Abstract
It is widely held that herbivore growth and production is limited by dietary nitrogen (N) that in turn constrains ecosystem elemental cycling. Yet, emerging evidence suggests that this conception of limitation may be incomplete, because chronic predation risk heightens herbivore metabolic rate and shifts demand from N-rich proteins to soluble carbohydrate–carbon (C). Because soluble C can be limiting, predation risk may cause ecosystem elemental cycling rates and stoichiometric balance to depend on herbivore physiological plasticity. We report on a stoichiometrically explicit ecosystem model that investigates this problem. The model tracks N, and soluble and recalcitrant C through ecosystem compartments. We evaluate how soluble plant C influences C and N stocks and flows in the presence and absence of predation risk. Without risk, herbivores are limited by N and respire excess C so that plant-soluble C has small effects only on elemental stocks and flows. With predation risk, herbivores are limited by soluble C and release excess N, so plant-soluble C critically influences ecosystem elemental stocks flows. Our results emphasize that expressing ecosystem stoichiometric balance using customary C : N ratios that do not distinguish between soluble and recalcitrant C may not adequately describe limitations on elemental cycling.
Keywords: carbon cycling, ecosystem functioning, nitrogen cycling, herbivore physiological stress, predation risk, stoichiometric balance
1. Introduction
Elemental cycling is a fundamental ecosystem process that determines rates of primary and secondary production, food chain length, trophic biomass and species diversity [1–3]. The classical view of ecosystem functioning holds that microbial species are the most critical driver of elemental cycling owing to their capacity to convert organic matter into mineral elements for plant uptake and production, and that plants and animals act as reservoirs that store and release those elements. Thus, rates of elemental cycling and hence primary and secondary production are presumed to be controlled largely by microbial action.
This classic view has been challenged with the rise of ecological stoichiometry [4,5], a more organismal-based field that aims to understand how elemental cycling and balance is affected by plant and animal species and their interactions with each other in ecosystems. Much current stoichiometric theory holds that elemental cycling within ecosystems is primarily controlled by elemental transfer between plants and herbivores owing to a mismatch between plant elemental supply and herbivore elemental demand for maintenance and production [4–6]. Plant species and parts are characterized by highly variable carbon (C): nitrogen (N): phosphorus (P) ratios with a relatively high abundance of low quality tissues, i.e. tissues with high C : N or C : P ratios [5,7,8]. Consumers, on the other hand, should regulate body elemental composition within strict, low C : N or C : P levels—known as homeostasis—to maximize survival, growth and reproduction [4,9]. This has lead to the widespread idea, at least for terrestrial systems, that C occurs in excess, whereas N and P are limiting and hence constrain elemental cycling rates [3–5,10].
Several lines of recent empirical evidence suggest, however, that the above reasoning may give an incomplete picture of controls over elemental cycling. First, empirical synthesis shows that there may be considerable intraspecific phenotypic plasticity in consumer body elemental composition [11,12], meaning that there may not be strict, homeostatic elemental requirements in many species. Second, elements do not flow freely, but rather are bound up with other elements to form macronutrients such as proteins, lipids and carbohydrates [13,14]. In terrestrial systems especially, plants contain two broad forms of organic compounds: soluble, aka labile (e.g. soluble glucose, dextrin, sucrose) and recalcitrant (e.g. cellulose lignin, and fibre from plant support and anti-herbivore defence tissues). Thus, although those plants have high overall C content, soluble organic compounds, such as carbohydrate used to fuel consumer energetic demands, may be limiting in comparison with the large proportion of their recalcitrant organic matter content [13–15]. Third, the tendency in ecosystem ecology to focus on transfer just at the plant–herbivore interface means that other important direct and indirect effects and feedbacks caused by interactions among species within the broader food web are not taken into consideration [15–19]. Specific to our case here, herbivores occupy intermediate levels within food chains and thus must often engage in behaviours that trade-off plant nutrient consumption against perceived predation risk to maximize fitness. Such trade-off behaviour can trigger a cascade of effects that influence ecosystem functioning [17,20,21]. Perceived predation risk can precipitate such effects, because it induces chronic stress in herbivore prey to increase the probability that prey individuals will survive a predator attack [22,23].
Predator-induced physiological stress responses may affect ecosystem elemental cycling in two ways, especially if soluble carbohydrate is in limiting supply. It elevates prey metabolic and respiration rate and accordingly energy demand, causing herbivores to shift preferences from N-rich proteins that support production (growth and reproduction) to carbohydrate–C to fuel the elevated metabolism and respiration [22–24]. Predation risk can also alter the efficiency and rate of elemental transfer between trophic levels in ecosystems because C is respired rather than converted to secondary production [19,22,23,25]. Furthermore, given that the amount of energy used for production correlates positively with N demand, and that herbivores have limited ability to store excess nutrients, stressed herbivores should also excrete N [23]. Ultimately, prey stressed by predation risk should increase their body C : N ratio [22,23]. Indeed, a prey body C : N difference of as little as 4 per cent between risk and risk-free conditions can perturb below-ground community function enough to alter subsequent organic matter decomposition and nutrient cycling by 60–200%—a large legacy effect of predation risk [26].
Accordingly, adaptive plasticity in herbivore physiology and elemental composition owing to the presence and absence of predation risk may cause important context-dependency in elemental cycling within ecosystems [23], and perhaps may explain context-dependent effects in response to other environmental stressors such as environmental warming [27]. Yet, how such evolutionary ecological processes at the individual organismal level scale to whole ecosystem functioning remains altogether unexplored by analytical tools. To this end, we derive a stoichiometrically explicit food chain model and use this model to analyse how predation risk may influence ecosystem elemental cycling through changes in prey physiological demand for and use of C and N.
Our work contributes to emerging theory aimed at understanding how species, especially animals, regulate ecosystem functioning through physiological effects that determine the nature of their trophic interactions in food webs [1,2,18,27–31]. This general class of theory recognizes that fundamental consumer demands for elements drive survival, growth and reproduction. But, since its original conception [1], analytical theory has been developed using two classes of approach. The first class [27,29–31] explores how variation in the degree of nutrient limitation and trophic interactions among species affects the temporal dynamics of consumer population abundances. The second class [2,18,28] explores how trophic interactions influence the temporal dynamics of elemental pool sizes within ecosystem compartments that have a species designation (e.g. primary producer, herbivore, carnivore, etc.). Because our interest here is to develop models of species interactions that inform how environmental context influences ecosystem elemental cycling, we have opted to build on the second class of approach by infusing mechanistic organismal physiological considerations into an ecosystem compartment formalism.
We address three general questions: (i) whether the evolutionary ecology of species interactions can influence ecosystem functioning [21]; (ii) whether changing resource quality controls nutrient flows among ecosystem compartments within the same ecosystem context of nutrient input and flux [32]; and (iii) whether or not food-web structure—i.e. presence of top predators within an ecosystem—influences ecosystem functioning by inducing changes in the quality (i.e. elemental C : N contents) of their prey species [4,23,33].
2. Model description
(a). Mechanism for plasticity
Current stoichiometric analyses of ecosystem elemental cycling hold that foodweb structure does not influence prey body C : N contents, because prey must maintian relatively tight homeostatic body C : N ratios to survive and reproduce [4]. This view, however, assumes that predator effects on prey are entirely consumptive [33]. However, the mere spectre of predation may elicit fear responses in prey leading to physiological stress that can be manifest as increased metabolism and respiration, and synthesis of heat shock proteins that together elevate maintenance energy demands [22,34–36]. In nutrient-limited systems, especially those with highly limiting soluble C, such increased maintenance costs reduce energy-C (i.e. soluble C-based energy) available for growth and reproduction because of the need to allocate finite resources among the competing demands of maintenance and production [37]. Hence, to meet heightened maintenance demands, stressed herbivores should reallocate energy-C from production to maintenance as well as increase their consumption of energy-C [22]. The amount of energy-C available for production correlates positively with protein–N demand. Because consumers have limited ability to store excess nutrients, threshold elemental ratio theory predicts that reallocation of energy-C from production to maintenance should cause N to be excreted [22,23,38]. Moreover, chronically heightened stress hormone levels increase the breakdown of body proteins to produce glucose, and hence may exacerbate nitrogen excretion [39] that in turn should increase herbivore body C : N ratios [22,26].
(b). General model structure
Our stoichiometrically explicit model builds on the general framework of Daufresne & Loreau [28,40]. The model tracks N, and soluble and recalcitrant forms of C through explicit compartments within herbivore and primary producer trophic levels, and soil pools (figure 1 and table 1). In real ecosystems, nutrients and elements also flow through predator and decomposer trophic levels [1,2,23]. However, as a starting approximation to motivate explicit thinking about herbivore physiological plasticity and nutrient cycling, we do not explicitly model flows through these two trophic levels. Because we are interested here in the novel pathway of risk-induced plasticity in elemental stoichiometry, we treat the presence and absence of predators only as a perturbation to elemental flows mediated by herbivore physiological stress responses, and thus do not permit predator consumptive effects. Moreover, evidence suggests that chronic predation risk effects alone can dominate ecosystem functioning despite the occurrence of predator consumptive effects [17,20,21]. We describe flows through decomposer pools by recognizing that the rate of microbial decomposition is often dependent upon the availability of soluble C in detritus [41]. Specifically, we emulate decomposition using a function that links the amount of soluble C released from plants and herbivores to the soil pool (i.e. microbial activity) to the rate at which N builds up in the soil pool and is available for plant uptake (see §2b(i)).
Figure 1.
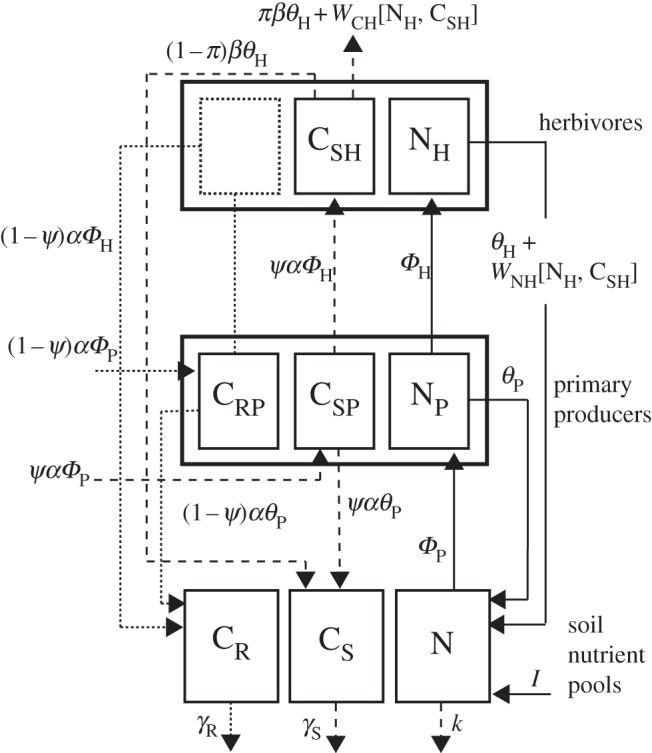
Stoichiometrically explicit model of a terrestrial herbivore–plant–soil nutrient ecosystem. The model tracks the pools of nitrogen (N), soluble carbon (CS) and recalcitrant carbon (CR). Under predation risk, herbivores have increased metabolism (higher C : N ratio) and therefore, actively seek plants with higher ratio of soluble carbon (e.g. sugars). We investigate stoichiometric plasticity in plants and herbivores by analysing models with and without predation risk and variable quantities of plant-soluble C. Solid lines are flows of N, dashed lines are flows of CS and dotted lines are flows of CR. See main text for full model description and parameter definitions.
Table 1.
Stoichiometrically explicit model of a terrestrial herbivore–plant–soil nutrient ecosystem with function and parameter definitions. See figure 1 for a conceptual diagram of the ecosystem model.
| model equations | description |
|---|---|
soil nutrients: 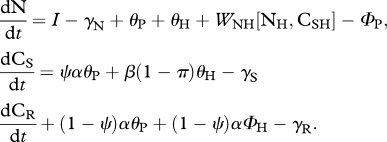
|
state variables: Ni, nitrogen stock in trophic level i; CSi, soluble carbon stock in trophic level i; CRi, recalcitrant carbon stock in trophic level i |
plants: 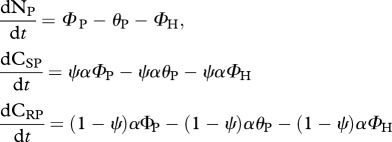
|
functions: γi, flux of element i lost from soil nutrient pool; θi, nitrogen flux recycled by trophic level i; WNH[NH, CSH], herbivore differential assimilation rate of nitrogen; WCH[NH, CSH], herbivore differential assimilation rate of soluble carbon; ΦP, nitrogen flux from the soil nutrient pool to plants; ΦH, nitrogen flux from plants to herbivores |
herbivores: 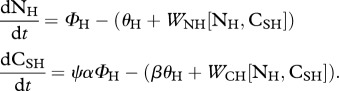
|
function definitions: γN
=
kN; γS = f(CS, N) = qSCSN; γR = qRCR;
θP = rPNP; θH = rHNH; ΦP = aPCSN; ΦH = aHNPNH;
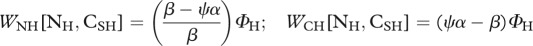
|
| parameters: I, independent nitrogen input rate to the soil nutrient pool; k, nitrogen loss rate from soil nutrient pool; qS, soluble carbon loss rate from soil nutrient pool; qR, recalcitrant carbon loss rate from soil nutrient pool; aP, nitrogen mineralization rate; aH, herbivore nutrition rate; ri, nitrogen-recycling rate of trophic level i; π, proportion of soluble carbon respired by herbivores; ψ, proportion of plant carbon that is soluble; α, CP : NP ratio; β, CSH : NH ratio |
The model couples C and N cycles. The carbon is separated into two components: a proportion that is soluble (ψ; CS) and a proportion that is recalcitrant (1 − ψ; CR). We separate carbon into soluble and recalcitrant components, because these two types of carbon are often differentially cycled within ecosystems [18,27,42,43]. We assume that primary producers take up inorganic nitrogen and produce soluble and recalcitrant carbon from the photosynthesis of atmospheric carbon (i.e. CO2). The primary producer community combines carbon and nitrogen into its own biomass and recycles carbon and nitrogen to the soil pools at a constant CiP : NP ratio, α [28,40]. We model the primary producer community as homeostatic, but we allow for stoichiometric plasticity by modifying the proportion of plant C that is soluble (ψ; see §2b(iii)). Herbivores take up nitrogen and soluble carbon and combine these two elements into their own biomass at a constant CSH : NH ratio, β [40]. We model herbivores as homeostatic, but investigate stoichiometric plasticity by allowing β to vary according to herbivore physiological state (i.e. not stressed versus stressed, see §2b(iv)). Recalcitrant carbon ingested by herbivores is typically in the form of indigestible fibre and lignin, which, we assume, based on analyses of herbivore feeding and nutrition, is egested [7]. Herbivores are assumed to recycle nitrogen through baseline egestion, excretion [44,45] and natural mortality θH [33,46], and by egestion and excretion through differential assimilation to maintain homeostasis, WNH[NH, CSH] [23,47]. Herbivores also recycle soluble carbon (βθH) at a constant CSH : NH ratio, β. A portion (π) of the recycled soluble carbon is naturally respired by herbivores; we assume the remainder (1 − π) is recycled to the soil-soluble carbon pool [33,46]. We assume that herbivores also respire CSH, if in excess, to maintain homeostasis, WCH[NH, CSH] [48]. Additionally, organic nitrogen (e.g. predator, decomposer carcasses) is supplied to the system by assuming a constant and independent source, I [33], and is lost from the system through a leaching flux, k. Soluble and recalcitrant carbon is lost from the soil carbon pools through respiration and leaching (γi) [43]. The dynamic equations for this ecosystem are defined in table 1 and represented in figure 1. See the electronic supplementary material, appendix S1 for variable, parameter as well as function definitions and dimensions.
(i). Nitrogen mineralization
Nitrogen is recycled from the primary producer and herbivore communities in organic form. Nitrogen is mineralized in proportion to the quantity of soluble carbon (CS) in the soil carbon pool according to ΦP = f(CS, N) = aPCSN, where aP is the nitrogen mineralization rate [43,49–51]. Mineralized (inorganic) nitrogen is then taken up by plants. Nitrogen mineralization governs soil respiration of soluble carbon [43]. Specifically, the respiration of CS is a function of the quantity of soil nitrogen (N) according to γS = f(CS, N) = qSCSN, where qS is the soil-soluble carbon respiration rate.
(ii). Herbivore uptake
We assume that herbivore nitrogen uptake follows simple Lotka–Volterra dynamics ΦH = aHNPNH, where aH is the herbivore nutrition rate for N [40]. Specifically, aH is a proportionality constant relating the amount of nitrogen taken from plants per unit nitrogen in herbivores per unit time (the electronic supplementary material, table S1 and appendix S1). Other models typically describe herbivore uptake using saturating functions [17,30]. However, those models describe uptake of plant biomass and assume complete assimilation of elements (i.e. assumes all elements are soluble or labile), some of which are later excreted. We instead model elemental assimilation as an emergent property of the proportion of recalcitrant and soluble C in the diet, and hence differential egestion of C. That is, reduced soluble N and C assimilation rate per unit plant matter ingested emerges when recalcitrant C comprises a high proportion of the herbivore diet.
(iii). Plant CSP : CRP : NP regulation
Primary producers exhibit great plasticity in their C : N ratios [23,38]. We model plants as homeostatic, but investigate stoichiometric plasticity in plants by modifying the proportion of plant C that is soluble (i.e. ψ) according to threshold elemental theory [23,38]. By modifying the proportion of plant C that is soluble, we emulate diversity in plant resource quality (i.e. plant CS : N ratio). This allows us to disentangle the dynamical feedbacks between trophic interactions (i.e. predation risk and herbivory) and resource quality and their corresponding effect on ecosystem elemental fluxes. Plant homeostasis implies that 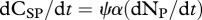 and
and 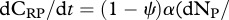
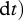 . Plant homeostasis is governed by the control of nutrient uptake [52], and we model this by making the inflow of CSP and CRP to plants equal to the inflow of NP multiplied by the plant α.
. Plant homeostasis is governed by the control of nutrient uptake [52], and we model this by making the inflow of CSP and CRP to plants equal to the inflow of NP multiplied by the plant α.
(iv). Herbivore CSH : HH regulation
Herbivores take up but do not assimilate recalcitrant carbon (CRP) typically found as fibre and lignin in plants [7]. Consequently, the recalcitrant carbon taken up by herbivores is egested with a similar ratio as that found in plants, α. This implies that herbivores selectively extract soluble carbon (CSP) and nitrogen (NP) from plant matter according to the plant ratio, α. Herbivore homeostasis implies that 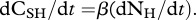
When there is no predation risk, herbivores take up nitrogen to fuel their growth and maintenance and they respire the excess CSH taken up in their diet [22]. Consequently, with no predation risk, WNH[NH, CSH] = 0. Substitution of equations dCSH/dt and dNH/dt with WNH[NH, CSH] = 0 provides the flux of CSH respired by herbivores with no predation risk to maintain homeostasis, WCH[NH, CSH] = (ψα − β)ΦH. Consequently, without predation risk, ψα > β.
To investigate the effect of predation risk on ecosystem function we do not explicitly model the dynamics of predators, we simply modify the CSH : NH ratio of herbivores such that βrisk
> βno risk (see §2a). Herbivores usually maintain homeostasis by differential assimilation, so that they release the element in surplus contained in the food they ingest [28,53]. Under predation risk, we assume that herbivores seek out soluble carbon to fuel their increased metabolism and therefore excrete excess NH [22]. Consequently, under predation risk, WCH[NH, CSH] = 0. Substitution of equations dCSH/dt and dNH/dt with WCH[NH, CSH] = 0 provides the flux of NH excreted by herbivores under predation risk to maintain homeostasis, 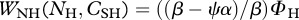 . Thus, under predation risk, β > ψα.
. Thus, under predation risk, β > ψα.
Empirical evidence suggests that herbivore respiration (π) is heightened under predation risk (reviewed in Hawlena & Schmitz [23]). Therefore, we investigate not only the consequences of differential elemental uptake by herbivores, but also the effect of increased herbivore respiration as a secondary mechanism for herbivore physiological plasticity under predation risk.
Note that models with and without predation risk respect empirical evidence that plants always have higher C : N than herbivores, i.e. α > β (5). With predation risk, however, ψα < β. ψα can be thought of as the functional C : N ratio of plants or more precisely the CS : N of plants. Consequently, under predation risk, the CS : N of herbivores is larger than the CS : N of plants but the overall C : N of herbivores is lower than the overall C : N of plants.
(c). Model analysis
We obtained a general solution for our models by setting the time derivatives to zero and solving the system of equations in table 1 for non-trivial equilibria. We analysed equilibrium conditions for the herbivore–plant–soil nutrient ecosystem model with and without predation risk. We investigate the behavioural modifications due to predation in ecosystems with plants with high and low relative quantities (i.e. ψ) of CSP and CRP under a range of herbivore respiration rates (π). We investigated the influence of the proportion of soluble carbon ψ on ecosystem stocks and fluxes by taking the partial derivative of nutrient stocks and fluxes with respect to ψ. Likewise, we investigated the influence of heightened herbivore respiration rates π under predation risk on ecosystem stocks and fluxes by taking the partial derivative of nutrient stocks and fluxes with respect to π. We illustrate our analytical results for specific parameters in figures 2–4 and the electronic supplementary material, figures S4a and S5a.
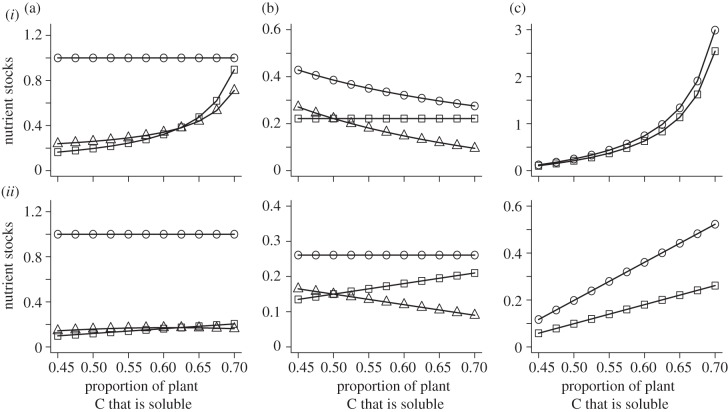
Figure 2.
Stocks of nitrogen (circles), soluble carbon (squares) and recalcitrant carbon (triangles) in (a) soils, (b) plants, and (c) herbivores for an increasing proportion of plant carbon that is soluble (ψ). Results are for models with predation risk ((i), β = 0.85) and for models without predation risk ((ii), β = 0.5). Note different scales on y-axis. Other parameter values are aP = aH = 1.15, I = 0.5, rP = rH = 0.3, qS = qR = k = 0.5, π = 0.5, α = 1.15. Qualitative results are not sensitive to particular parameter values. See the electronic supplementary material, appendix S3 for analytic results.
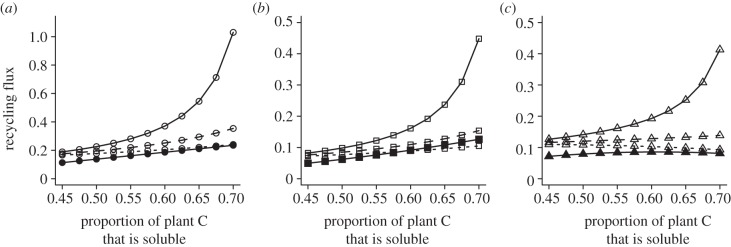
Figure 4.
(a) Total flux of nitrogen, (b) soluble carbon and (c) recalcitrant carbon recycled to the soil nutrient pools. Results are for models with predation risk (open symbols, β = 0.85) and without predation risk (filled symbols, β = 0.5). We show results under predation risk for three levels of herbivore respiration (π = 0.5 (solid lines), 0.6 (dashed lines), 0.7 (dotted lines)). All other parameters are as defined in figure 2. See the electronic supplementary material, appendix S3 and S5 for analytical results.
3. Results
(a). Effects of plant CS : CR ratio and predation risk on ecosystem stocks
For 1 > π > 0 and positive values for all other parameters, the ecosystem without predation risk persists if 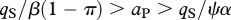 or
or 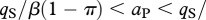
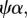 whereas the ecosystem with predation risk persists if
whereas the ecosystem with predation risk persists if 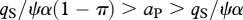 . The electronic supplementary material, appendix S2 provides the equilibrium solutions for ecosystem stocks in models with and without predation risk.
. The electronic supplementary material, appendix S2 provides the equilibrium solutions for ecosystem stocks in models with and without predation risk.
In an ecosystem without predation risk, variation in the proportion of soluble versus recalcitrant carbon but with identical overall C : N ratio leads to minor changes in soil nutrient pools. An increase in the proportion of plant C that is soluble produces a slight increase in the soil and herbivore carbon stocks but no effect on the soil and plant N stocks (figure 2). Herbivore nitrogen stocks increase with the CSP : CRP ratio of plants via an indirect effect of nitrogen mineralization in soils which depends on soil-soluble carbon (figure 2 and the electronic supplementary material, appendix S3).
The role of soluble plant C in driving ecosystem dynamics dramatically changes when we include herbivore physiological plasticity under predation risk (figure 2 and the electronic supplementary material, appendix S3). Now herbivores become soluble-C limited and they excrete the excess N from their diets. We predict a large increase in herbivore N and soluble-C stocks and a corresponding decrease in plant recalcitrant C stocks with an increase in the proportion of plant C that is soluble. With more plant C that is soluble, herbivores need to recycle less N to maintain homeostasis. This reduction in herbivore N recycling leads to stronger regulation of plant nutrient stocks and a decrease in plant N stocks (figure 2). An increase in plant-soluble C also leads to a large increase in soluble and recalcitrant C soil stocks but no effect on the N soil stock. These results emphasize a potentially important role of predators for carbon sequestration in ecosystems dominated by plants with a high proportion of soluble C.
Overall, herbivore N stocks are predicted to be larger in ecosystems with predation risk than without predation risk (see the electronic supplementary material, figure S4a and appendix S4). Herbivore N stocks in ecosystems with and without predation risk also are larger when there is a higher proportion of plant C that is soluble (see the electronic supplementary material, figure S4a and appendix S4).
Plant stocks are not influenced by heightened herbivore respiration under predation risk but herbivore and soil nutrient stocks decline under increased herbivore respiration (see the electronic supplementary material, table S5a and appendix S5). Consequently, the effects of an increase in the proportion of plant C that is soluble on ecosystem stocks are dampened by heightened herbivore respiration under predation risk (figure 3 and the electronic supplementary material, figure S5a and appendix S5).
Figure 3.
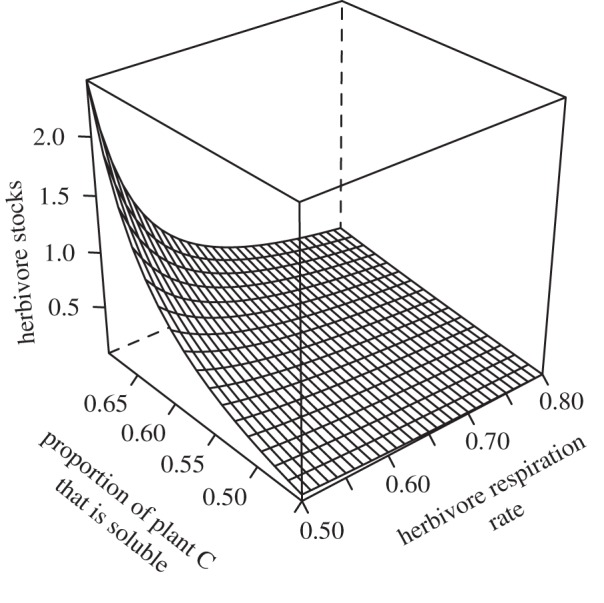
Herbivore stocks (measured as nitrogen in herbivore trophic level) under predation risk for increasing proportion of plant C that is soluble (ψ) and increasing herbivore respiration rate (π). Parameter values are β = 0.85, α = 1.15 and all other parameters are as defined in figure 2.
(b). Effects of plant CS : CR ratio and predation risk on ecosystem elemental fluxes
In an ecosystem without predation risk, whole ecosystem elemental fluxes are N = θP + θH, CS = ψαθP + (1 − π)βθH + WCH[NH, CSH], CR = (1 − ψ)αθP + (1 − ψ)αΦH. Without predation risk, ecosystem fluxes of N, soluble C and recalcitrant C increase monotonically with an increase in the proportion of plant C that is soluble (figure 4 and the electronic supplementary material, appendix S3).
In an ecosystem with predation risk, whole ecosystem elemental fluxes are N = θP + θH + WNH[NH, CSH], CS = ψαθP + (1 − π)βθH and CR = (1 − ψ)αθP + (1 − ψ)αΦH. Now, ecosystem fluxes of N increase significantly with an increase in the proportion of plant C that is soluble, whereas ecosystem fluxes of soluble C and recalcitrant C show more modest increases. Fluxes of nitrogen and carbon decline with increased herbivore respiration under predation risk (see the electronic supplementary material, table S5b and appendix S5), because the negative relationship between percentage soluble C and respiration rate (figure 3) means that increased respiration can dampen any increase in ecosystem fluxes due to higher availability of soluble plant-C (figure 4 and the electronic supplementary material, appendix S5).
4. Discussion
A fundamental assumption of ecological stoichiometry is that C reflects all carbohydrate-based energy. As a result, the majority of ecological models, even stoichiometrically explicit ones, do not distinguish between different carbohydrate-C sources [5]. This is in spite of substantial variation in the molecular structure of those compounds (e.g. glucose versus hemicelluloses), and hence in a consumer's ability to use them. Moreover, growing empirical evidence demonstrates that disregarding variation in digestibility of different organic compounds may mask important ecosystem dynamics. We included a simple representation of this variation in a consumer's ability to use different organic compounds by modelling soluble and recalcitrant C as separate pools (figure 1).
Changes in the relative quantity of soluble versus recalcitrant C in plants influence mineralization, recycling and photosynthesis in our model. In an ecosystem with predation risk, plant-soluble C influences herbivore differential assimilation which has cascading effects on ecosystem elemental stocks and fluxes (figures 2 and 4). Specifically, an increase in the proportion of plant C that is soluble leads to a large increase in herbivore and soil nutrient stocks and whole ecosystem elemental fluxes in ecosystems with predation risk. However, this increase in ecosystem stocks and fluxes is dampened if herbivores experience heightened respiration owing to predation risk (figures 3 and 4). In ecosystems without risk, an increase in the proportion of plant C that is soluble leads to relatively modest increases in ecosystem stocks and fluxes (figures 2 and 4). The positive effect of an increase in the proportion of plant C that is soluble occurs, because herbivore nitrogen stocks increase with the CSP : CRP ratio of plants via an indirect effect of nitrogen mineralization in soils that depends on soil-soluble carbon (figure 2). This is consistent with an established body of literature that demonstrates N availability and not C-energy is driving ecosystem processes and properties in ecosystems comprised of soil, plant and herbivore trophic compartments but without considerations of predation or predation risk [5]. Collectively, these results answer our three initial questions in the affirmative, namely that (i) the evolutionary ecology of species interactions (i.e. adaptive physiological response to stress) can influence ecosystem functioning by changing; (ii) the way resource quality controls nutrient flows among ecosystem compartments of an ecosystem, and that such effects do depend on (iii) food-web structure, in particular, the presence of top predators within an ecosystem influences ecosystem functioning by inducing changes in the quality (i.e. elemental C : N contents) of their prey. This shows further that consumer-mediated recycling can be a key mechanism determining the nature of cascading trophic effects within ecosystems [26,33,44,54].
Our results demonstrate the need for caution in interpreting findings that are based on the commonly used C : N ratio. This index is based on the assumption that N is the limiting element in consumer diets and hence that lower C : N ratio reflects a better diet. This does not consider physiological and resource choice plasticity in herbivores under predation risk and that soluble carbon can be a limiting nutrient in ecosystems. Moreover, in some cases, herbivores may be chronically limited by non-protein energy and the impaired performance owing to this limitation forces them to ingest a surplus of N in which case high dietary N again does not reflect higher dietary quality [55,56]. Thus, our model highlights the diverse effects of energy-C limitation on ecosystem function and shows that a simplistic index that does not distinguish between soluble and recalcitrant C may not be adequate to reflect the structure and dynamics of ecosystems.
We modelled non-consumptive effects of predation as a cost function in which physiological adjustment to escape predation increases respiration and metabolic demands for soluble-C and consequently alters herbivore nutrient (elemental) balance. To maintain homeostasis herbivores may shift their diet to match these new requirements. As a result, under predation risk, herbivores actively use a food source richer in soluble C, and this food source can support larger herbivore biomass [22]. This leads to the counterintuitive result that predation risk can actually increase herbivores biomass (figure 2 and the electronic supplementary material, figure S4a). However, if the physiological stress of predation risk leads to higher herbivore respiration, the magnitude of increase in herbivore biomass will also be reduced (figure 3 and the electronic supplementary material, figure S5a and appendix S5). Similarly, consumptive effects of predators, which we have not included in our model, can regulate herbivore populations and dampen the positive effect of predation risk and herbivore physiological plasticity on herbivore biomass (S. J. Leroux, D. Hawlena and O. J. Schmitz 2012, unpublished results).
An increase in soluble carbon in plants also has an additional indirect positive feedback on herbivore biomass, because predation risk is predicted to increase nitrogen-recycling flux and hence the productivity that can ultimately support larger herbivore biomass (figures 2 and 4). Indeed, this predicted effect of predation risk on N cycling has been observed in field experiments involving a hunting spider predator, a dominant grasshopper herbivore and a variety of grasses and forbs, where treatments in which the grasshoppers faced predation risk had soil N-cycling rates that were double that of risk-free conditions [57]. This intriguing result emphasizes the need for more empirical research to test whether elemental feedbacks between above- and below-ground processes, and herbivore biomass-C, are indeed mediated by a key mechanism in which herbivore response to predation risk is manifest as a trade-off between availability of environmental-soluble carbon for energy offset by heightened respiration (figure 3). Our model also treats nitrogen mineralization as a simple linear donor control function of soil-soluble C and N but future work should investigate the effect of more complex mineralization functions on ecosystem function.
The effect of perceived predation risk is larger in environments in which a lower proportion of the plant carbon is invested in structural compounds (figures 2–4). Consequently, we predict more dramatic effects of predation risk on ecosystem function in aquatic environments in which plants have much lower proportions of structural compounds (i.e. recalcitrant carbohydrates such as cellulose and lignin). Similarly, our model predicts dramatic consequences of predation risk in terrestrial systems characterized by plants with lower proportions of structural compounds (e.g. grassland) relative to the effect of predation risk expected in ecosystem characterized by woody plants (e.g. temperate forest).
Systems with low proportions of structural carbohydrates may also be most prone to the biggest changes in ecosystem function as a result of predator extirpation. We predict that loss of apex predators in these systems will lead to reductions in nitrogen and soluble carbon fluxes with potential cascading effects on food-web structure (i.e. lower herbivore biomass).
The kinds of effects we describe may not apply solely to cases of perceived predation risk. Indeed, animals show similar physiological responses when facing other life-threatening stressors such as drought, competition and environmental warming, because the physiological machinery driving the stress responses are evolutionarily conserved across many taxa [23]. We therefore expect that the physiological effects that modify the animal trophic function described here are generalizable to other contexts in which animals must adjust their body elemental balance to maximize fitness [27].
5. Summary
We derived a stoichiometrically explicit ecosystem model to investigate the interaction of predation risk, herbivore physiological plasticity and plant quality on biogeochemical cycling and food-web dynamics. Our analysis contributes towards increased appreciation [33,44,45,58,59] that consumers among several trophic levels in ecosystems can be critically important in driving elemental cycling within ecosystems. Foraging plasticity can alter the ratio of elements contained within their body as well as ratios of elements cycled and stored throughout the ecosystem [22,23,60]. We show that predators can exert important indirect control over the elemental content and supply of detritus inputs to the soil. The attributes of detrital quality determine the rate at which microbes perform ecosystem processes such as decomposition and mineralization [26]. Predictions of this theory are also consistent with results of experiments examining the linkages among predation risk, herbivore metabolism and body C : N ratio, and C and N mineralization rates in the soil [26,57]. Thus, our theory helps to increase our understanding of the way plasticity in herbivore organismal physiology in response to predation risk can explain context-dependency in ecosystem functioning.
Acknowledgements
We thank M. Cherif, J. Marleau and the anonymous reviewers for comments. The work was supported by a postdoctoral research fellowship from NSERC (Canada) to S.J.L., a postdoctoral fellowship from funds from the Yale School of Forestry and Environmental Studies to D.H. and NSF grant (no. DEB 0816504) to O.J.S.
References
- 1.DeAngelis D. L. 1992. Dynamics of nutrient cycling and food webs. Population and community biology series. London, UK: Chapman and Hall [Google Scholar]
- 2.Loreau M. 1995. Consumers as maximizers of matter and energy flow in ecosystems. Am. Nat. 145, 22–42 [Google Scholar]
- 3.DeAngelis D. L., Bartell S. M., Brenkert A. L. 1989. Effects of nutrient recycling and food chain length on resilience. Am. Nat. 134, 778–805 10.1086/285011 (doi:10.1086/285011) [DOI] [Google Scholar]
- 4.Elser J. J., et al. 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 10.1038/35046058 (doi:10.1038/35046058) [DOI] [PubMed] [Google Scholar]
- 5.Sterner R. W., Elser J. J. 2002. Ecological stoichiometry. Princeton, NJ: Princeton University Press [Google Scholar]
- 6.Hillebrand H., et al. 2009. Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 12, 516–527 (doi:10.1111/j.1461–0248.2009.01304.x) [DOI] [PubMed] [Google Scholar]
- 7.Robbins C. T. 1983. Wildlife feeding and nutrition. San Diego, CA: Academic Press [Google Scholar]
- 8.Karasov W. H., Martinez del Rio C. 2007. Physiological ecology. Princeton, NJ: Princeton Press University [Google Scholar]
- 9.Fagan W. F., Siemann E., Mitter C., Denno R. F., Huberty A. F., Woods H. A., Elser J. J. 2002. Nitrogen in insects: implications for trophic complexity and species diversification. Am. Nat. 160, 784–802 10.1086/343879 (doi:10.1086/343879) [DOI] [PubMed] [Google Scholar]
- 10.Anderson T. R., Hessen D. O., Elser J. J., Urabe J. 2005. Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am. Nat. 165, 1–15 10.1086/426598 (doi:10.1086/426598) [DOI] [PubMed] [Google Scholar]
- 11.Bertram S. M., Bowen M., Kyle M., Schade J. D. 2008. Extensive natural intraspecific variation in stoichiometric (C : N : P) composition in two terrestrial insect species. J. Insect Sci. 8, 1–7 10.1673/031.008.2601 (doi:10.1673/031.008.2601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson J., Fink P., Goto A., Hood J. M., Jonas J., Kato S. 2010. To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119, 741–751 10.1111/j.1600-0706.2009.18545.x (doi:10.1111/j.1600-0706.2009.18545.x) [DOI] [Google Scholar]
- 13.Anderson T. R., Boersma M., Raubenheimer D. 2004. Stoichiometry: linking elements to biochemicals. Ecology 85, 1193–1202 10.1890/02-0252 (doi:10.1890/02-0252) [DOI] [Google Scholar]
- 14.Raubenheimer D., Simpson S. J., Mayntz D. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 23, 4–16 10.1111/j.1365-2435.2009.01522.x (doi:10.1111/j.1365-2435.2009.01522.x) [DOI] [Google Scholar]
- 15.Moe S. J., Stelzer R. S., Forman M. R., Harpole W. S., Daufresne T., Yoshida T. 2005. Recent advances in ecological stoichiometry: insights for population and community ecology. Oikos 109, 29–39 10.1111/j.0030-1299.2005.14056.x (doi:10.1111/j.0030-1299.2005.14056.x) [DOI] [Google Scholar]
- 16.Dickman E. M., Newell J. M., Gonzalez M. J., Vanni M. J. 2008. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl Acad. Sci. USA 105, 18 408–18 412 10.1073/pnas.0805566105 (doi:10.1073/pnas.0805566105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz O. J. 2008. Herbivory from individuals to ecosystems. Annu. Rev. Ecol. Syst. 39, 133–152 10.1146/annurev.ecolsys.39.110707.173418 (doi:10.1146/annurev.ecolsys.39.110707.173418) [DOI] [Google Scholar]
- 18.Cherif M., Loreau M. 2009. When microbes and consumers determine the limiting nutrient of autotrophs: a theoretical analysis. Proc. R. Soc. B 276, 487–497 10.1098/rspb.2008.0560 (doi:10.1098/rspb.2008.0560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumann M. 1998. The fallacy of the missing middle: physics fisheries. Fisheries Oceanogr. 7, 63–65 10.1046/j.1365-2419.1998.00048.x (doi:10.1046/j.1365-2419.1998.00048.x) [DOI] [Google Scholar]
- 20.Schmitz O. J. 2010. Resolving ecosystem complexity. Princeton, NJ: Princeton University Press [Google Scholar]
- 21.Schmitz O. J., Grabowski J. H., Peckarsky B. L., Preisser E. L., Trussell G. C., Vonesh J. R. 2008. From individuals to ecosystem function: toward an integration of evolutionary and ecosystem ecology. Ecology 89, 2436–2445 10.1890/07-1030.1 (doi:10.1890/07-1030.1) [DOI] [PubMed] [Google Scholar]
- 22.Hawlena D., Schmitz O. J. 2010. Herbivore physiological response to predation risk and implications for ecosystem nutrient dynamics. Proc. Natl Acad. Sci. USA 107, 15 503–15 507 10.1073/pnas.1009300107 (doi:10.1073/pnas.1009300107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawlena D., Schmitz O. J. 2010. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 176, 537–556 10.1086/656495 (doi:10.1086/656495) [DOI] [PubMed] [Google Scholar]
- 24.McPeek M. A., Grace M., Richardson J. M. L. 2001. Physiological and behavioral responses to predators shape the growth/predation risk trade-off in damselflies. Ecology 82, 1535–1545 10.1890/0012-9658(2001)082[1535:PABRTP]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[1535:PABRTP]2.0.CO;2) [DOI] [Google Scholar]
- 25.Trussell G. C., Ewanchuk P. J., Matassa C. M. 2006. The fear of being eaten reduces energy transfer in a simple food chain. Ecology 87, 2979–2984 10.1890/0012-9658(2006)87[2979:TFOBER]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2979:TFOBER]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 26.Hawlena D., Strickland M. S., Bradford M. A., Schmitz O. J. 2012. Fear of predation slows plant-litter decomposition. Science 336, 1434–1438 10.1126/science.1220097 (doi:10.1126/science.1220097) [DOI] [PubMed] [Google Scholar]
- 27.Hall S. R. 2009. Stoichiometrically explicit food webs: feedbacks between resource supply, elemental constraints, and species diversity. Annu. Rev. Ecol. Syst. 40, 503–528 10.1146/annurev.ecolsys.39.110707.173518 (doi:10.1146/annurev.ecolsys.39.110707.173518) [DOI] [Google Scholar]
- 28.Daufresne T., Loreau M. 2001. Plant–herbivore interactions and ecological stoichiometry: when do herbivores determine plant nutrient limitation? Ecol. Lett. 4, 196–206 10.1046/j.1461-0248.2001.00210.x (doi:10.1046/j.1461-0248.2001.00210.x) [DOI] [Google Scholar]
- 29.Muller E. B., Nisbet R. M., Kooijman S., Elser J. J., McCauley E. 2001. Stoichiometric food quality and herbivore dynamics. Ecol. Lett. 4, 519–529 10.1046/j.1461-0248.2001.00240.x (doi:10.1046/j.1461-0248.2001.00240.x) [DOI] [Google Scholar]
- 30.Grover J. P. 2003. The impact of variable stoichiometry on predator–prey interactions: a multinutrient approach. Am. Nat. 162, 29–43 10.1086/376577 (doi:10.1086/376577) [DOI] [PubMed] [Google Scholar]
- 31.Kuijper L. D. J., Kooi B. W., Anderson T. R., Kooijman S. 2004. Stoichiometry and food-chain dynamics. Theor. Popul. Biol. 66, 323–339 10.1016/j.tpb.2004.06.011 (doi:10.1016/j.tpb.2004.06.011) [DOI] [PubMed] [Google Scholar]
- 32.Marcarelli A. M., Baxter C. V., Mineau M. M., Hall R. O., Jr 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92, 1215–1225 10.1890/10-2240.1 (doi:10.1890/10-2240.1) [DOI] [PubMed] [Google Scholar]
- 33.Schmitz O. J., Hawlena D., Trussell G. C. 2010. Predator control of ecosystem nutrient dynamics Ecol. Lett. 13, 1199–1209 10.1111/j.1461-0248.2010.01511.x (doi:10.1111/j.1461-0248.2010.01511.x) [DOI] [PubMed] [Google Scholar]
- 34.Rovero F., Hughes R. N., Chelazzi G. 2000. When time is of the essence: choosing a currency for prey-handling costs. J. Anim. Ecol. 69, 683–689 10.1046/j.1365-2656.2000.00426.x (doi:10.1046/j.1365-2656.2000.00426.x) [DOI] [Google Scholar]
- 35.Pauwels K., Stoks R., De Meester L. 2005. Coping with predator stress: interclonal differences in induction of heat-shock proteins in the water flea Daphnia magna. J. Evol. Biol. 18, 867–872 10.1111/j.1420-9101.2005.00890.x (doi:10.1111/j.1420-9101.2005.00890.x) [DOI] [PubMed] [Google Scholar]
- 36.Steiner U. K., Van Buskirk J. 2009. Predator-induced changes in metabolism cannot explain the growth/predation risk tradeoff. PLoS ONE 4, e6160. 10.1371/journal.pone.0006160 (doi:10.1371/journal.pone.0006160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuRant S. E., Hopkins W. A., Talent L. G. 2007. Energy acquisition and allocation in an ectothermic predator exposed to a common environmental stressor. Comp. Biochem. Physiol. C 145, 442–448 10.1016/j.cbpc.2007.01.014 (doi:10.1016/j.cbpc.2007.01.014) [DOI] [PubMed] [Google Scholar]
- 38.Sterner R. W. 1997. Modelling interactions of food quality and quantity in homeostatic consumers. Freshwater Biol. 38, 473–481 10.1046/j.1365-2427.1997.00234.x (doi:10.1046/j.1365-2427.1997.00234.x) [DOI] [Google Scholar]
- 39.Stoks R., De Block M., McPeek M. A. 2005. Alternative growth and energy storage responses to mortality threats in damselflies. Ecol. Lett. 8, 1307–1316 10.1111/j.1461-0248.2005.00840.x (doi:10.1111/j.1461-0248.2005.00840.x) [DOI] [Google Scholar]
- 40.Daufresne T., Loreau M. 2001. Ecological stoichiometry, primary producer–decomposer interactions, and ecosystem persistence. Ecology 82, 3069–3082 10.1890/0012-9658(2001)082[3069:esppdi]2.0.co;2 (doi:10.1890/0012-9658(2001)082[3069:esppdi]2.0.co;2) [DOI] [Google Scholar]
- 41.Ekblad A., Nordgren A. 2002. Is growth of soil microorganisms in boreal forests limited by carbon or nitrogen availability? Plant Soil 242, 115–122 10.1023/a:1019698108838 (doi:10.1023/a:1019698108838) [DOI] [Google Scholar]
- 42.Wetzel R. G. 1995. Death, detritus, and energy flow in aquatic ecosystems. Freshwater Biol. 33, 83–89 10.1111/j.1365-2427.1995.tb00388.x (doi:10.1111/j.1365-2427.1995.tb00388.x) [DOI] [Google Scholar]
- 43.Coleman M. D., Isebrands J. G., Tolsted D. N., Tolbert V. R. 2004. Comparing soil carbon of short rotation poplar plantations with agricultural crops and woodlots in North Central United States. Environ. Manage. 33, S299–S308 10.1007/s00267-003-9139-9 (doi:10.1007/s00267-003-9139-9) [DOI] [Google Scholar]
- 44.Vanni M. J. 2002. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 33, 341–370 10.1146/annurev.ecolysis.33.010802.150519 (doi:10.1146/annurev.ecolysis.33.010802.150519) [DOI] [Google Scholar]
- 45.Wardle D. A., Bardgett R. D. 2004. Human-induced changes in large herbivorous mammal density: the consequences for decomposers. Front. Ecol. Environ. 2, 145–153 10.2307/3868240 (doi:10.2307/3868240) [DOI] [Google Scholar]
- 46.Bump J. K., Peterson R. O., Vucetich J. A. 2009. Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses. Ecology 90, 3159–3167 10.1890/09-0292.1 (doi:10.1890/09-0292.1) [DOI] [PubMed] [Google Scholar]
- 47.Frost P. C., Benstead J. P., Cross W. F., Hillebrand H., Larson J. H., Xenopoulos M. A., Yoshida T. 2006. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol. Lett. 9, 774–779 10.1111/j.1461-0248.2006.00919.x (doi:10.1111/j.1461-0248.2006.00919.x) [DOI] [PubMed] [Google Scholar]
- 48.Zanotto F. P., Gouveia S. M., Simpson S. J., Raubenheimer D., Calder P. C. 1997. Nutritional homeostasis in locusts: is there a mechanism for increased energy expenditure during carbohydrate overfeeding? J. Exp. Biol. 200, 2437–2448 [DOI] [PubMed] [Google Scholar]
- 49.Reinertsen S. A., Elliott L. F., Cochran V. L., Campbell G. S. 1984. Role of available carbon and nitrogen in determining the rate of wheat straw decomposition. Soil Biol. Biochem. 16, 459–464 10.1016/0038-0717(84)90052-x (doi:10.1016/0038-0717(84)90052-x) [DOI] [Google Scholar]
- 50.Weintraub M. N., Schimel J. P. 2003. Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in arctic tundra soils. Ecosystems 6, 129–143 10.1007/s10021-002-0124-6 (doi:10.1007/s10021-002-0124-6) [DOI] [Google Scholar]
- 51.Gilmour J. T., Clark M. D., Sigua G. C. 1985. Estimating net nitrogen mineralization from carbon dioxide evolution. Soil Sci. Soc. Am. J. 49, 1398–1402 10.2136/sssaj1985.03615995004900060013x (doi:10.2136/sssaj1985.03615995004900060013x) [DOI] [Google Scholar]
- 52.Tilman D. 1982. Resource competition and community structure. Monogr. Popul. Biol. 17, 1–296 [PubMed] [Google Scholar]
- 53.Sterner R. W. 1990. The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am. Nat. 136, 209–229 10.1086/285092 (doi:10.1086/285092) [DOI] [Google Scholar]
- 54.Leroux S. J., Loreau M. 2010. Consumer-mediated recycling and cascading trophic interactions. Ecology 91, 2162–2171 10.1890/09-0133.1 (doi:10.1890/09-0133.1) [DOI] [PubMed] [Google Scholar]
- 55.Rothman J. M., Raubenheimer D., Chapman C. A. 2011. Nutritional geometry: gorillas prioritize non-protein energy while consuming surplus protein. Biol. Lett. 7, 847–849 10.1098/rsbl.2011.0321 (doi:10.1098/rsbl.2011.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cease A. J., Elser J. J., Ford C. F., Hao S. G., Kang L., Harrison J. F. 2012. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 335, 467–469 10.1126/science.1214433 (doi:10.1126/science.1214433) [DOI] [PubMed] [Google Scholar]
- 57.Schmitz O. J. 2006. Predators have large effects on ecosystem properties by changing plant diversity, not plant biomass. Ecology 87, 1432–1437 10.1890/0012-9658(2006)87[1432:PHLEOE]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1432:PHLEOE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 58.Kitchell J. F., Oneill R. V., Webb D., Gallepp G. W., Bartell S. M., Koonce J. F., Ausmus B. S. 1979. Consumer regulation of nutrient cycling. BioScience 29, 28–34 10.2307/1307570 (doi:10.2307/1307570) [DOI] [Google Scholar]
- 59.Estes J. A., et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306 10.1126/science.1205106 (doi:10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 60.Wingfield J. C., Ramenofsky M. 1999. Hormones and the behavioral ecology of stress. In Stress physiology in animals (ed. Baum P. H. M.), pp. 1–51 Sheffield, UK: Sheffield Academic Press [Google Scholar]