Abstract
Purpose
This study examined the effect of Parkinson’s disease (PD) on the intonational marking of final and nonfinal syntactic boundaries and investigated whether the effect of PD on intonation was sex-specific.
Method
Eight women and 8 men with PD and 16 age- and sex-matched control participants read a passage at comfortable pitch, rate, and loudness. Nuclear tones from final and nonfinal syntactic boundaries in clauses and lists were extracted. Measures of F0 were made on each tone contour.
Results
Individuals with PD demonstrated impaired differentiation of syntactic boundary finality/nonfinality with contour direction. They produced a lower proportion of falling contours in final boundaries and a higher proportion of falling contours in nonfinal boundaries than control participants. While not mediated by syntax, the effect of PD on F0 standard deviation (F0 SD) and pitch range (PRST) was sex-specific. Women with PD produced greater F0 SD and PRST than men with PD and women without PD. Men with PD produced lower PRST than men without PD.
Conclusions
Impaired intonational marking of syntactic boundaries likely contributes to dysprosody and reduced communicative effectiveness in PD. The effect of PD on intonation was sex-specific. The results were not fully explained by PD-related motor execution impairments.
Keywords: Parkinson’s disease, Prosody disorders, Syntax, Dysarthria, Intonation
Introduction
The appropriate use of prosody—or variations in the suprasegmental aspects of pitch, length, and loudness—is integral to effective communication (Cruttenden, 1997; Monrad-Krohn, 1947). The loss of normal prosody (i.e., dysprosody) often occurs as a component of hypokinetic dysarthria in individuals with Parkinson’s disease (PD) (Darley, Aronson, & Brown, 1969a, 1969b; Plowman-Prine et al., 2009; Sapir et al., 2001). When perceptually evident, dysprosody can result in reduced communicative effectiveness, speech naturalness, and social-linguistic competence (Pell, Cheang, & Leonard, 2006; Yorkston, Beukelman, Strand, & Bell, 1999).
Dysprosody in individuals with PD has been attributed, in part, to a perceived monopitch quality of their speech (Darley et al., 1969a, 1969b). Monopitch implies a generally flat pitch pattern, or intonation contour, across speech segments. As fundamental frequency (F0) is the physical correlate of pitch, investigators have assumed that reduced F0 variability across relatively long samples of speech (e.g., sentences and whole passages) underlies the perception of monopitch and contributes to the perception of dysprosody (Canter, 1963; Goberman, Coelho, & Robb, 2005; Harel, Cannizzaro, Cohen, Reilly, & Snyder, 2004; Harel, Cannizzaro, & Snyder, 2004). Consistent with this assumption, reduced F0 variability has been documented in individuals with PD (Canter, 1963; Gamboa et al., 1997; Harel, Cannizzaro, Cohen, Reilly, & Snyder, 2004; Jiménez-Jiménez et al., 1997).
However, reduced F0 variability in individuals with PD has not been consistently reported. Prior work has found that F0 variability during connected speech does not significantly differ between individuals with and without PD (Goberman et al., 2005; Flint, Black, Campbell-Taylor, Gailey, & Levinton, 1992) or is reduced in only a subset of individuals with PD (Holmes, Oates, Phyland, & Hughes, 2000). Collectively, these findings suggest that alterations in F0 variability, as measured across speech segments, are highly variable and do not adequately capture the complexities of pitch use by individuals with PD.
One probable reason for the equivocal findings related to F0 variability is that gross measures of F0 variability in connected speech reflect the use of intonation for many communicative functions without clearly identifying the contribution of any single function to the percepts of monopitch and dysprosody. As an alternative approach, focused examinations of specific functions of intonation may improve our knowledge of the nature and consequences of impaired intonation and dysprosody in speakers with PD.
Previous research has shown, in fact, that speakers with PD are impaired in specific aspects of intonation, such as the use of pitch patterns to mark contrastive stress and to indicate the sentence mode (Blonder, Gur, & Gur, 1989; Cheang & Pell, 2007; LeDorze, Ryalls, Brassard, Boulanger, & Ratté, 1998). Deficits in other areas, such as the strength and placement of focus markers and the production of phonemic stress patterns, appear to be more variable (Cheang & Pell, 2007; Darkins, Fromkin, & Benson, 1988; Hertrich & Ackermann, 1993; Penner, Miller, Hertrich, Ackermann, & Schumm, 2001). Moreover, when deficits do occur in these targeted uses of intonation, communication is adversely affected. For example, listeners inaccurately perceive the stress patterns and sentence mode contours produced by individuals with PD (Pell et al., 2006). Since pitch is a key component of both stress and sentence mode markers, this finding supports the hypothesis that linguistic competence and intelligibility are at risk as a consequence of abnormal intonation in individuals with PD.
One of the most important functions of intonation is to enhance speech intelligibility by marking the boundaries of syntactic units, such as groups of words organized in clause and list constructions (Cooper & Sorensen, 1981; Selkirk, 1984). This facilitates the discernment of syntactic structure and the appropriate parsing of spoken language (Beach, 1991; Crystal, 1986; Streeter, 1978; Warren, 1996; Wingfield, Lombardi, & Sokol, 1984). The syntactic-parsing function of intonation is carried out by nuclear tones (Cruttenden, 1997). According to the nuclear tone theory of intonation, nuclear tones are distinctive pitch patterns (i.e., contours) that occur at the end of major syntactic units. A nuclear tone begins on the last primary stressed syllable of a syntactic unit and extends to the end boundary of the final word in that unit (Cruttenden, 1997; Crystal, 1986; Vanderslice & Ladefoged, 1972). Each tone carries the most prominent pitch change in the F0 envelope associated with a given syntactic unit.
Syntactic units may occur at the end (i.e., final units) or in the middle (i.e., nonfinal units) of a sentence or utterance. For example, in the sentence, Although we never showed Papa our appreciation on a daily basis, I know that he felt our love, or so I hope, the word hope concludes a final unit and basis concludes a nonfinal unit. Functionally, tone contours mark syntactic boundaries in speech in much the same way that punctuation does in written language. However, some syntactic units that are marked in speech may not be marked by punctuation in writing.
Tone contours carry out this boundary-marking function through two main features of the pitch movement: the direction of the primary pitch change (i.e., rising or falling) and the amount of pitch change (i.e., wide or narrow pitch range). The near-universal tendencies are for speakers to mark the final units of statements with falling contours and wide pitch ranges and to mark nonfinal units with rising contours and narrow pitch ranges (Cooper & Sorensen, 1977, 1981; Cruttenden, 1981). These syntactic boundary markers signal to the listener that a major syntactic unit (either final or nonfinal) has been completed, which can then be processed for meaning.
Elucidating the effect of impaired intonation in PD on syntactic boundary marking is essential. Reduced or inappropriate marking may interfere with a listener’s ability to parse the speaker’s intended syntactic units. In turn, this may contribute to reduced communicative effectiveness in individuals with PD. Given the decrements in F0 production and intelligibility that can occur in PD and the tight link between intonation and intelligibility in syntactic boundary marking, investigation of the intonation-syntax interface may help to identify a cause of the percepts of monopitch and dysprosody. It may also help to clarify the communicative consequences of impaired intonation in this population.
Sex Differences in Parkinson’s Disease
Relatively few investigations of intonation in individuals with PD have considered sex differences. Yet, evidence indicates that there are significant sex differences in the manifestation and progression of PD (Hertrich & Ackermann, 1995; Lyons, Hubble, Tröster, Pahwa, & Koller, 1998; Rahn III, Chou, Jiang, & Zhang, 2007; Skodda, Rinsche, & Schlegel, 2008). Several studies have reported differential effects of PD on F0 in women and men, with a greater effect of PD on F0 variability in women (Doyle, Raade, St. Pierre, & Desai, 1995; Holmes et al., 2000). However, men with PD report significantly more problems with pitch than do women with PD (Scott, Borgman, Engler, Johnels, & Aquilonius, 2000). Overall, growing evidence indicates that PD impacts vocal function, including F0 variability, differently in women and men. However, it does not appear that one sex is consistently affected to a greater degree across all measures. Evidence also indicates that sex affects how speakers without PD produce pitch changes due to factors such as underlying differences in laryngeal mass and inertia (Sundberg, 1979; Xu & Sun, 2002). These fundamental differences, combined with evidence of sex-specific effects of PD on vocal function, suggest that investigation of potential sex differences in intonation will improve our understanding of how dysprosody manifests in women and men with PD.
Purpose
The present study had two purposes. The first purpose was to examine the effect of PD on the intonational marking of final and nonfinal syntactic boundaries in clause and list syntactic constructions using objective measures of F0. It was hypothesized that, compared to age- and sex-matched control participants, individuals with PD would exhibit difficulty with the acoustic differentiation of final and nonfinal syntactic boundaries.
The second purpose of this study was to explore whether the effect of PD on intonation differed by speaker sex. It was hypothesized that some measures of intonation would be differentially affected in women and men with PD. Due to the limited nature of the available literature base, no specific suppositions were made regarding the direction or extent of these differences.
Methods
Participants
Sixteen individuals with a neurological diagnosis of idiopathic PD and 16 age- and sex-matched control individuals participated in this study. Each group consisted of 8 women and 8 men. The mean ages of the women with PD and the men with PD were 70 years, 11 months (SD = 8 years, 10 months) and 77 years, 3 months (SD = 7 years, 4 months), respectively. The average time since diagnosis with PD was approximately 3 years, 11 months and 4 years, 6 months for the women and men, respectively. All participants with PD were ambulatory and living independently in the community. Three participants with PD reported a prior history of speech treatment. Further information about the participants with PD is provided in Table 1. Individuals with PD were tested within 1–3 hours of taking their anti-parkinsonian medication in order to control for its potential influence on speech function.
Table 1.
Information about participants with Parkinson’s disease.
| Participant | Sex | Age | Time post-diagnosis | Medications | Monotonicity | Speech rate | Reduced loudness | Overall severity |
|---|---|---|---|---|---|---|---|---|
| WPD1 | F | 72,6 | 1 | Mirapex | 2.83 | 2.67 | 2.67 | 2.72 |
| WPD2 | F | 69, 9 | 9 | Eldepryl | 1.33 | 1.33 | 2.00 | 1.55 |
| WPD3 | F | 50, 11 | 2 | Comtan, Sinemet | 1.33 | 1.00 | 1.00 | 1.11 |
| WPD4 | F | 74, 4 | 5 | Bromocriptine, Eldepryl, Sinemet | 2.00 | 1.83 | 1.00 | 1.61 |
| WPD5 | F | 79, 9 | 6 | Eldepryl, Requip, Stalevo | 1.33 | 1.83 | 1.00 | 1.39 |
| WPD6 | F | 75, 4 | 4–5 | Carbidopa-Levodopa | 1.67 | 1.33 | 2.67 | 1.89 |
| WPD7 | F | 75, 11 | 1 | None | 2.67 | 3.00 | 1.67 | 2.45 |
| WPD8 | F | 68, 9 | 3 | Comtan, Mirapex, Sinemet | 3.00 | 1.67 | 2.00 | 2.22 |
| MPD1 | M | 83, 5 | 5 | None | 6.33 | 5.33 | 6.50 | 6.05 |
| MPD2 | M | 76, 5 | 5 | Comtan, Requip, Sinemet | 5.67 | 5.67 | 4.33 | 5.22 |
| MPD3 | M | 68, 10 | 3–4 | Permax, Stalevo | 3.00 | 1.33 | 2.00 | 2.11 |
| MPD4 | M | 90, 2 | 3 | Amantadine, Carbidopa | 4.67 | 1.67 | 2.00 | 2.78 |
| MPD5 | M | 74, 7 | 3 | Carbidopa, Comtan, Permax | 3.00 | 2.67 | 3.67 | 3.11 |
| MPD6 | M | 72, 9 | 9 | Sinemet | 3.83 | 3.00 | 1.33 | 2.72 |
| MPD7 | M | 70, 0 | 4 | Sinemet | 3.50 | 3.50 | 1.67 | 2.89 |
| MPD8 | M | 82, 0 | 4 | Amantadine, Sinemet | 2.33 | 1.33 | 3.33 | 2.33 |
Age is in years, months. Time post-diagnosis is in years. Rating scale: 1.00–1.99 = normal, 2.00–3.99 = mild, 4.00–5.99 = moderate, and 6.00–7.00 = severe.
The mean ages of the control women and the control men were 70 years, 8 months (SD = 8 years, 8 months) and 76 years, 10 months (SD = 5 years, 10 months), respectively. All, control participants had normal speech, language, and voice, as judged by the second author, a certified speech-language pathologist. They also demonstrated typical hearing for their age group, as indicated by passing a hearing screening at 40 dB HL at 500, 1000, and 2000 Hz bilaterally (Ventry & Weinstein, 1983).
All participants reported no history of neurological disease (except PD), respiratory problems, head or neck cancer or surgery, or formal speaking or singing training. They had been nonsmokers for a minimum of 5 years and were free from colds, infections, and allergy symptoms at the time of testing. All participants also passed a cognitive screening, either the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) or the Cognitive-Linguistic Quick Test (Helm-Estabrooks, 2001).
Equipment
The acoustic signal was transduced via a Quest model 1700 condenser microphone for all participants except one, whose signal was transduced with a Countryman model E6i headset condenser microphone. The frequency responses of the microphones are essentially the same. All data were collected using a constant 6-inch mouth-to-microphone distance, with a 45° angle between the microphone and the speaker’s mouth. Calibration was completed with a sound pressure level (SPL) meter, and the gain used in data collection was factored into calibration of the acoustic signal. For 22 participants, the microphone signal was recorded to digital audiotape and later digitized to a computer via Praat (Boersma & Weenink, 2003). For the remaining participants, the signal was digitally recorded to a compact flash card and later transferred to a computer. All signals were recorded at 44.1 kHz and resampled to 18 kHz. According to standard acoustic sampling procedures, signals were low-pass filtered at 9 kHz (well above the highest frequency of interest) for anti-aliasing.
Speech Task
Prior to the initiation of data collection, each participant was familiarized with the “Papa Passage” (Sapienza & Stathopoulos, 1995). The passage has a Flesch-Kincaid grade level of 5.7, which indicates that it should be readable by individuals with less than a sixth-grade education level (Flesch, 1948; Kincaid, Fishburne, Rogers, & Chissom, 1975). After familiarization, each participant read the passage two times at her/his comfortable pitch, rate, and loudness.
Perceptual Ratings of the Speech of Participants with Parkinson’s Disease
The speech of each individual with PD was perceptually rated on the characteristics of monotonicity, speech rate, and reduced loudness by three certified speech-language pathologists. These speech-language pathologists were not affiliated with the study. Each had significant experience in treating individuals with motor speech disorders and in making perceptual speech judgments. The characteristics of monotonicity, speech rate, and reduced loudness were selected to represent the three components of prosody: pitch, length, and loudness.
Ratings were made on sentences extracted from recordings of the reading passage. Monotonicity and speech rate were rated from the same sentence production, which was equalized for intensity. Reduced loudness was rated from a different sentence production, which was not equalized for intensity. Each characteristic was rated separately. In order to facilitate the rating of the speech characteristics of the participants with PD, speech samples from two of the control participants (one woman and one man) were used in the listening paradigm as anchors reflecting age-appropriate “normal” on the rating scale. Based on the samples of the control participants’ speech, each rater determined a comfortable volume to use during the listening paradigm. This level was set prior to hearing or rating the speech samples of the participants with PD, and it was maintained throughout the listening paradigm. The anchors were played before each speech characteristic was rated. The speech samples were presented in random order, blocking for sex. Ratings were made on a 1–7 integer scale, with 1 = normal, 2–3 = mild, 4–5 = moderate, and 6–7 = severe.
Ratings of monotonicity, speech rate, and reduced loudness for each participant, averaged across the three speech-language pathologists, are presented in Table 1. Overall perceptual speech severity for each individual with PD was determined by averaging the ratings for these three speech characteristics across the three speech-language pathologists. Although the speech-language pathologists were aware of the diagnosis of the participants with PD, they rated one or more of the speech characteristics of many of the participants with PD as normal. This indicates that their ratings were not systematically biased by knowledge of an individual’s diagnosis. Differences between women and men with PD on the rated perceptual characteristics and on overall speech severity were assessed with Wilcoxon tests. Results indicated that the men with PD had greater overall perceptual speech severity (mildly to moderately impaired) than the women with PD (normal to mildly impaired, p = .02). They also demonstrated greater monotonicity (mildly to moderately impaired) than the women with PD (normal to moderately impaired, p = .01). There were no significant differences between the women and men with PD on the perceptual characteristics of speech rate and reduced loudness (both p > .10).
Acoustic Stimuli and Analysis Perspective
In order to examine the effect of PD on the intonational marking of syntactic boundaries, final and nonfinal boundaries in clause and list syntactic constructions were identified in the reading passage (see the Appendix). Three final clause, two nonfinal clause, one final list, and two nonfinal list boundaries were selected from the passage for analysis. The final clause boundaries occurred at the end of their respective clauses and utterances, whereas the nonfinal clause boundaries did not occur at the end of utterances but did occur at the end of clauses (i.e., dependent clauses). The final list boundary occurred at the end of the three-item list. Regardless of embedding in a longer unit of speech, lists are characterized by recognizable intonational structures, which make lists identifiable as such to listeners (Selting, 2007). The final item is typically expected to have a falling contour to designate it as the last item (i.e., completing the list). This is the case even if the surrounding unit of speech continues. The two nonfinal list boundaries differed in their relative nonfinal positions (i.e., first [nonfinal list 1] or second/middle [nonfinal list 2] boundary in the three-item list). These boundaries were examined separately, each representing a unique boundary, as it was expected that the intonational marking would differ between the two nonfinal boundary positions in the list sequence (Wright, 2002).
Appendix.
Reading passage with the word at each examined syntactic boundary marked.
| Papa Passage |
| (Sapienza & Stathopoulos, 1995) |
| Papa was a great man. Working all his life as a carpenter, he built homes for other people. Papa was an excellent craftsman. Anyone who worked with Papa knew that he was an honest man. Papa gave himself to his work, toiling daily for small amounts of money. No one disliked Papa. In fact, neighbors used to bring Papa apples (nonfinal list 1), pears (nonfinal list 2), and other fruits (final list), especially around the holidays (final clause). |
| I remember Papa for his kind ways (final clause). What I remember was the manner in which Papa dressed (nonfinal clause), the way he carried himself. Papa was such a strong man. Devoted to his family, especially his children, Papa worked night and day to provide for us. Although we never showed Papa our appreciation on a daily basis (nonfinal clause), I know that he felt our love, or so I hope (final clause). |
A nuclear tones analysis perspective was utilized. Typically, the nuclear tones of the examined syntactic boundaries were contained within the word that occurred at each boundary, as each word contained both the last primary stressed syllable and the end boundary of the clause or list unit. Thus, these words typically carried the nuclear tones that marked the boundaries. For example, in the case of the final clause boundary associated with the three-syllable word holidays, the nuclear tone extended from hol, the last primary stressed syllable in the syntactic unit, to the end of the word. In the case of the final clause boundary associated with the word ways, both the last primary stressed syllable and the end boundary of the syntactic unit occurred in the same syllable. That is, the nuclear tone spanned across three syllables in holidays and across a single syllable in ways.
The first author verified where each examined nuclear tone began and ended by listening to each boundary word within its connected speech context and identifying the last primary stressed syllable of the syntactic unit. In some cases, the last primary stressed syllable of the syntactic unit did not occur in the expected word. On a minority of productions of the final list boundary associated with fruits, the first syllable in the preceding word, other, received primary stress. Also, in the case of the final clause boundary associated with hope, occasionally the syllable with primary stress was so or I. In these cases, the nuclear tone extended across multiple words (e.g., so I hope). Thus, the nuclear tone was relative to the stress pattern the speaker produced. In all cases, the unit of analysis was consistent—the nuclear tone. The contour over the entire tone was examined, whether it was contained in a single word or in multiple words.
The words and phrases that carried the examined nuclear tones varied in phonetic characteristics such as the number and length of syllables. No attempt was made to control for these characteristics based on evidence that speech timing variables typically do not account for the melodic features of interest in this study, namely, the width and direction of pitch change over the tone contour (Allen, 1983; Ashby, 1978; Snow, 1994, 1995, 1998b, 2006).
Measurements
The conventions of Allen and Hawkins (1980) and Snow (1994, 1998a) were adopted for purposes of defining the measurement boundaries of the tone contours. Specifically, the measured portions of the contours included the vowel and any sonorant segments that preceded or followed it. Contour measures ended at the last vocalic segment preceding the right-edge boundary. For example, in the case of an item like ways, the measured portion did not include the voiced obstruent [z]. The tone contours were extracted from the passage via spectrographic analysis in Praat (Boersma & Weenink, 2003). Each contour was examined for accuracy by the first author. Any contour portions that appeared to deviate from the rest of the contour, such as a sharp increase in F0 that was incongruous with the surrounding portions of the contour, were checked. To check these portions, approximately three successive glottal cycles were visualized on Praat’s waveform display, and F0 for these cycles was calculated. When possible, this process was repeated for several sets of approximately three glottal cycles in an incongruous contour portion. If the measurer-calculated F0 differed appreciably from the contour determined by Praat, the affected portion of the contour was removed. No contour portions were added. Portions with glottal fry were excluded. To be included in the analysis, at least 75–80% of the expected contour had to be present. Contours meeting this requirement could not be extracted on 12.5%, 7.5%, 14.8% and 7.0 % of the productions by women with PD, men with PD, control women, and control men, respectively.
The following measurements were made for each tone contour using Praat (Boersma & Weenink, 2003): minimum F0 (Hz), maximum F0 (Hz), time of the minimum F0 (s), time of the maximum F0 (s), and standard deviation of F0 (F0 SD, Hz). The mean SPL (dB) of the intensity contour associated with each tone contour was also measured. Pitch range in semitones (PRST) for each tone contour was computed with the following formula:
| (1) |
Contour direction (rising or falling) was determined by the relationship between the time of the minimum and maximum F0. When the minimum F0 occurred before the maximum F0, the contour direction was classified as rising. When the maximum F0 occurred before the minimum F0, the contour direction was classified as falling. For each participant, a single proportion of falling contours value for each syntactic boundary (final clause, nonfinal clause, final list, nonfinal list 1, and nonfinal list 2) was computed with the following formula:
| (2) |
In order to assess the use of contour direction to distinguish final and nonfinal syntactic boundaries, the difference in the proportion of falling contours between the final and nonfinal boundaries of each type of syntactic construction was calculated for each participant with the following formula:
| (3) |
This resulted in one data point for each participant for each syntactic boundary comparison (final clause – nonfinal clause, final list – nonfinal list 1, and final list – nonfinal list 2). A larger positive difference in the proportion of falling contours indicates that the two boundaries were more clearly distinguished. In addition to facilitating the direct comparison of the contour direction used in final and nonfinal boundaries, difference measures were utilized because they were more normally distributed than both the raw and the arcsin transformed proportion of falling contours values at each boundary.
Statistics
The dependent variables F0 SD and PRST were assessed with three-factor repeated measures analyses of covariance. Participant group (PD and control) and sex were the between-subjects factors, and syntactic boundary (final clause, nonfinal clause, final list, nonfinal list1, and nonfinal list 2) was the within-subjects factor. Mean SPL associated with each tone served as a covariate in order to control for the influence of SPL on F0. The difference in the proportion of falling contours comparisons (final clause – nonfinal clause, final list – nonfinal list 1, and final list – nonfinal list 2) were assessed with two-factor analyses of variance, with participant group and sex as the between-subjects factors. Tukey’s honestly significant difference tests were completed post hoc for all significant main and interaction effects. The alpha level for all tests was set at p < .05.
Intermeasurer reliability measurements were completed by a second measurer for one randomly chosen woman and man from each group (12.5% of the data). Mean differences (MD) and Pearson product-moment correlations (r) between the original and the reliability measurements were as follows: minimum F0: MD = −0.16 Hz, r = 0.99; maximum F0: MD = −1.08 Hz, r = 0.99; time of the minimum F0: MD = −0.003, r = 0.96; time of the maximum F0: MD = −0.002, r = .96; F0 SD: MD = 0.20 Hz, r = 0.99; and SPL: MD = −0.341 dB, r = 0.99. There was intermeasurer agreement on contour direction for 98.8% of the contours. Reliability measurements were not completed for PRST or the proportion difference comparisons since these values were derived from the other measurements. The small mean differences and high correlations indicate good reliability.
Results
Fundamental Frequency Standard Deviation (F0 SD) and Pitch Range in Semitones (PRST)
There were significant main effects of sex for F0 SD [F(1, 28) = 41.34, p < .01] and of syntactic boundary for both F0 SD [F(4, 110) = 12.37, p < .01] and PRST [F(4, 110) = 16.25, p < .01). There were also significant Group × Sex and Sex × Syntactic boundary interaction effects for both F0 SD and PRST.
Regarding the Group × Sex interaction effect for F0 SD [F(1, 28) = 15.76, p < .01] and PRST [F(1, 28) = 21.81, p < .01] (see Table 2 for descriptive statistics):
Table 2.
Descriptive statistics for the fundamental frequency standard deviation and pitch range produced by each participant group.
| Fundamental frequency SD (Hz) | Pitch range (semitones) | |||||
|---|---|---|---|---|---|---|
| (F0 SD) | (PRST) | |||||
| Group | M | (SE) | n | M | (SE) | n |
| Women with PD | 17.52 | (0.99) | 112 | 5.07 | (0.28) | 112 |
| Control women | 12.72 | (0.97) | 108 | 3.90 | (0.28) | 108 |
| Men with PD | 7.19 | (1.03) | 110 | 3.23 | (0.29) | 110 |
| Control men | 10.25 | (0.95) | 119 | 4.70 | (0.27) | 119 |
Note. SD = standard deviation. M = adjusted mean. SE = adjusted standard error. n = number of observations.
Women with PD generally produced the greatest F0 SD and PRST. F0 SD was significantly greater for women with PD compared to all other groups. PRST was significantly greater for women with PD compared to men with PD and control women.
Men with PD generally produced the lowest F0 SD and PRST. Their F0 SD was significantly lower than those of women with PD and control women. There was no significant difference in F0 SD between men with PD and control men. The PRST of men with PD was significantly lower than that of women with PD and control men.
There was no significant difference in F0 SD or PRST between control women and men.
Relative to the Sex × Syntactic boundary interaction effect for F0 SD [F(4, 110) = 4.74, p < .01] and PRST [F(4, 110) = 3.12, p = .02] (see Table 3 for descriptive statistics):
Table 3.
Descriptive statistics for the fundamental frequency standard deviation and pitch range produced by each sex in each syntactic boundary.
| Syntactic boundary | Fundamental frequency SD (Hz) | Pitch range (semitones) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (F0 SD) | (PRST) | |||||||||||
| Women | Men | Women | Men | |||||||||
| M | (SE) | n | M | (SE) | n | M | (SE) | n | M | (SE) | n | |
| Final clause | 15.01 | (1.14) | 71 | 9.91 | (1.07) | 82 | 4.67 | (0.33) | 71 | 4.45 | (0.30) | 82 |
| Nonfinal clause | 15.29 | (1.30) | 55 | 10.87 | (1.28) | 57 | 4.74 | (0.37) | 55 | 4.67 | (0.36) | 57 |
| Final list | 11.39 | (1.72) | 31 | 5.87 | (1.76) | 30 | 3.25 | (0.49) | 31 | 2.75 | (0.50) | 30 |
| Nonfinal list 1 | 25.83 | (1.72) | 31 | 10.49 | (1.72) | 31 | 7.39 | (0.49) | 31 | 4.87 | (0.49) | 31 |
| Nonfinal list 2 | 8.07 | (1.70) | 32 | 6.45 | (1.79) | 29 | 2.37 | (0.49) | 32 | 3.09 | (0.51) | 29 |
Note. SD = standard deviation. M = adjusted mean. SE = adjusted standard error. n = number of observations.
Women generally produced greater F0 SD and PRST than men across most syntactic boundaries. However, only three comparisons reached statistical significance. F0 SD in the final clause and nonfinal list 1 boundaries was significantly greater for women than for men. PRST in the nonfinal list 1 boundary was also significantly greater for women than for men.
For women, F0 SD and PRST differed across syntactic boundaries. Women produced the greatest F0 SD and PRST in the nonfinal list 1 boundary, significantly greater than in any of the other boundaries. The F0 SD and PRST produced by women were also significantly greater in the final clause and nonfinal clause boundaries than in the nonfinal list 2 boundary. There were no significant differences in F0 SD or PRST across boundaries for men.
Differences in the Proportion of Falling Contours
For the differences in the proportion of falling contours between final and nonfinal syntactic boundaries, there was a main effect of group for two comparisons and a main effect of sex for all three comparisons.
The effect of group was significant for the final clause – nonfinal clause [F(1, 27) = 26.73, p < .01] and final list – nonfinal list 1 [F(1, 28) = 9.55, p < .01] comparisons (see Table 4 for descriptive statistics). Compared to controls, individuals with PD produced a significantly lower difference in the proportion of falling contours between the final and nonfinal boundaries in both comparisons. That is, participants with PD treated these final and nonfinal boundaries more similarly with respect to contour direction than did control participants.
Table 4.
Descriptive statisticsfor the difference in the proportion of falling contours produced by each group and sex for each syntactic boundary comparison.
| Syntactic boundary comparison | Group | Sex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parkinson’s disease | Control | Women | Men | |||||||||
| M | (SE) | n | M | (SE) | n | M | (SE) | n | M | (SE) | n | |
| Final clause – nonfinal clause | −0.04 | (0.07) | 15 | 0.39 | (0.06) | 16 | 0.29 | (0.09) | 15 | 0.07 | (0.07) | 16 |
| Final list – nonfinal list 1 | 0.38 | (0.14) | 16 | 0.84 | (0.10) | 16 | 0.84 | (0.08) | 16 | 0.38 | (0.15) | 16 |
| Final list – nonfinal list 2 | 0.13 | (0.13) | 15 | 0.44 | (0.13) | 16 | 0.50 | (0.13) | 16 | 0.07 | (0.12) | 15 |
Note. The difference in the proportion of falling contours for each syntactic boundary comparison represents the proportion of falling contours in the first boundary minus the proportion of falling contours in the second boundary. M = mean. SE = standard error. n = number of observations.
The effect of sex was significant for the final clause – nonfinal clause [F(1, 27) = 6.30, p = .02], final list – nonfinal list 1 [F(1, 28) = 9.55, p < .01], and final list – nonfinal list 2 [F(1, 27) = 6.66, p = .02]comparisons (see Table 4 for descriptive statistics). Men produced a significantly lower difference in the proportion of falling contours between the final and nonfinal boundaries in each of the three comparisons. In other words, men did not distinguish final and nonfinal syntactic boundaries as clearly as women did.
Discussion
The current study had two purposes, to examine the effect of PD on the intonational marking of final and nonfinal syntactic boundaries and to explore whether the effect of PD on intonation was sex-specific. A priori hypotheses were supported in that there was an effect of PD on the intonational marking of final and nonfinal syntactic boundaries, and the effect of PD on some measures of intonation differed by speaker sex.
Effect of Parkinson’s Disease on Syntactic Boundary Marking
Individuals with PD did not distinguish final and nonfinal syntactic boundaries with contour direction as clearly as control participants did. Although both groups demonstrated some degree of variability, as expected, the control participants followed the theory-based predictions more often than the participants with PD. In fact, individuals with PD often used contour directions that were incongruent with how control participants marked the boundaries.
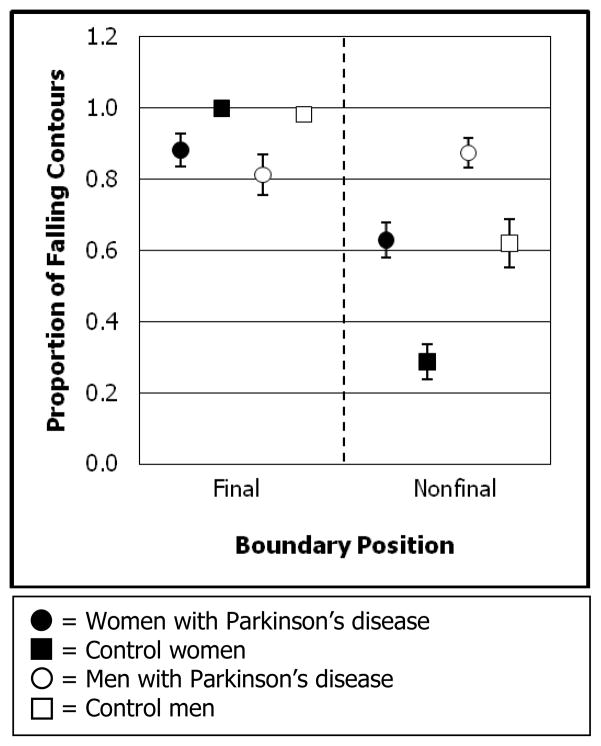
Compared to control participants, individuals with PD produced a lower proportion of falling contours in the final boundaries and a higher proportion of falling contours in the nonfinal boundaries. The raw proportion of falling contours data indicate that the control women and the men with PD demonstrated the most and the least differentiation, respectively, of final and nonfinal boundaries with contour direction (see Figure 1). The clear differentiation of these boundaries by the control women was due to their use of a considerably higher proportion of falling contours in final boundaries compared to nonfinal boundaries. The poor differentiation of these boundaries by the men with PD was due to their use of a lower proportion of falling contours in final boundaries than in nonfinal boundaries. This was the opposite final – nonfinal difference than was present for control participants, and it was the most aberrant use of contour direction in the study. The women with PD demonstrated an intermediate level of differentiation. While they distinguished these boundaries better than the men with PD, the women with PD still followed the overall group (PD) effect of reduced differentiation of boundary finality with contour direction.
Figure 1.
Proportion of falling contours produced by each participant group across all final boundaries and across all nonfinal boundaries. Symbols represent means. Lines indicate standard errors. Note that, for final boundaries, standard error bars are absent for control women because of zero standard error. For final boundaries, standard error bars for control men are obscured by the symbol due to low standard error.
The underlying reason for the contour direction-syntax mismatch in the PD data remains to be determined. It is possible that changes in motor execution (e.g., muscular rigidity) contributed to the findings. However, PD-related motor execution impairments alone do not fully explain why individuals with PD demonstrated reduced differentiation of these boundaries.
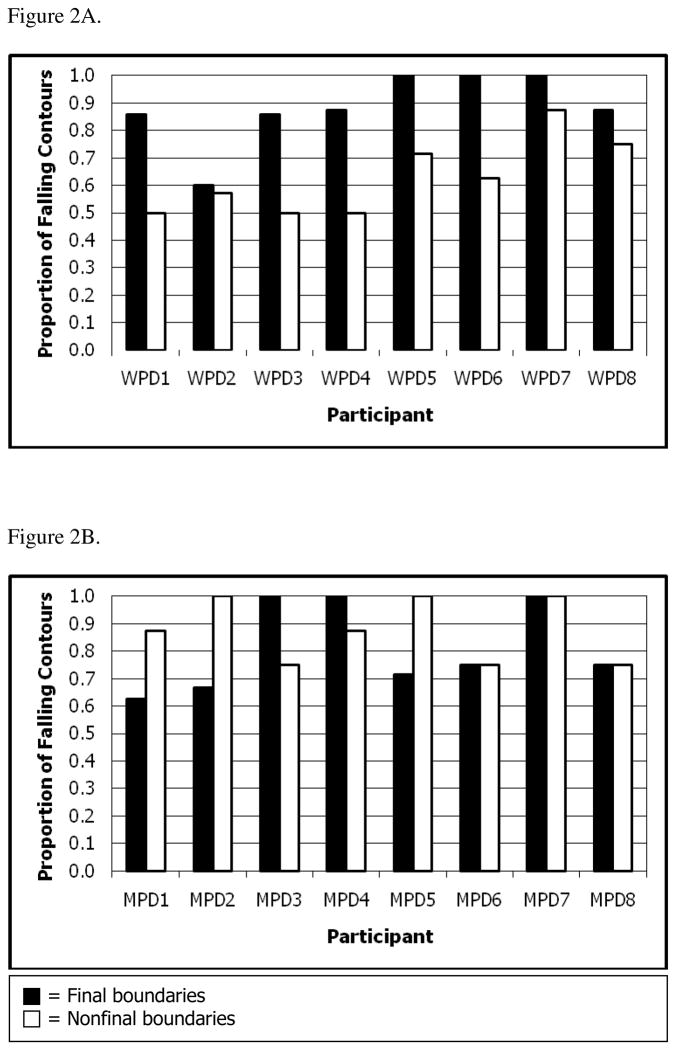
If the impaired intonational marking of syntactic boundaries was driven by speech motor execution difficulties, speakers with PD would consistently substitute physiologically easy pitch patterns for difficult ones. Physiologically easy pitch patterns include falling contours in final positions and rising contours in nonfinal positions (Cooper & Sorensen, 1977, 1981; Snow, 1998a). Contrary to this prediction, speakers with PD used more physiologically difficult contour directions in both final and nonfinal boundary contexts (i.e., they used more rising contours in final boundaries and more falling contours in nonfinal boundaries, compared to control participants). This was particularly true of the men with PD, who were more severely impaired from a speech perspective. While a few individuals with PD were more homogenous in their use of contour direction, most used both falling and rising contours (see Figure 2). This indicates that they were able to produce and switch between the two contour directions; however, they did not do so appropriately relative to the syntax of the passage. Thus, while participant performance may have been influenced by underlying changes in motor execution related to PD, the data indicate that other factors had a significant influence on the performance of the individuals with PD.
Figure 2.
Proportion of falling contours produced across all final boundaries and across all nonfinal boundaries by A) each woman with PD (WPD) and B) each man with PD (MPD).
There are several potential causes of the reduced differentiation of syntactic boundaries by individuals with PD that are not related to motor execution. For example, the observed difficulty with appropriately marking final and nonfinal syntactic boundaries may be related to impairments at the language-motor interface. Support for this supposition is provided by prior work which indicates that the basal ganglia are key in the integration of pitch and linguistic information (Darkins et al., 1988) and are important for the communicative use of prosody (Van Lancker Sidtis, Pachana, Cummings, & Sidtis, 2006). Further, potential subclinical deficits in syntax comprehension (Lieberman 1990, 1992) may have affected participants’ ability to analyze the passage and determine linguistically appropriate pitch patterns. The current findings, together with those of prior studies, suggest a role of language-motor interface impairments in the inappropriate intonational marking of syntactic boundaries by individuals with PD.
It is also possible that a deficit in speech motor planning contributed to the current findings. That is, even if intonation and linguistic information are appropriately integrated at a more conceptual level, the ability to accurately plan the motor commands to produce the correct pitch change may still be impaired. Indirect support for this potential cause is offered by evidence of motor planning deficits in individuals with PD (Cunnington et al., 1996; Dalrymple-Alford, Kalders, Jones, & Watson, 1994; Dujardin, Defebvre, Grunberg, Becquet, & Destée, 2001; Sharpe, Cermak & Sax, 1983). The most convincing support for the role of a speech motor planning deficit comes from the work of Spencer and Rogers (2005), who documented deficits in the construction and maintenance of speech motor programs by individuals with PD. These findings, together with the specific boundary-marking deficit observed in the present study, suggest a role of speech motor planning deficits in the impaired intonational marking of syntactic boundaries by individuals with PD.
Impairments in marking syntactic boundaries by individuals with PD may also relate to deficits in attention, working memory, or self-monitoring. These deficits would interfere with the coordination and performance of the concurrent tasks involved in oral reading (Dalrymple-Alford et al., 1994; Dujardin et al., 2001), such as processing text while planning and executing speech movements and monitoring performance. This possibility is supported by the finding that reading aloud, rather than being automatic, requires central attention and processing resources (Reynolds & Besner, 2006). Attention, memory, and self-monitoring deficits are relatively common in persons with PD (Culbertson, Moberg, Duda, Stern & Weintraub, 2004; Kliegel, Phillips, & Kopp, 2005; McNamara, Obler, Au, Durso, & Albert, 1992; Tun, Wingfield, Stine, & Mecsas, 1992) and can occur even among those who are newly diagnosed and who pass a screening for global cognitive functioning (Muslimović, Post, Speelman, & Schmand, 2005). As a whole, these findings indicate that subclinical cognitive difficulties may have interfered with participants’ performance of the experimental task.
Finally, the potential influence of depression on prosody has received attention in the literature. However, it is unlikely that depression, if present in any of the participants, affected the results. While findings are not unanimous, numerous studies have found that depression is not significantly related to impaired prosody in persons with PD (Darkins et al., 1988; Pell et al., 2006; Sapir et al., 2001). Moreover, speakers with depression can still mark final-nonfinal contrasts with contour direction and relative differences in pitch range (Snow & Stoel-Gammon, 1991). Therefore, the current findings cannot be accounted for by the potential presence of depression in the participants with PD.
Regardless of the exact cause of the deficit, the impaired use of contour direction to mark syntactic boundaries may relate to listeners’ frequently documented perception of the speech of individuals with PD as monopitch and dysprosodic. Most importantly, the reduced differentiation of final and nonfinal boundaries likely contributes to reduced intelligibility and communicative effectiveness in individuals with PD.
Sex-specific Effect of Parkinson’s Disease on Intonation
There was a sex-specific effect of PD on F0 variability (as measured by F0 SD) and pitch range (as measured by PRST). Compared to age- and sex-matched control participants, women with PD demonstrated an expansion of tone contour F0 variability and pitch range, while men with PD demonstrated a compression of pitch range. These differences held across all syntactic boundaries. The findings suggest that PD manifests differently, relative to F0 change, in women and men. These findings are consistent with the existing evidence that PD affects vocal function and F0 variability differently in women and men (Doyle et al., 1995; Hertrich & Ackermann, 1995; Holmes et al, 2000; Rahn III et al., 2007; Scott et al., 2000).
While it is possible that the observed sex-specific effect of PD is due, in part, to the particular sample of individuals examined (women with PD had less severely affected speech than men with PD), the results strongly suggest that this is not the case. If the findings were due to the women with PD having less severe speech symptoms, we would predict that their variability and range either would not differ from those of the control women or would be reduced compared to the control women, but not as reduced as the men with PD compared to the control men. However, the women with PD produced greater F0 variability and pitch range than the control women, whereas the men with PD used a smaller pitch range (but not F0 variability) than the control men. These results are counter to expectations based on a speech severity explanation, and they support the interpretation of the findings as indicative of a true sex difference in the effect of PD.
The current results, together with those of previous studies (Doyle et al., 1995; Hertrich & Ackermann, 1995; Holmes et al., 2000; Scott et al., 2000), provide preliminary evidence for a sex-specific effect of PD on intonation. They reinforce the importance of accounting for speaker sex in investigations of intonation in individuals with PD and call into question the validity of extrapolating findings relative to intonation in PD from one sex to the other.
Caveats and Future Directions
This was the first study to explore the effect of PD on the intonational marking of syntactic boundaries. The conclusions drawn from the data are preliminary and await future findings. In regard to the effect of PD, there are three main issues to which the present study does not speak: the use of other prosodic components (i.e., length and loudness) to help mark syntactic boundaries, intonational syntactic boundary marking in self-generated language, and how the observed acoustic abnormalities are perceived. Future research should investigate these issues. Additionally, future work should focus on further elucidating the specific causal factors that contribute to impaired intonational marking of syntactic boundaries in individuals with PD.
Conclusions
The differentiation of final and nonfinal syntactic boundaries with intonation was impaired in individuals with PD. This impairment was characterized by the decreased use of falling contours in final boundaries and the increased use of falling contours in nonfinal boundaries, in both clause and list constructions. For individuals with PD, the use of contour direction was, at times, incongruent with expectations based on the syntax of the language and with how control participants marked the boundaries. This difficulty with syntactic boundary differentiation, which was not fully explained by changes in motor execution related to PD, may contribute to the dysprosody and reduced communicative effectiveness that can occur in individuals with PD. The current findings underscore the benefit of examining targeted, functional uses of intonation in order to better understand the constellation of communicative difficulties encountered by individuals with PD.
The effect of PD on two measures of intonation, F0 variability and pitch range in nuclear tone contours, was sex-specific. The present findings suggest that PD may manifest differently, relative to F0 change, in women and men. They also highlight the importance of considering speaker sex in examinations of vocal function and intonation in individuals with PD. Future research should focus on further clarifying the conclusions drawn from the results of this preliminary study.
Acknowledgments
This research was funded by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grant R03DC05731 and by a Research Support Incentive Grant from the Center on Aging and the Life Course at Purdue University. The first author was supported by Predoctoral Fellowship T32DC00030 from the National Institutes of Health, National Institute on Deafness and Other Communication Disorders and by a Lynn Fellowship from Purdue University during the completion of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, or Purdue University.
References
- Allen GD. Some suprasegmental contours in French two-year-old children’s speech. Phonetica. 1983;40:269–292. [Google Scholar]
- Allen GD, Hawkins S. Phonological rhythm: Definition and development. In: Yeni-Komshian GH, Kavanagh JF, Ferguson CA, editors. Child phonology: Vol. 1. Production. New York: Academic Press; 1980. pp. 227–258. [Google Scholar]
- Ashby MG. A study of two English nuclear tones. Language and Speech. 1978;21:326–336. doi: 10.1177/002383097802100407. [DOI] [PubMed] [Google Scholar]
- Beach CM. The interpretations of prosodic patterns at points of syntactic structure ambiguity: Evidence for cue trading relations. Journal of Memory and Language. 1991;30:644–663. [Google Scholar]
- Blonder LX, Gur RE, Gur RC. The effects of right and left hemiparkinsonism on prosody. Brain and Language. 1989;36:193–207. doi: 10.1016/0093-934x(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat (Version 4.1). [Computer program] Amsterdam: Institute of Phonetic Sciences; 2003. [Google Scholar]
- Canter GJ. Speech characteristics of patients with Parkinson’s disease: I. Intensity, pitch, and duration. Journal of Speech and Hearing Disorders. 1963;28:221–229. doi: 10.1044/jshd.2803.221. [DOI] [PubMed] [Google Scholar]
- Cheang HS, Pell MD. An acoustic investigation of Parkinsonian speech in linguistic and emotional contexts. Journal of Neurolinguistics. 2007;20:221–241. [Google Scholar]
- Cooper WE, Sorensen JM. Fundamental frequency contours at syntactic boundaries. Journal of the Acoustical Society of America. 1977;62:683–692. doi: 10.1121/1.381556. [DOI] [PubMed] [Google Scholar]
- Cooper WE, Sorensen JM. Fundamental frequency in sentence production. New York: Springer-Verlag; 1981. [Google Scholar]
- Cruttenden A. Falls and rises: Meanings and universals. Journal of Linguistics. 1981;17:77–91. [Google Scholar]
- Cruttenden A. Intonation. 2. Cambridge, England: Cambridge University Press; 1997. [Google Scholar]
- Crystal D. Prosodic development. In: Fletcher PJ, Garman M, editors. Studies in First Language Development. New York: Cambridge University Press; 1986. pp. 174–197. [Google Scholar]
- Culbertson WC, Moberg PJ, Duda JE, Stern MB, Weintraub D. Assessing the executive function deficits of patients with Parkinson’s disease: Utility of the Tower of London-Drexel. Assessment. 2004;11:27–39. doi: 10.1177/1073191103258590. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Thickbroom GW, Laing BA, Mastaglia FL, Bradshaw JL, et al. Effects of magnetic stimulation over supplementary motor area on movement in Parkinson’s disease. Brain. 1996;119:815–822. doi: 10.1093/brain/119.3.815. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Kalders AS, Jones RD, Watson RW. A central executive deficit in patients in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:360–367. doi: 10.1136/jnnp.57.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkins AW, Fromkin VA, Benson DF. A characterization of the prosodic loss in Parkinson’s disease. Brain and Language. 1988;34:315–327. doi: 10.1016/0093-934x(88)90142-3. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. Journal of Speech and Hearing Research. 1969a;12:462–496. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research. 1969b;12:246–269. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- Doyle PC, Raade AS, St Pierre A, Desai S. Fundamental frequency and acoustic variability associated with production of sustained vowels by speakers with hypokinetic dysarthria. Journal of Medical Speech-Language Pathology. 1995;3:41–50. [Google Scholar]
- Dujardin K, Defebvre L, Grunberg C, Becquet E, Destée A. Memory and executive function in sporadic and familial Parkinson’s disease. Brain. 2001;124:389–398. doi: 10.1093/brain/124.2.389. [DOI] [PubMed] [Google Scholar]
- Flesch R. A new readability yardstick. Journal of Applied Psychology. 1948;32:221–233. doi: 10.1037/h0057532. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Black SE, Campbell-Taylor I, Gailey GF, Levinton C. Acoustic analysis in the differentiation of Parkinson’s disease and major depression. Journal of Psycholinguistic Research. 1992;21:383–399. doi: 10.1007/BF01067922. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gamboa J, Jiménez-Jiménez FJ, Nieto Montojo, Ortí-Pareja Molina, et al. Acoustic voice analysis in patients with Parkinson’s disease treated with dopaminergic drugs. Journal of Voice. 1997;11:314–320. doi: 10.1016/s0892-1997(97)80010-0. [DOI] [PubMed] [Google Scholar]
- Goberman A, Coelho C, Robb M. Prosodic characteristics of parkinsonian speech: The effect of levodopa-based medication. Journal of Medical Speech-Language Pathology. 2005;13:51–68. [Google Scholar]
- Harel BT, Cannizzaro MS, Cohen H, Reilly N, Snyder PJ. Acoustic characteristics of Parkinsonian speech: A potential biomarker of early disease progression and treatment. Journal of Neurolinguistics. 2004;17:439–453. [Google Scholar]
- Harel B, Cannizzaro M, Snyder PJ. Variability in fundamental frequency during speech in prodromal and incipient Parkinson’s disease: A longitudinal case study. Brain and Cognition. 2004;56:24–29. doi: 10.1016/j.bandc.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Helm-Estabrooks N. Cognitive-Linguistic Quick Test. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Hertrich I, Ackermann H. Acoustic analysis of speech prosody in Huntington’s and Parkinson’s disease: A preliminary report. Clinical Linguistics and Phonetics. 1993;7:285–297. [Google Scholar]
- Hertrich I, Ackermann H. Gender-specific vocal dysfunctions in Parkinson’s disease: Electroglottic and acoustic analyses. Annals of Otology, Rhinology, and Laryngology. 1995;104:197–202. doi: 10.1177/000348949510400304. [DOI] [PubMed] [Google Scholar]
- Holmes RJ, Oates JM, Phyland DJ, Hughes AJ. Voice characteristics in the progression of Parkinson’s disease. International Journal of Language and Communication Disorders. 2000;35:407–418. doi: 10.1080/136828200410654. [DOI] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Gamboa J, Nieto A, Guerrero J, Ortí-Pareja M, Molina JA, et al. Acoustic voice analysis in untreated patients with Parkinson’s disease. Parkinsonism and Related Disorders. 1997;3:111–116. doi: 10.1016/s1353-8020(97)00007-2. [DOI] [PubMed] [Google Scholar]
- Kincaid JP, Fishburne LRP, Rogers RL, Chissom BS. Derivation of new readability formulas (automated readability index, Fog count, Flesch reading ease formula) for Navy enlisted personnel (Research Branch Report 8–75) Millington, TN: Naval Technical Training Command; 1975. [Google Scholar]
- Kliegel M, Phillips LH, Lemke U, Kopp UA. Planning and realization of complex intentions in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1501–1505. doi: 10.1136/jnnp.2004.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDorze G, Ryalls J, Brassard C, Boulanger N, Ratté D. A comparison of the prosodic characteristics of the speech of people with Parkinson’s disease and Friedreich’s ataxia with neurologically normal speakers. Folia Phoniatrica et Logopaedica. 1998;50:1–9. doi: 10.1159/000021444. [DOI] [PubMed] [Google Scholar]
- Lieberman P, Friedman J, Feldman LS. Syntax comprehension deficits in Parkinson’s disease. Journal of Nervous and Mental Disease. 1990;178:360–365. doi: 10.1097/00005053-199006000-00003. [DOI] [PubMed] [Google Scholar]
- Lieberman P, Kako E, Friedman J, Tajchman G, Feldman LS, Jiminez EB. Speech production, syntax comprehension, and cognitive deficits in Parkinson’s disease. Brain and Language. 1992;43:169–189. doi: 10.1016/0093-934x(92)90127-z. [DOI] [PubMed] [Google Scholar]
- Lyons KE, Hubble JP, Tröster AI, Pahwa R, Koller WC. Gender differences in Parkinson’s disease. Clinical Neuropharmacology. 1998;21:118–121. [PubMed] [Google Scholar]
- McNamara P, Obler LK, Au R, Durso R, Albert ML. Speech monitoring skills in Alzheimer’s disease, Parkinson’s disease, and normal aging. Brain and Language. 1992;42:38–51. doi: 10.1016/0093-934x(92)90055-j. [DOI] [PubMed] [Google Scholar]
- Monrad-Krohn GH. Dysprosody or altered “melody of language. Brain: A Journal of Neurology. 1947;70:405–415. doi: 10.1093/brain/70.4.405. [DOI] [PubMed] [Google Scholar]
- Muslimović D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Pell MD, Cheang HS, Leonard CL. The impact of Parkinson’s disease on vocal-prosodic communication from the perspective of listeners. Brain and Language. 2006;97:123–134. doi: 10.1016/j.bandl.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Penner H, Miller N, Hertrich I, Ackermann H, Schumm F. Dysprosody in Parkinson’s disease: An investigation of intonation patterns. Clinical Linguistics and Phonetics. 2001;15:551–566. [Google Scholar]
- Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, et al. Perceptual characteristics of Parkinsonian speech: A comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation. 2009;24:131–144. doi: 10.3233/NRE-2009-0462. [DOI] [PubMed] [Google Scholar]
- Rahn DA, III, Chou M, Jiang JJ, Zhang Y. Phonatory impairment in Parkinson’s disease: Evidence from nonlinear dynamic analysis and perturbation analysis. Journal of Voice. 2007;21:64–71. doi: 10.1016/j.jvoice.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Besner D. Reading aloud is not automatic: Processing capacity is required to generate a phonological code from print. Journal of Experimental Psychology. 2006;32:1303–1323. doi: 10.1037/0096-1523.32.6.1303. [DOI] [PubMed] [Google Scholar]
- Sapienza CM, Stathopoulos ET. Speech task effects on acoustic and aerodynamic measures of women with vocal nodules. Journal of Voice. 1995;9:413–418. doi: 10.1016/s0892-1997(05)80203-6. [DOI] [PubMed] [Google Scholar]
- Sapir S, Pawlas AA, Ramig LO, Countryman S, O’Brien C, Hoehn MM, et al. Voice and speech abnormalities in Parkinson disease: Relation to severity of motor impairment, duration of disease, medication, depression, gender, and age. Journal of Medical Speech-Language Pathology. 2001;9:213–226. [Google Scholar]
- Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson’s disease symptom profile. Acta Neurologica Scandinavica. 2000;102:37–43. doi: 10.1034/j.1600-0404.2000.102001037.x. [DOI] [PubMed] [Google Scholar]
- Selkirk EO. Phonology and syntax: The relation between sound and structure. Cambridge, MA: M.I.T. Press; 1984. [Google Scholar]
- Selting M. Lists as embedded structures and the prosody of list construction as an interactional resource. Journal of Pragmatics. 2007;39:483–526. [Google Scholar]
- Sharpe MH, Cermak SA, Sax DS. Motor planning in Parkinson patients. Neuropsychologia. 1983;21:455–462. doi: 10.1016/0028-3932(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Skodda S, Rinsche H, Schlegel U. Progression of dysprosody in Parkinson’s disease over time—A longitudinal study. Movement Disorders. 2008;24:716–722. doi: 10.1002/mds.22430. [DOI] [PubMed] [Google Scholar]
- Snow D. Phrase-final syllable lengthening and intonation in early child speech. Journal of Speech and Hearing Research. 1994;37:831–840. doi: 10.1044/jshr.3704.831. [DOI] [PubMed] [Google Scholar]
- Snow D. Formal regularity of the falling tone in children’s early meaningful speech. Journal of Phonetics. 1995;23:387–405. [Google Scholar]
- Snow D. Children’s imitations of intonation contours: Are rising tones more difficult than falling tones? Journal of Speech, Language, and Hearing Research. 1998a;41:576–587. doi: 10.1044/jslhr.4103.576. [DOI] [PubMed] [Google Scholar]
- Snow D. Prosodic markers of syntactic boundaries in the speech of 4-year-old children with normal and disordered language development. Journal of Speech, Language, and Hearing Research. 1998b;41:1158–1170. doi: 10.1044/jslhr.4105.1158. [DOI] [PubMed] [Google Scholar]
- Snow D. Regression and reorganization of intonation between 6 and 23 months. Child Development. 2006;77:281–296. doi: 10.1111/j.1467-8624.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- Snow D, Stoel-Gammon C. Grammatical and emotional aspects of intonation in early child language. Paper presented at the Twelfth Annual Child Phonology Conference; Iowa City: University of Iowa; 1991. [Google Scholar]
- Spencer KA, Rogers MA. Speech motor programming in hypokinetic and ataxic dysarthria. Brain and Language. 2005;94:347–366. doi: 10.1016/j.bandl.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Streeter LA. Acoustic determinants of phrase boundary perception. Journal of the Acoustical Society of America. 1978;64:1582–1592. doi: 10.1121/1.382142. [DOI] [PubMed] [Google Scholar]
- Sundberg J. Maximum speed of pitch changes in singers and untrained subjects. Journal of Phonetics. 1979;7:71–79. [Google Scholar]
- Tun PA, Wingfield A, Stine EAL, Mecsas C. Rapid speech processing and divided attention: Processing rate versus processing resources as an explanation of age effects. Psychology and Aging. 1992;7:546–550. doi: 10.1037//0882-7974.7.4.546. [DOI] [PubMed] [Google Scholar]
- Vanderslice R, Ladefoged P. Binary suprasegmental features and transformational word-accentuation rules. Language. 1972;48:819–838. [Google Scholar]
- Van Lancker Sidtis D, Pachana N, Cummings JL, Sidtis JJ. Dysprosodic speech following basal ganglia insult: Toward a conceptual framework for the study of the cerebral representation of prosody. Brain and Language. 2006;97:135–153. doi: 10.1016/j.bandl.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37–42. [PubMed] [Google Scholar]
- Warren P. Prosody and parsing: An introduction. Language and Cognitive Processes. 1996;11:1–16. [Google Scholar]
- Wingfield A, Lombardi L, Sokol S. Prosodic features and the intelligibility of accelerated speech: Syntactic versus periodic segmentation. Journal of Speech and Hearing Research. 1984;27:128–134. doi: 10.1044/jshr.2701.128. [DOI] [PubMed] [Google Scholar]
- Wolfe VI, Garvin JS, Bacon M, Waldrop W. Speech changes in Parkinson’s disease during treatment with L-Dopa. Journal of Communication Disorders. 1975;8:271–279. doi: 10.1016/0021-9924(75)90019-2. [DOI] [PubMed] [Google Scholar]
- Wright E. Unpublished master’s thesis. Purdue University, West Lafayette; Indiana: 2002. Non-final intonation in preschoolers with normal and disordered language development. [Google Scholar]
- Xu Y, Sun X. Maximum speed of pitch change and how it may relate to speech. Journal of the Acoustical Society of America. 2002;111:1399–1413. doi: 10.1121/1.1445789. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Beukelman DR, Strand EA, Bell KR. Management of Motor Speech Disorders in Children and Adults. 2. Austin, TX: Pro-Ed; 1999. [Google Scholar]