s) for these effects were thought to be mediated via activation of macrophage rich tissues and toll receptors. It is important to note that our study confirmed these previous results and showed that in the spleen, lung and GI tract, 18FDG accumulation was stimulated by LPS injection in sham mice.
In conclusion, our studies suggest that burn injury 7 days previously predisposes mice to alteration in 18FDG accumulation produced by LPS injection and increases the lethality from LPS injection. The changes in 18FDG accumulation may relate in part, to increased lethality of LPS injection in previously burned mice. The use of 18FDG and other PET tracers combined with PET imaging may allow for the longitudinal monitoring of animals subjected to combined burn and LPS treatment to better understand the changes produced in specific organs of animals that do not survive.
Acknowledgments
Supported in part by grants from the NIH and Shriners Hospitals for Children
References
- 1.Martin GS, Mannino DM, Eaton S, et al. the epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Pruitt BA., Jr Infection and the burn patient. Br J Surg. 1990;77:1081. doi: 10.1002/bjs.1800771002. [DOI] [PubMed] [Google Scholar]
- 4.Saffle JR, Sullivan JJ, Tuohig GM, Larson CM. Multiple organ faiure in patients with thermal Injury. Crit Care Med. 1993;(21):1673–83. doi: 10.1097/00003246-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Moore FA, Moore EE. Evolving concepts in the pathogenesis of multiple organ failure. Surg Clin North Am. 1995;75:257. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 6.Rue LW, III, Cioffi WG, Mason AD, et al. The risk of pneumonia in thermally injured patients requiring ventilatory support. J Burn care Rehabilitation. 1995;16:262. doi: 10.1097/00004630-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Geyik MF, Aldemir M, Hosoblu S, et al. Epidemiology of burn unit infections in children. Am J Infect Control. 2003;31:342. doi: 10.1016/s0196-6553(02)48226-0. [DOI] [PubMed] [Google Scholar]
- 8.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;20:683–94. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao SC, Zhu SH, Xia ZF, Ma B, Cheng DS. Successful treatment of a critical burn patient with obstinate hyperglycemia and septic shock from pan-drug-resistant strains. Med Sci Monit. 2009 Nov;15(11):CS163–5. [PubMed] [Google Scholar]
- 10.Shangraw RE, Jahoor F, Miyoshi H, Neff WA, Stuart CA, Herndon DN, Wolfe RR. Differentiation between septic and postburn insulin resistance. Metabolism. 1989 Oct;38(10):983–9. doi: 10.1016/0026-0495(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 11.Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008 Oct;144(4):629–35. doi: 10.1016/j.surg.2008.07.001. discussion 635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu AS, Lin HD, Huang SC, Phelps ME, Wu HM. Quantitation of cerebral glucose metabolism rate in mice using 18F-FDG and small-animal PET. J Nuc Med. 2009;50(6):966–73. doi: 10.2967/jnumed.108.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter EA, Bonab AA, Hamrahi V, Pitman J, Macintosh LJ, Cyr EM, Paul K, Yerxa J, Jung W, Tompkins RG, Fischman AJ. Effects of burn injury, cold stress and cutaneous wound injury on the morphology and energy metabolism of murine brown adipose tissue (BAT) in vivo. Life Sci. 2011 Jul 18;89(3-4):78–85. doi: 10.1016/j.lfs.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter E, Tompkins RG, Babich J, Correia B, Bailey E, Fischman A. Thermal injury in rats alters glucose utilization by skin, wound, and small intestine, but not by skeletal muscle. Metabolism. 1996;45(9):1161–1167. doi: 10.1016/s0026-0495(96)90017-7. [DOI] [PubMed] [Google Scholar]
- 15.Carter E, Tompkins R, Hsu H, Christian B, Alpert N, Weise S, Fischman A. Metabolic alterations in muscle of thermally injured rabbits, measured by positron emission tomography. Life Sciences. 1997;61(1):39–44. doi: 10.1016/s0024-3205(97)00355-x. [DOI] [PubMed] [Google Scholar]
- 16.Beffa DC, Fischman AJ, Fagan SP, Hamrahi VF, Paul KW, Kaneki M, Yu YM, Tompkins RG, Carter EA. Simvastatin treatment improves survival in a murine model of burn sepsis: Role of interleukin 6. Burns. 2011;37(2):222–6. doi: 10.1016/j.burns.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy TJ, Peterson HM, Krlynovich S, Zang Y, Kurt-Jones EA, Mannick JA, Lederer JA. Linking the “two-hit” responses following injury to enhanced TLR4 reactivity. J Leukocyte Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 18.Huber NL, Bailey SR, Schuster RM, Schuster RM, Ogle CK, Lentsch AB, Pritts TA. Remote thermal injury increases LPS-induced intestinal IL-6 production. J Surg Res. 2010;160:190–195. doi: 10.1016/j.jss.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes. 2010;34(Suppl 1):S36–42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crum R, Borrow B, Schackford S, Hansbrough J, Brown MR. The neurohormonal response to burn injury in patients resuscitated with hypertonic saline. J Trauma. 1988;28(8):1181–7. doi: 10.1097/00005373-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Barrett L, Orie N, Taylor V, Stidwill RP, Clapp L, Singer M. Differential effects of vasopressin and norepinephrine on vascular reactivity in a long-term rodent model of sepsis. Crit Care med. 2007;35(10):2337–43. doi: 10.1097/01.ccm.0000281861.72907.17. [DOI] [PubMed] [Google Scholar]
- 22.Fry DE. Multiple system organ failure. In: Fry DE, editor. Multiple system organ failure. St Louis: Mosby-Year Book; 1992. pp. 3–14. [Google Scholar]
- 23.Wang X, Andersson R. The role of endothelial cells in systemic inflammatory response syndrome and MSOF. Eur J Sur. 1995;(161):703–713. [PubMed] [Google Scholar]
- 24.Carter EA, Derojas-Walker T, Tamir S, Tannenbaum SR, Yu YM, Tompkins RG. Nitric oxide production is intensely and persistently increased in tissue by thermal injury. Biochem J. 1994;304:201–204. doi: 10.1042/bj3040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter EA, Tompkins RG, Babich JW, Correia JA, Fischman AJ. Decreased cerebral glucose utilization in rats during the ebb phase of thermal injury. J Trauma. 1996;40:930–935. doi: 10.1097/00005373-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Meszaros K, Lang CH, Bagby GL, Spitzer JJ. In vivo glucose utilization by individual tissues during nonlethal hypermetabolic sepsis. FASEB. 1988;2:3083–3086. doi: 10.1096/fasebj.2.15.3056766. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Kozlowski J, Goodrich AL, Markman N, Chen DL, Schuster DP. Molecular imaging of lung glucose utake after endotoxin in mice. Am J Physiol Lung Mol Physiol. 2005;289:760–768. doi: 10.1152/ajplung.00146.2005. [DOI] [PubMed] [Google Scholar]


