Abstract
Allomones are widely used by insects to impede predation. Frequently these chemical stimuli are released from specialized glands. The larvae of Chrysomelina leaf beetles produce allomones in gland reservoirs into which the required precursors and also the enzymes are secreted from attached gland cells. Hence, the reservoirs can be considered as closed bio-reactors for producing defensive secretions. We used RNA interference (RNAi) to analyse in vivo functions of proteins in biosynthetic pathways occurring in insect secretions. After a salicyl alcohol oxidase was silenced in juveniles of the poplar leaf beetles, Chrysomela populi, the precursor salicyl alcohol increased to 98 per cent, while salicyl aldehyde was reduced to 2 per cent within 5 days. By analogy, we have silenced a novel protein annotated as a member of the juvenile hormone-binding protein superfamily in the juvenile defensive glands of the related mustard leaf beetle, Phaedon cochleariae. The protein is associated with the cyclization of 8-oxogeranial to iridoids (methylcyclopentanoid monoterpenes) in the larval exudates made clear by the accumulation of the acylic precursor 5 days after RNAi triggering. A similar cyclization reaction produces the secologanin part of indole alkaloids in plants.
Keywords: RNAi, insects, leaf beetle, secretome, salicyl alcohol oxidase, monoterpene cyclization
1. Introduction
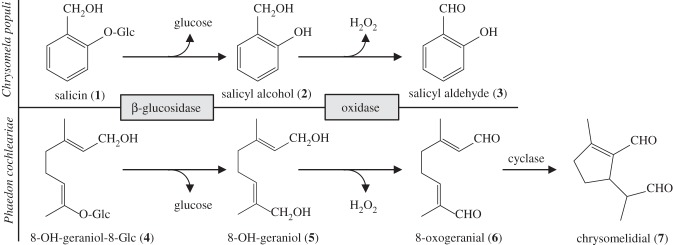
Insects are extraordinarily inventive when it comes to producing defensive compounds for repelling their enemies. To circumvent auto-intoxicative effects, these natural products frequently originate in the epidermis-derived exocrine glands [1]. The gland cells produce secretions that are fortified with defensive compounds [2,3]. It has been demonstrated that insects convert either intrinsic precursors or food-derived compounds into biologically active allelochemicals [4–7]. The precursors can be activated in the defensive glands or in the secretions. Immature leaf beetles of the subtribe Chrysomelina, for example, produce their deterrents in biphasic secretions, and store them in nine unique pairs of impermeable reservoirs in their backs [8,9]. The larval exudates containing salicyl aldehyde (3) have been of particular interest [10,11]. The hydrophobic aldehyde forms an organic layer, accounting for 15 per cent of the total discharge volume, while the aqueous phase constitutes 85 per cent [12]. The latter contains the precursor salicyl alcohol (2) and a flavine-dependent salicyl alcohol oxidase (SAO); the SAO uses molecular oxygen as an electron acceptor for alcohol oxidation, yielding the aldehyde and hydrogen peroxide [12–14] (figure 1). Salicyl aldehyde is considered as a potent repellent against generalist predators [11,15] and as an antimicrobial agent [16]. The larvae feed on salicaceaous plants and sequester the secondary metabolite salicin (1) [17–19]. After shuttling salicin to the defensive glands, the glucoside is cleaved by a β-glucosidase into 2 and glucose for further metabolism [20] (figure 1). According to phylogenetic analyses, the synthesis of 3 from sequestered precursors has evolved from the de novo production of defensive iridoids (methylcyclopentanoid monoterpenes containing an iridane skeleton) [21]. Also the last steps of the iridoid pathway in the secretions are thought to be similar to those found in sequestering species [20] (figure 1). At first, the sugar moiety is cleaved from 8-hydroxygeraniol-8-O-β-d-glucoside (4), and an oxygen-dependent oxidase converts the aglucone into 8-oxogeranial (6) [20,22–24]. A subsequent cyclization reaction yields iridoids (7) [25].
Figure 1.
Enzymatic reactions in the defensive secretions of juvenile C. populi and P. cochleariae adapted from Michalski et al. [14]. Glc, Glucose.
Despite the many current genome- and transcriptome-sequencing projects, up to now it has only been shown for SAO sequences to be entangled in allomone production in the defensive secretions of the leaf beetle species Chrysomela tremulae, Chrysomela populi, Chrysomela lapponica and Phratora vitellinae [13,14,26]. To demonstrate the in vivo relevance of a target sequence, gene silencing by RNA interference (RNAi) is a suitable method. RNAi is an endogenous mechanism, derived from an anti-viral immune response [27], and can be found virtually in all eukaryotic species. It can be triggered artificially by double-stranded RNA (dsRNA), whose nucleotide sequence is identical to that of the target gene [28]. The RNAi effect is attended by decreased transcript and protein levels, and consequently by loss-of-function phenotypes. In addition to embryogenesis, pattern formation, reproduction and behaviour, RNAi allows biosynthetic pathways in insects to be successfully analysed [29–31].
Here, we describe how RNAi can be used to target the biosynthesis of discrete components in the defensive discharges of juvenile Chrysomelina. We first validated this technique by silencing the known SAO sequence in the sequestering species C. populi (CpopSAO). After knocking down the SAO, the alcohol precursor of 3 accumulated in the gland. This showed that we are able to interrupt the deterrent biosynthesis in vivo. Next, we extended the method to the related de novo iridoid-producing species, P. cochleariae. In the secretions of its larvae C10-precursors are converted to the methylcyclopentanoid monoterpene chrysomelidial. Particularly, the cyclization mechanism is of importance because it occurs not only in insects but also in plants. Here, the cyclization leads ultimately to secologanin, one of the building blocks for more than 2500 indole alkaloids that have been isolated mainly from three plant families [32]. Although an enzyme with cyclase activity for secologanin biosynthesis has long been predicted, a corresponding sequence has yet to be published. In the P. cochleariae secretome, we identified a novel protein which is involved in the cyclization reaction of the monoterpenoid 8-oxogeranial to chrysomelidial.
2. Material and methods
See electronic supplementary material for complete secretome analyses by data-independent liquid chromatography/mass spectrometry detection (LC/MSE), cloning procedures, detailed quantitative real-time PCR procedure (qPCR), all primer sequences and accession numbers.
(a). Beetle rearing and secretion analyses
Chrysomela populi (L.) was collected near Dornburg, Germany (latitude 51.015, longitude 11.64), on Populus maximowiczii×Populus nigra. In the laboratory, beetles were kept in a 16 L : 8 D cycle, 18 ± 2°C in light and 13 ± 2°C in darkness. Phaedon cochleariae (F.) was laboratory-reared on Brassica oleracea convar. capitata var. alba (Gloria F1) in 16 L : 8 D cycle conditions and 15 ± 2°C. According to [33], we obtained the relative growth rate (RGR) of six biological replicates of each group of five larvae by RGR = [(final weight − weight of neonate larva)/(weight of neonate larva × developmental time (days))]. Each replicate group was weighed every 24 ± 3 h and data were compared with two-tailed t-test. Larval secretions were collected in glass capillaries (inner diameter, 0.28 mm; outer diameter, 0.78 mm, length 100 mm; Hirschmann, Eberstadt, Germany). Sealed capillaries containing samples were stored at −20°C until needed. Secretions were weighed in the sealed capillaries on an ultra-microbalance (Mettler-Toledo, Greifensee, Switzerland) three times; the weight of the capillaries was subtracted and the final weight was averaged.
(b). Production of double-stranded RNA
Sequenced plasmids pIB-CpopSAO (GeneBank: HQ245154.1) and pIB-PcTo-like (GeneBank: JQ728549) were used to amplify a 1.5 kb CpopSAO fragment and a 450 bp PcTo-like fragment, respectively. The gfp sequence was amplified from pcDNA3.1/CT- GFP-TOPO (Life Technology, Darmstadt, Germany). The amplicons were subject to in vitro transcription assays according to instructions from the Ambion MEGAscript RNAi kit (Life Technologies, Darmstadt, Germany). The resulting dsRNA was eluted after nuclease digestion three times with 50 µl of injection buffer (3.5 mM Tris–HCl, 1 mM NaCl, 50 nM Na2HPO4, 20 nM KH2PO4, 3 mM KCl, 0.3 mM EDTA, pH 7.0). The concentration of dsRNA was calculated with A = 1 = 45 mg ml−1 and adjusted to 1 µg µl−1. The quality of dsRNA was checked by TBE-agarose-electrophoresis.
(c). Injection of double-stranded RNA
First instar of C. populi with 5 mm body length was injected with 0.1–3 µg of dsRNA approximately 10 days after hatching. Phaedon cochleariae second instar with 4 mm body length was injected with 0.3 µg of dsRNA approximately 5 days after hatching. Injections were accomplished with ice-chilled larvae using a Nano2000 injector (WPI, Sarasota, FL, USA) directed by a three-axis micromanipulator. The larvae were injected parasagittally between the pro- and mesothorax.
(d). Off-target prediction
According to the mechanism of RNAi [28], the top and bottom strands of dsRNAs of CpopSAO, PcTo-like and gfp were diced in silico into all possible 21 bp fragments [34]. The resulting siRNAs were subjected to BLASTn (stand-alone NCBI-BLAST) [35] by invoking Blastall v. 2.2.21 (parameters: -p blastn -e 1e-1 -G 7 -T -b 80 -v 80) searching against our in-house transcriptome databases of C. populi and P. cochleariae. Hits less than 20 nts in length were ignored and hits more than or equal to 20 nts were considered as putative off targets.
(e). CpopSAO and PcTo-like transcript abundance
Cq values of genes of interest from three biological replicates were normalized by CpRPL45 and CpActin for C. populi and PcRP-L8 and PcRP-S18 for P. cochleariae, respectively. Real-time PCR data were acquired on an Mx3000P Real-Time PCR system using Brilliant II SYBR Green qPCR Master Mix (Agilent, Santa Clara, CA, USA).
(f). Gas chromatography/mass spectrometry analysis of low-molecular-weight compounds in chrysomelid secretions
Secretions of C. populi were diluted in 1 : 150 (w/v) ethyl acetate and secretions of P. cochleariae were diluted in 1 : 100 (w/v) dichloromethane. Of each diluted secretion, 1 μl was subjected to GC/EIMS analysis (ThermoQuest Finnigan Trace GC/MS 2000, Frankenhorst, Germany) equipped with Phenomenex (Aschaffenburg, Germany) ZB–5–W/Guardian–column, 25 m. Substances were separated using helium as a carrier (1.5 ml min−1). Conditions for C. populi secretions: 50°C (1 min), 10°C per minute to 80°C, 60°C per minute to 280°C (1 min). Inlet temperature was 220°C, transfer line was 280°C. Substances were identified according to standard substances 2 and 3. Conditions for P. cochleariae secretions: 50°C (2 min), 10°C per minute to 80°C, 5°C per minute to 200°C, 30°C per minute to 300°C (1 min). Inlet temperature was 220°C and transfer line was 280°C. Substances were identified according to [36] and the reference compounds 8-oxogeranial and chrysomelidial. The synthesis of 8-oxogeranial and chrysomelidial was carried out as in [25,37], respectively. Peak areas from GC-chromatograms were obtained using an ICIS-algorithm (Xcalibur bundle v. 2.0.7, Thermo Scientific).
(g). Statistical analyses
Two-tailed Student's t-tests for unequal variation were used to value the significance levels of transcript abundances and to weight differences comparing values of three different biological replicates from the non-injected control (NIC) group with those of either the RNAi group or the gfp control. Multi-dimensional ANOVA tests were carried out to validate significant differences in time series and between different RNAi treatments. The level of significance was reached at a p-value of 0.05. Calculations were done with R (http://www.r-project.org/).
3. Results
(a). Targeting the defensive glands of juvenile poplar leaf beetles by RNA interference
Recently, a 1872-bp CpopSAO cDNA (Genbank/HQ245154.1) encoding a 69 kDa protein for conversion of 2 into 3 was identified from the larval defensive glands of C. populi [13,14] (figure 2a). It belongs to the glucose–methanol–choline (GMC) family of oxidoreductases [38]. Given that the expression of CpopSAO was detectable exclusively in glandular tissues (figure 2b), silencing this gene would affect only the process of glandular biosynthesis.
Figure 2.
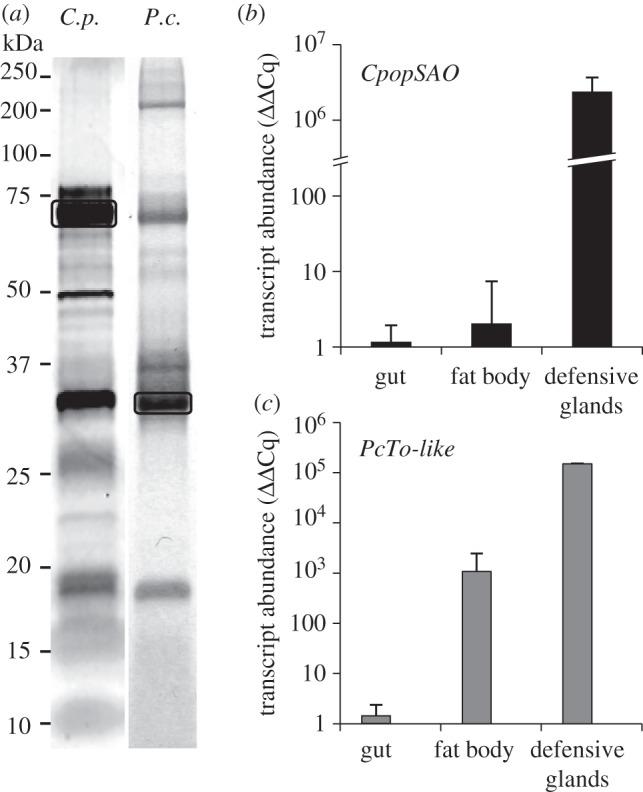
Protein and transcript abundance in juvenile leaf beetles. (a) Proteins in defensive exudates separated by one-dimensional SDS–PAGE. left: 1 mg secretions of C. populi (C.p.), silver stained, box marks CpopSAO; right: 0.65 mg secretions of P. cochleariae (P.c.), Coomassie stained, box marks PcTo-like. (b) Expression pattern of CpopSAO ±s.d. in different C. populi tissues, n = 3. (c) Expression pattern of PcTo-like ±s.d. in different P. cochleariae tissues, n = 2. Both y-axes are in log10 scale.
To induce RNAi in C. populi larvae, we injected 1.0 µg of 1.5 kb CpopSAO dsRNA into late first instar. A 719-bp dsRNA fragment of gfp served as a control for effects caused by dsRNA; although the RNAi machinery will be induced, genes should not be silenced. Furthermore, we included an NIC group in our experiments. By monitoring the developmental traits and the secretion production in C. populi and comparing the results with those from control groups, we found that silencing CpopSAO did not influence either growth rate or pupae weight (see the electronic supplementary material, figure S1a). But the larvae treated with CpopSAO dsRNA produced slightly more secretions than did the larvae of the control groups (see the electronic supplementary material, figure S1b), which might be owing to the different osmotic characteristics between 2 and 3 [12]. Because we did not detect significant differences between NIC and gfp controls in any experiments delineated below, we continue showing only the data of the gfp controls.
Transcript abundance was measured in glandular tissue using qPCR after 1, 3 and 12 days. Comparing tissue from these samples to tissue from the gfp controls, we noticed significant reductions to 7.6 per cent mRNA level (p = 0.002) just 24 h after injection. After day 3, the transcript level was diminished to 1.6 per cent (p = 0.004), and after day 12, to 0.5 per cent (figure 3a).
Figure 3.
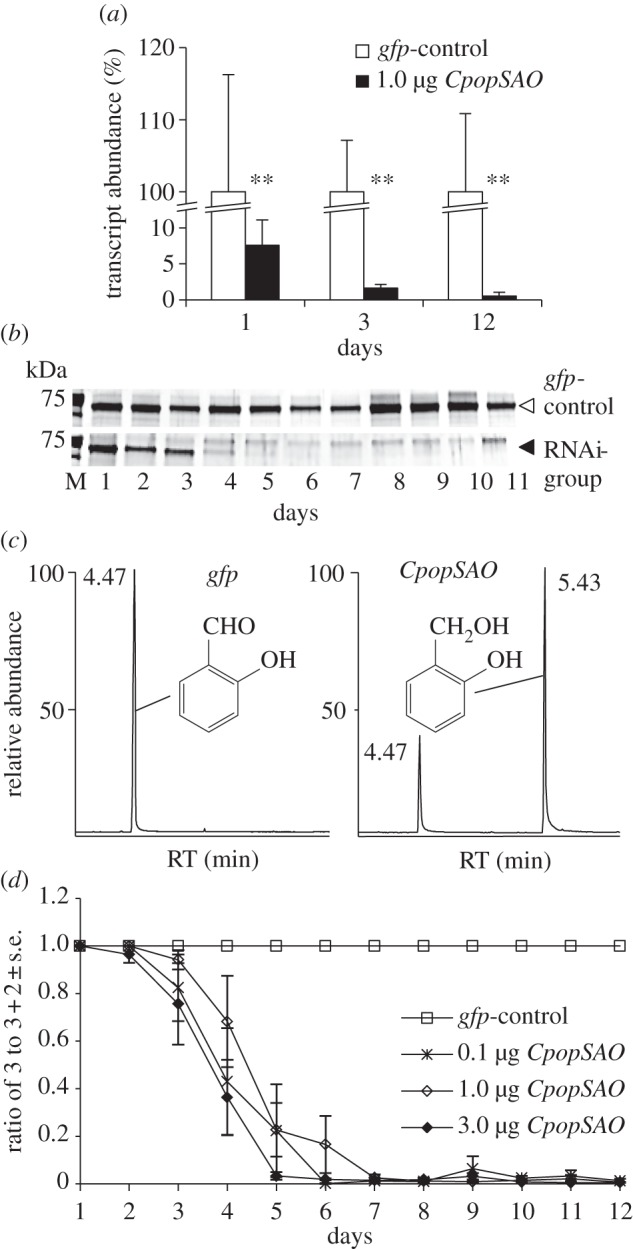
RNAi effects in juvenile C. populi. (a) White bars: transcript abundance of CpopSAO after injecting 1.0 µg gfp dsRNA, n = 3±s.d.. Black bars: transcript abundance of CpopSAO after injecting 1.0 µg CpopSAO dsRNA, n = 3. 100% = ΔCq of gfp control. Asterisks indicate level of significance: **p < 0.01 (b) CpopSAO protein abundance in defence secretions was monitored over time. A total of 0.85 mg secretions per lane was separated on silver-stained SDS gels. The 70-kDa band corresponds to CpopSAO. Secretions originating from control treatment with 1 µg dsRNA of gfp (white arrowhead) and RNAi treatment with 1 µg dsRNA of CpopSAO (black arrowhead) are shown. (c) GC-chromatogram of secretions on day 5 after treatment; left: injecting 1.0 µg gfp dsRNA resulted in the production of 3, right: injecting 1.0 µg CpopSAO dsRNA resulted in the production of 2 and 3. (d) GC-chromatogram peak-area-based plot of secretions after dsRNA injection of gfp and different amounts of CpopSAO (n = 5).
In accordance with the literature, SAO corresponds to the dominant band at 70 kDa in the secretions of C. populi (figure 2a) [12,14]. The composition of the secretome after dsRNA treatment was monitored in a time series in silver-stained one-dimensional SDS gels. Owing to the silencing effect, the quantity of CpopSAO was apparently reduced just 2 days after dsRNA injection and the protein was barely visible after day 5 (figure 3b).
The effects on the biosynthesis of 3 in the defensive secretions were determined by GC/MS analysis. For these experiments, 0.1, 1.0 and 3.0 µg of CpopSAO dsRNA were injected into larvae from the same clutch. As in the protein reduction, we detected 2 in the defensive secretions just 2 days after the injection of 3.0 µg CpopSAO dsRNA (figure 3c). Compound 2 was not detectable in gfp control secretions. In addition, no unexpected chemical compound arose owing to the dsRNA treatments. By setting the peak area of 3 in ratio equalling the sum of the main peak areas, a diagram of the RNAi-dependent reduction of 3 can be plotted (figure 3d). We have tested dsRNA amounts ranging from 0.1 to 3.0 µg. After RNAi induction, significantly less aldehyde was observed for the 3.0 µg CpopSAO group (p = 0.015) on the 4th day and for all tested CpopSAO groups on the 5th day (0.1 µg, p = 0.016; 1 µg, p = 0.002; 3 µg, p =  0.001). Biological variation prevented us from observing dose-dependent RNAi effects in these experiments; the amount of 2 did not differ significantly between the RNAi samples.
0.001). Biological variation prevented us from observing dose-dependent RNAi effects in these experiments; the amount of 2 did not differ significantly between the RNAi samples.
(b). Off-target prediction and validation for CpopSAO
Owing to strong sequence identities, co-silencing non-target genes can cause unintended side-effects [39,40]. Therefore, we performed off-target predictions for the desired dsRNA sequences of CpopSAO and gfp. Predicted off-target genes were validated by qPCR using cDNA derived from successful RNAi experiments. Because of a lack of genome sequences, the potential silencing effects of targeting the nucleus where fragments of the long dsRNA may bind to non-transcribed regulatory sequences [41] or introns [42] could be neither predicted nor validated.
For gfp dsRNA, no critical candidates were detected in the transcriptome library of C. populi. Off-target analyses in the C. populi sequence library, however, identified 25–21 bp contiguous regions of CpopSAO dsRNA that were identical to sequences of eight unique transcripts (see the electronic supplementary material, table ST2 for putative off-target hits). Three of them encode putative proteins having the GMC-oxidoreductase motif in the C-terminal region (CpGMClike I-III) and five were annotated as hypothetical proteins (CpCOMP3092; CpCOMP6024; CpCOMP36289; CpCOMP38777; CpCOMP51471).
CpopSAO shares with CpGMClike-I two similar regions spanning 22 and 25 nucleotides (nts) each; these regions are interrupted by one mismatch (22/1 and 25/1) and, with CpGMClike-III, one similar sequence stretch without mismatch (24/0). CpGMClike-II and the five remaining transcripts possess sequence regions of 22/0 to 20/0 nts identical to CpopSAO. In all tissues, all putative off-target genes exhibited generally low expression levels with relative Cq values median less than 2 × 10−3. qPCR assays were carried out for all eight targets 12 days after larvae were treated with 1.0 µg 1500-bp CpopSAO dsRNA; only for the CpGMClike-I and CpGMClike-II did these treatments reveal significant differences of transcript level in the gut tissue (p = 0.049; p = 0.032). No other tested transcripts showed changes of mRNA abundance in the examined tissues (see the electronic supplementary material, figure S2). Since C. populi larvae transport the plant-derived precursor into the defensive glands for final transformation, we assume that the off-target effects on the putative GMC-oxidoreductases in gut tissue of unknown function do not distort the RNAi effects observed in the secretions.
(c). Identification of unknown proteins in the defence-related secretome of Phaedon cochleariae
After successfully introducing the ‘lack-of-function approach’ to the defensive secretions of C. populi by silencing an enzyme for which we had a clear expectation of the resulting phenotype, we used the method to identify proteins in unknown secretions. For this reason, we chose the larval exudates from the related de novo iridoid-producing species P. cochleariae. We assigned to the abundant 35-kDa band a putative protein whose deduced sequence contains 243 amino acids and a 22 amino acid signal peptide; the existence of such a sequence suggests that the mature protein is secreted (figure 2a). It possesses a conserved domain (pfam06585) characteristic for the juvenile hormone-binding protein (JHBP) superfamily. Sequence comparisons using the BLAST algorithm [35] revealed that the P. cochleariae amino acid sequence shares only very limited identity with functionally characterized insect proteins, for example, 12 per cent identity with the JHBP from Bombyx mori [43] and 16 per cent with the takeout (To) 1 from Epiphyas postvittana [44] (figure 2a). Higher identities up to 25 per cent were found only with insect proteins not yet fully characterized in their functions, such as those with the To-like protein (NP_001191952) from Acyrtosyphon pisum or the JHBP-like (XP_001359416) from Drosophila pseudoobscura pseudoobscura. None of the mentioned insect species is known to produce cyclic monoterpenoids.
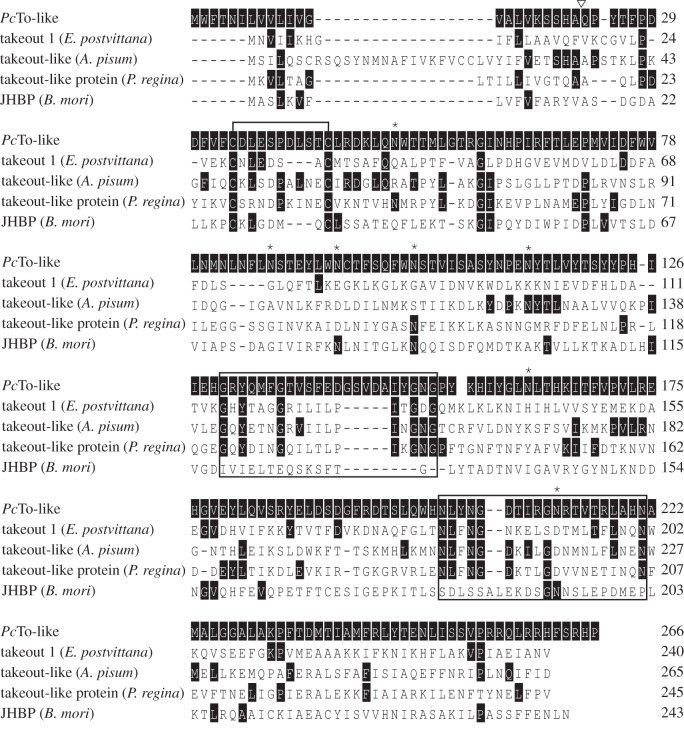
The JHBP superfamily combines both the To protein family and the JHBP family. There are two major differences between the families: one is the number of disulphide bonds (To proteins have one and JHBPs have two) and the second are the conserved C-terminal sequence motives that are only present in To proteins. In silico analyses predicted in the P. cochleariae sequence seven N-glycosylation sites and only one disulphide bond. Along with identifying the two To-specific motives [45] (figure 4) in the C-terminal region, we conclude that our protein can be attributed to the To family. Therefore, we named it PcTo-like.
Figure 4.
Amino acid alignments (ClustalW) of PcTo-like from P. cochleariae and other members from the To/JHBP family (Epiphyas postvittana To1: GeneBank: ACF39401; Acyrthosiphon pisum To-like: GeneBank: XP_001952685; Phormia regina To-like protein: GeneBank: BAD83405; Bombyx mori JHBP: GeneBank: AAF19267). Solid black shading depicts amino acids identical to PcTo-like sequence. White inverted triangles indicate the predicted signal peptide of PcTo-like. Asterisks mark the predicted N-glycosylation sites. Conserved cysteine residues that form disulphide bonds are marked with a bracket above the sequence. The two black boxes show the location of the To-typical motives.
Despite the generally low sequence similarity, most To proteins and JHBPs are classified as ligand-binding proteins for juvenile hormones or similar hydrophobic terpenoids [44,46–49]. Because the precursor of the cyclic iridoid is also a terpenoid, we hypothesize that the putative protein could be involved in the iridoid biosynthesis in the defensive secretions. The assumption that the putative protein has relevance in the defensive glands is corroborated by the high transcript level which has been detected mainly in the glandular tissue of juvenile mustard leaf beetles (figure 2c). Low mRNA levels were also detectable in the fat body tissue.
(d). RNA interference effects in larvae of Phaedon cochleariae
A total of 0.3 µg of dsRNA derived from a 450-bp fragment of the PcTo-like sequence was injected into second instars of P. cochleariae. Transcript quantification 5 days after dsRNA injection confirmed a significant reduction of the mRNA in glandular tissue (p = 0.043) down to 1.0 per cent (±0.9%) compared with mRNA levels in gfp injections (figure 5a).
Figure 5.
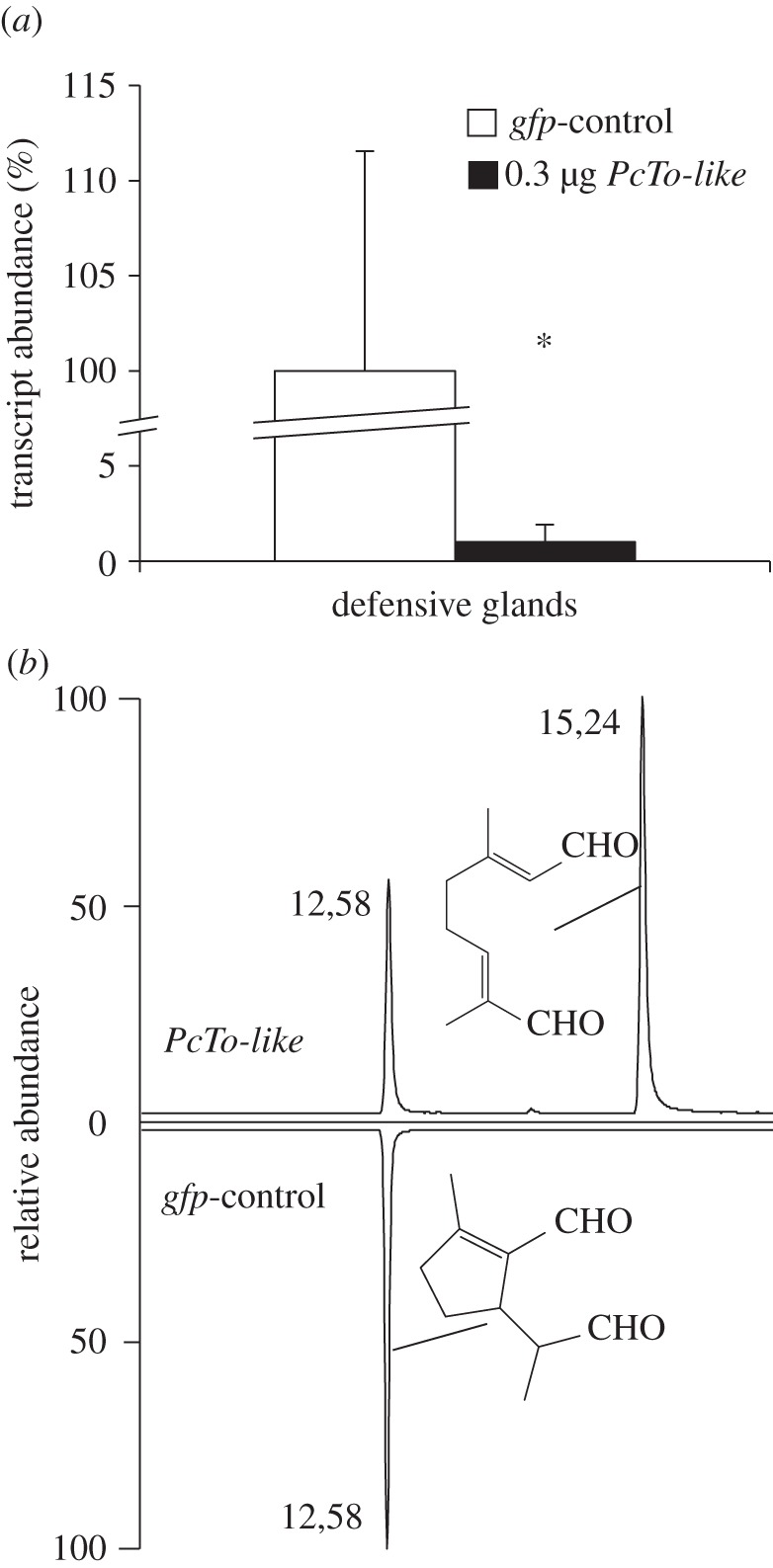
RNAi effects in juvenile P. cochleariae. (a) White bars: transcript abundance of PcTo-like after injecting 0.3 µg gfp dsRNA, n = 3 ±SD. Black bars: transcript abundance of PcTo-like after injecting 0.3 µg PcTo-like dsRNA, n = 3. 100% = ΔCq of gfp-control. Asterisks indicate level of significance: *p < 0.05. (b) GC-chromatogram of diluted secretions on day 5 after treatment, above: injecting 0.3 µg PcTo-like dsRNA resulted in the production of 6 and 7; below: injecting 0.3 µg gfp dsRNA resulted in the production of 7.
Phenotypic analyses after injection of dsRNA on the composition of low-molecular-weight compounds in the secretions were carried out by using GC/MS. The quality of the metabolites in samples collected 1 and 2 days after PcTo-like RNAi induction did not vary from the quality of the metabolites in those collected from gfp controls. In both treatments, we detected only the end-product 7. The first deviation in the composition of the secretions was measured 3 days after dsRNA injection. Only in samples triggered by PcTo-like RNAi did minor amounts of the postulated intermediate 6 in addition to 7 emerge. After 5 days, however, 6 clearly accumulated in addition to 7 owing to the RNAi effect (figure 4b). Therefore, we conclude that the PcTo-like has to be involved in the cyclization of monoterpene precursors into iridoids.
Off-target effects were predicted using the method described for CpopSAO, and predicted off-target effects were avoided from final dsRNA sequence by choosing the template for dsRNA outside of areas of predicted off-target effects.
4. Discussion
The results of our larval RNAi experiments clearly demonstrate selective excision of a component in a biosynthetic pathway. To the best of our knowledge, RNAi has never been used to target enzymes in insect defensive secretions. Owing to the silencing of CpopSAO, the chemical composition in the larval exudates of C. populi was massively altered, starting as early as 48 h after treatment. This shows a distinct function of this enzyme in vivo. Before, Kirsch et al. [13] showed activity only in in vitro assays. Evidently, RNAi is a valuable technique for identifying in vivo relevance for unknown proteins in defensive glands. Although insects contain a large number of exocrine glands in which bioactive compounds are produced, to date few studies have relied on RNAi to provide evidence for the in vivo function of enzymes in insect glands. One example is the production of sex pheromones in special glands of the silkmoth Bombyx mori. By injecting the pupae with dsRNA, Ohnishi et al. [50,51] were able to dissect the components of the biosynthetic pathway as well as assign a function to a transport protein within the glands of adults. Another RNAi target was the production of pheromone in jewel wasps, Nasonia vitripennis. Silencing an epoxide hydrolase in these insects resulted in pheromone reduction by 55 per cent and suppressed the targeted gene transcripts by 95 per cent [52]. Freshly emerged males were injected and 2 days later levels of transcript and pheromones were analysed. As our results demonstrate, RNAi effects are easily detectable in exocrine glands. In the secretions of immature P. cochleariae, we were able to assign in vivo relevance to a cDNA encoding a protein which is important for the cyclization of iridoids. The iridoid pathway in insects was already proposed by using deuterium labelled precursors by Weibel et al. [53]. In his work, the stereospecifity of the cyclization was analysed and allocated to an enzymatic conversion. However, to date JHBPs and To proteins have been established as being carriers of hydrophobic ligands [44,48]. Several lines of evidence indicate that JHBPs form complexes with juvenile hormones (JHs) which provide protection of the chemically labile JHs against nonspecific enzymatic degradation and/or adsorption to lipophilic surfaces during the delivery process from the production site to the target tissue [46,47,49]. Up to now only the crystal structure of To 1 from E. postvittana with ubiquinone provided direct evidence for ligand binding in To proteins [44]. Most of the putative To proteins await elucidation of their mode of action. Therefore, the actual mechanism how PcTo-like acts in the defensive exudates has to be analysed in vitro with purified recombinant protein. On-going experiments will reveal more functional enzymes in Chrysomelina and clarify the molecular machinery for the biosynthesis of deterrents in larval defence secretions.
To perform RNAi experiments, it is essential to ensure the specificity. Off-target effects can arise when siRNAs diced from long dsRNA fragments possess sufficient sequence similarity to non-target mRNA and thus triggering degradation of similar sequences [39]. For sequenced organisms, genome-scale off-target prediction programs are available [34]. These approaches are not suitable for organisms whose genomes have yet to be sequenced. In the last few years, several approaches have been used to detect off targets for those species, such as screening for target specificity by rapidly amplifying cDNA ends [54]. Another approach has used microarrays to compare the cDNAs from treated groups with those from non-treated groups; such comparisons offer proof of differentially expressed transcripts via qPCR [55]. Transcriptome sequences have rarely been used for approaches based on local alignment algorithms but represent an economical way to make off-target predictions [56]. In our case, we showed that in silico dicing of long dsRNA pieces to 21-bp fragments and subsequent BLASTn searches in our transcriptome libraries also lead to the identification of putative off-target transcripts. Subsequent qPCR analysis after successful RNAi induction revealed the co-silencing of predicted transcripts in C. populi. Two of eight mRNAs were significantly altered in gut tissue (see the electronic supplementary material, figure S2). But the observed off-target silencing could be assigned neither to the length of the fragments nor to the amount of pmol of the putative siRNAs (see the electronic supplementary material, table ST2). Furthermore, the composition and internal stability of the sequence fragments are supposed to have an impact on successful RNAi triggering [57] and could be included in the prediction. Although publications concerning off-target prediction have increased in the last 2 years, as yet no standard method is available. But as our results indicate, off-target validation is crucial for a realistic discussion of RNAi effects.
Acknowledgements
The authors would like to express their gratitude to Heiko Vogel for making available 454-sequences from P. cochleariae. We also gratefully acknowledge Angelika Berg, Kerstin Ploss, Gerhard Pauls and Antje Loele for technical assistance. We thank Gregor Bucher for helpful discussions on aspects of this work. Very special thanks are due to Prof. Jacques M. Pasteels and Emily Wheeler for their critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (BU 1862/2-1) and the Max Planck Society.
R.R.B., P.R., M.S., M.K., N.W., W.B. and A.B. designed study. R.R.B. established RNAi in leaf beetles and performed RNAi treatments of CpopSAO and its control treatments, performed off-target validation, collected all corresponding data except the off-target prediction and analysed output data. P.R. identified PcTo-like, performed RNAi treatments of PcTo-like and control treatments, collected all corresponding data and analysed output data. S.F., M.S. and M.G. generated transcriptome libraries. M.S. established and performed off-target prediction and contributed to interpretation of LC/MSE output data. M.K. designed GC/MS assays, synthesized 6 and 7 and contributed to interpretation of output data. N.W. performed LC/MSE analysis, collected and contributed to interpretation of output data. W.B. and A.B. contributed substantially to interpretation of all output data. R.R.B., P.R. and A.B. wrote first draft of the manuscript, and all authors contributed substantially to revisions.
References
- 1.Quennedey A. 1998. Insect epidermal gland cells: ultrastructure and morphogenesis. In Microscopic anatomy of invertebrates, vol. 11A: insecta (eds Harrison W. H., Locke M.) pp. 177–207. New York: Wiley-Liss [Google Scholar]
- 2.Dettner K. 1987. Chemosystematics and evolution of beetle chemical defenses. Annu. Rev. Entomol. 32, 17–48 10.1146/annurev.en.32.010187.000313 (doi:10.1146/annurev.en.32.010187.000313) [DOI] [Google Scholar]
- 3.Pasteels J. M., Gregoire J. C., Rowell-Rahier M. 1983. The chemical ecology of defense in arthropods. Annu. Rev. Entomol. 28, 263–289 10.1146/annurev.en.28.010183.001403 (doi:10.1146/annurev.en.28.010183.001403) [DOI] [Google Scholar]
- 4.Burse A., Frick S., Discher S., Tolzin-Banasch K., Kirsch R., Strauss A., Kunert M., Boland W. 2009. Always being well prepared for defense: the production of deterrents by juvenile Chrysomelina beetles (Chrysomelidae). Phytochemistry 70, 1899–1909 10.1016/j.phytochem.2009.08.002 (doi:10.1016/j.phytochem.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 5.Duffey S. S. 1980. Sequestration of plant natural-products by insects. Annu. Rev. Entomol. 25, 447–477 10.1146/annurev.en.25.010180.002311 (doi:10.1146/annurev.en.25.010180.002311) [DOI] [Google Scholar]
- 6.Opitz S. E. W., Mueller C. 2009. Plant chemistry and insect sequestration. Chemoecology 19, 117–154 10.1007/s00049-009-0018-6 (doi:10.1007/s00049-009-0018-6) [DOI] [Google Scholar]
- 7.Laurent P., Braekman J. C., Daloze D., Pasteels J. 2003. Biosynthesis of defensive compounds from beetles and ants. Eur. J. Org. Chem. 2733–2743 10.1002/ejoc.200300008 (doi:10.1002/ejoc.200300008) [DOI] [Google Scholar]
- 8.Hinton H. E. 1951. On a little-known protective device of some chrysomelid pupae (Coleoptera). Proc. R. Entomol. Soc. A 26, 67–73 10.1111/j.1365-3032.1951.tb00123.x (doi:10.1111/j.1365-3032.1951.tb00123.x) [DOI] [Google Scholar]
- 9.Pasteels J. M., Rowell-Rahier M. 1989. Defensive glands and secretions as taxonomical tools in the Chrysomelidae. Entomography 6, 423–432 [Google Scholar]
- 10.Pasteels J. M., Daloze D., Rowell-Rahier M. 1986. Chemical defense in chrysomelid eggs and neonate larvae. Physiol. Entomol. 11, 29–37 10.1111/j.1365-3032.1986.tb00388.x (doi:10.1111/j.1365-3032.1986.tb00388.x) [DOI] [Google Scholar]
- 11.Pasteels J. M., Rowell-Rahier M., Braekman J. C., Dupont A. 1983. Salicin from host plant as precursor of salicyl aldehyde in defensive secretion of chrysomeline larvae. Physiol. Entomol. 8, 307–314 [Google Scholar]
- 12.Brueckmann M., Termonia A., Pasteels J. M., Hartmann T. 2002. Characterization of an extracellular salicyl alcohol oxidase from larval defensive secretions of Chrysomela populi and Phratora vitellinae (Chrysomelina). Insect Biochem. Mol. Biol. 32, 1517–1523 10.1016/S0965-1748(02)00072-3 (doi:10.1016/S0965-1748(02)00072-3) [DOI] [PubMed] [Google Scholar]
- 13.Kirsch R., Vogel H., Muck A., Reichwald K., Pasteels J. M., Boland W. 2011. Host plant shifts affect a major defense enzyme in Chrysomela lapponica. Proc. Natl Acad. Sci. USA 108, 4897–4901 10.1073/pnas.1013846108 (doi:10.1073/pnas.1013846108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalski C., Mohagheghi H., Nimtz M., Pasteels J. M., Ober D. 2008. Salicyl alcohol oxidase of the chemical defense secretion of two chrysomelid leaf beetles—molecular and functional characterization of two new members of the glucose–methanol–choline oxidoreductase gene family. J. Biol. Chem. 283, 19 219–19 228 10.1074/jbc.M802236200 (doi:10.1074/jbc.M802236200) [DOI] [PubMed] [Google Scholar]
- 15.Wallace J. B., Blum M. S. 1969. Refined defensive mechanisms in Chrysomela scripta. Ann. Entomol. Soc. Am. 62, 503–506 [Google Scholar]
- 16.Gross J., Podsiadlowski L., Hilker M. 2002. Antimicrobial activity of exocrine glandular secretion of Chrysomela larvae. J. Chem. Ecol. 28, 317–331 10.1023/a:1017934124650 (doi:10.1023/a:1017934124650) [DOI] [PubMed] [Google Scholar]
- 17.Rowell-Rahier M., Pasteels J. M. 1986. Economics of chemical defense in Chrysomelinae. J. Chem. Ecol. 12, 1189–1203 10.1007/bf01639004 (doi:10.1007/bf01639004) [DOI] [PubMed] [Google Scholar]
- 18.Kuhn J., Pettersson E. M., Feld B. K., Burse A., Termonia A., Pasteels J. M., Boland W. 2004. Selective transport systems mediate sequestration of plant glucosides in leaf beetles: a molecular basis for adaptation and evolution. Proc. Natl Acad. Sci. USA 101, 13 808–13 813 10.1073/pnas.0402576101 (doi:10.1073/pnas.0402576101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Discher S., Burse A., Tolzin-Banasch K., Heinemann S. H., Pasteels J. M., Boland W. 2009. A versatile transport network for sequestering and excreting plant glycosides in leaf beetles provides an evolutionary flexible defense strategy. ChemBioChem 10, 2223–2229 10.1002/cbic.200900226 (doi:10.1002/cbic.200900226) [DOI] [PubMed] [Google Scholar]
- 20.Pasteels J. M., Duffey S., Rowell-Rahier M. 1990. Toxins in chrysomelid beetles possible evolutionary sequence from de novo synthesis to derivation from food-plant chemicals. J. Chem. Ecol. 16, 211–222 10.1007/bf01021280 (doi:10.1007/bf01021280) [DOI] [PubMed] [Google Scholar]
- 21.Termonia A., Hsiao T. H., Pasteels J. M., Milinkovitch M. C. 2001. Feeding specialization and host-derived chemical defense in Chrysomeline leaf beetles did not lead to an evolutionary dead end. Proc. Natl Acad. Sci. USA 98, 3909–3914 10.1073/pnas.061034598 (doi:10.1073/pnas.061034598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daloze D., Pasteels J. M. 1994. Isolation of 8-hydroxygeraniol-8-O-beta-d-glucoside, a probable intermediate in biosynthesis of iridoid monoterpenes, from defensive secretions of Plagiodera versicolora and Gastrophysa viridula (Coleoptera, Chrysomelidae). J. Chem. Ecol. 20, 2089–2097 10.1007/BF02066245 (doi:10.1007/BF02066245) [DOI] [PubMed] [Google Scholar]
- 23.Soetens P., Pasteels J. M., Daloze D. 1993. A simple method for in vivo testing of glandular enzymatic activity on potential precursors of larval defensive compounds in Phratora species (Coleoptera, Chrysomelinae). Experientia 49, 1024–1026 10.1007/BF02125653 (doi:10.1007/BF02125653) [DOI] [Google Scholar]
- 24.Veith M., Oldham N. J., Dettner K., Pasteels J. M., Boland W. 1997. Biosynthesis of defensive allomones in leaf beetle larvae—stereochemistry of salicylalcohol oxidation in Phratora vitellinae and comparison of enzyme substrate and stereospecificity with alcohol oxidases from several iridoid producing leaf beetles. J. Chem. Ecol. 23, 429–443 10.1023/B:JOEC.0000006369.26490.c6 (doi:10.1023/B:JOEC.0000006369.26490.c6) [DOI] [Google Scholar]
- 25.Veith M., Lorenz M., Boland W., Simon H., Dettner K. 1994. Biosynthesis of iridoid monoterpenes in insects—defensive secretions from larvae of leaf beetles (Coleoptera, Chrysomelidae). Tetrahedron 50, 6859–6874 10.1016/S0040-4020(01)81338-7 (doi:10.1016/S0040-4020(01)81338-7) [DOI] [Google Scholar]
- 26.Kirsch R., Vogel H., Muck A., Vilcinskas A., Pasteels J. M., Boland W. 2011. To be or not to be convergent in salicin-based defence in chrysomeline leaf beetle larvae: evidence from Phratora vitellinae salicyl alcohol oxidase. Proc. R. Soc. B 278, 3225–3232 10.1098/rspb.2011.0175 (doi:10.1098/rspb.2011.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding S.-W., Li H., Lu R., Li F., Li W.-X. 2004. RNA silencing: a conserved antiviral immunity of plants and animals. Virus Res. 102, 109–115 10.1016/j.virusres.2004.01.021 (doi:10.1016/j.virusres.2004.01.021) [DOI] [PubMed] [Google Scholar]
- 28.Fire A., Xu S. Q., Montgomery M. K., Kostas S. A., Driver S. E., Mello C. C. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 10.1038/35888 (doi:10.1038/35888) [DOI] [PubMed] [Google Scholar]
- 29.Belles X. 2010. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu. Rev. Entomol. 55, 111–128 10.1146/annurev-ento-112408-085301 (doi:10.1146/annurev-ento-112408-085301) [DOI] [PubMed] [Google Scholar]
- 30.Mito T., Nakamura T., Bando T., Ohuchi H., Noji S. 2011. The advent of RNA interference in entomology. Entomol. Sci. 14, 1–8 10.1111/j.1479-8298.2010.00408.x (doi:10.1111/j.1479-8298.2010.00408.x) [DOI] [Google Scholar]
- 31.Terenius O., et al. 2011. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 57, 231–245 10.1016/j.jinsphys.2010.11.006 (doi:10.1016/j.jinsphys.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 32.Szabo L. F. 2008. Rigorous biogenetic network for a group of indole alkaloids derived from strictosidine. Molecules 13, 1875–1896 10.3390/molecules13081875 (doi:10.3390/molecules13081875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhnle A., Muller C. 2011. Responses of an oligophagous beetle species to rearing for several generations on alternative host–plant species. Ecol. Entomol. 36, 125–134 10.1111/j.1365-2311.2010.01256.x (doi:10.1111/j.1365-2311.2010.01256.x) [DOI] [Google Scholar]
- 34.Naito Y., Yamada T., Matsumiya T., Ui-Tei K., Saigo K., Morishita S. 2005. dsCheck: highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res. 33, W589–W591 10.1093/nar/gki419 (doi:10.1093/nar/gki419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul S. F., Madden T. L., Schaeffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 10.1093/nar/25.17.3389 (doi:10.1093/nar/25.17.3389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldham N. J., Veith M., Boland W., Dettner K. 1996. Iridoid monoterpene biosynthesis in insects—evidence for a de novo pathway occurring in the defensive glands of Phaedon armoraciae (Chrysomelidae) leaf beetle larvae. Naturwissenschaften 83, 470–473 10.1007/BF01144016 (doi:10.1007/BF01144016) [DOI] [Google Scholar]
- 37.Uesato S., Ogawa Y., Doi M., Inouye H. 1987. Biomimetic synthesis of (±)-chrysomelidial, (±)-dehydroiridoidal, and (±)-iridoidal. J. Chem. Soc.-Chem. Commun. 1020–1021 10.1039/c39870001020 (doi:10.1039/c39870001020) [DOI] [Google Scholar]
- 38.Cavener D. R. 1992. GMC oxidoreductases: a newly defined family of homologous proteins with diverse catalytic activities. J. Mol. Biol. 223, 811–814 10.1016/0022-2836(92)90992-s (doi:10.1016/0022-2836(92)90992-s) [DOI] [PubMed] [Google Scholar]
- 39.Jackson A. L., Bartz S. R., Schelter J., Kobayashi S. V., Burchard J., Mao M., Li B., Cavet G., Linsley P. S. 2003. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 21, 635–637 10.1038/nbt831 (doi:10.1038/nbt831) [DOI] [PubMed] [Google Scholar]
- 40.Seinen E., Burgerhof J. G. M., Jansen R. C., Sibon O. C. M. 2011. RNAi-induced off-target effects in Drosophila melanogaster: frequencies and solutions. Brief. Funct. Genom. 10, 206–214 10.1093/bfgp/elr017 (doi:10.1093/bfgp/elr017) [DOI] [PubMed] [Google Scholar]
- 41.Morris K. V., Chan S. W. L., Jacobsen S. E., Looney D. J. 2004. Small interfering RNA-induced transcriptional gene silencing in human cells. Science 305, 1289–1292 10.1126/science.1101372 (doi:10.1126/science.1101372) [DOI] [PubMed] [Google Scholar]
- 42.Bosher J. M., Dufourcq P., Sookhareea S., Labouesse M. 1999. RNA interference can target pre-mRNA: consequences for gene expression in a Caenorhabditis elegans operon. Genetics 153, 1245–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermunt A. M. W., Kamimura M., Hirai M., Kiuchi M., Shiotsuki T. 2001. The juvenile hormone binding protein of silkworm haemolymph: gene and functional analysis. Insect Mol. Biol. 10, 147–154 10.1046/j.1365-2583.2001.00249.x (doi:10.1046/j.1365-2583.2001.00249.x) [DOI] [PubMed] [Google Scholar]
- 44.Hamiaux C., Stanley D., Greenwood D. R., Baker E. N., Newcomb R. D. 2009. Crystal structure of Epiphyas postvittana takeout 1 with bound ubiquinone supports a role as ligand carriers for takeout proteins in insects. J. Biol. Chem. 284, 3496–3503 10.1074/jbc.M807467200 (doi:10.1074/jbc.M807467200) [DOI] [PubMed] [Google Scholar]
- 45.So W. V., Sarov-Blat L., Kotarski C. K., McDonald M. J., Allada R., Rosbash M. 2000. Takeout, a novel Drosophila gene under circadian clock transcriptional regulation. Mol. Cell. Biol. 20, 6935. 10.1128/MCB.20.18.6935-6944.2000 (doi:10.1128/MCB.20.18.6935-6944.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.deKort C. A. D., Granger N. A. 1996. Regulation of JH titers: the relevance of degradative enzymes and binding proteins. Arch. Insect Biochem. Physiol. 33, 1–26 (doi:10.1002/(sici)1520-6327(1996)33:1<1::aid-arch1>3.0.co;2-2) [DOI] [Google Scholar]
- 47.Prestwich G. D., Wojtasek H., Lentz A. J., Rabinovich J. M. 1996. Biochemistry of proteins that bind and metabolize juvenile hormones. Arch. Insect Biochem. Physiol. 32, 407–419 (doi:10.1002/(sici)1520-6327(1996)32:3/4<407::aid-arch13>3.0.co;2-g) [DOI] [PubMed] [Google Scholar]
- 48.Suzuki R., Fujimoto Z., Shiotsuki T., Tsuchiya W., Momma M., Tase A., Miyazawa M., Yamazaki T. 2011. Structural mechanism of JH delivery in hemolymph by JHBP of silkworm, Bombyx mori. Sci. Rep. 1, 133. 10.1038/srep00133 (doi:10.1038/srep00133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winiarska B., Dwornik A., Debski J., Grzelak K., Bystranowska D., Zalewska M., Dadlez M., Ozyhar A., Kochman M. 2011. N-linked glycosylation of G. mellonella juvenile hormone binding protein—Comparison of recombinant mutants expressed in P. pastoris cells with native protein. Biochim. Biophys. Acta: Proteins Proteom. 1814, 610–621 10.1016/j.bbapap.2011.02.002 (doi:10.1016/j.bbapap.2011.02.002) [DOI] [PubMed] [Google Scholar]
- 50.Ohnishi A., Hashimoto K., Imai K., Matsumoto S. 2009. Functional characterization of the Bombyx mori fatty acid transport protein (BmFATP) within the silkmoth pheromone gland. J. Biol. Chem. 284, 5128–5136 10.1074/jbc.M806072200 (doi:10.1074/jbc.M806072200) [DOI] [PubMed] [Google Scholar]
- 51.Ohnishi A., Hull J. J., Matsumoto S. 2006. Targeted disruption of genes in the Bombyx mori sex pheromone biosynthetic pathway. Proc. Natl Acad. Sci. USA 103, 4398–4403 10.1073/pnas.0511270103 (doi:10.1073/pnas.0511270103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Latief M., Garbe L. A., Koch M., Ruther J. 2008. An epoxide hydrolase involved in the biosynthesis of an insect sex attractant and its use to localize the production site. Proc. Natl Acad. Sci. USA 105, 8914–8919 10.1073/pnas.0801559105 (doi:10.1073/pnas.0801559105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weibel D. B., Oldham N. J., Feld B., Glombitza G., Dettner K., Boland W. 2001. Iridoid biosynthesis in staphylinid rove beetles (Coleoptera : Staphylinidae, Philonthinae). Insect Biochem. Mol. Biol. 31, 583–591 10.1016/s0965-1748(00)00163-6 (doi:10.1016/s0965-1748(00)00163-6) [DOI] [PubMed] [Google Scholar]
- 54.Sabirzhanov B., Sabirzhanova I. B., Keifer J. 2011. Screening target specificity of siRNAs by rapid amplification of cDNA ends (RACE) for non-sequenced species. J. Mol. Neurosci. 44, 68–75 10.1007/s12031-011-9514-6 (doi:10.1007/s12031-011-9514-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lew-Tabor A. E., Kurscheid S., Barrero R., Gondro C., Moolhuijzen P. M., Valle M. R., Morgan J. A. T., Covacin C., Bellgard M. I. 2011. Gene expression evidence for off-target effects caused by RNA interference-mediated gene silencing of Ubiquitin-63E in the cattle tick Rhipicephalus microplus. Int. J. Parasitol. 41, 1001–1014 10.1016/j.ijpara.2011.05.003 (doi:10.1016/j.ijpara.2011.05.003) [DOI] [PubMed] [Google Scholar]
- 56.Qiu S., Adema C. M., Lane T. 2005. A computational study of off-target effects of RNA interference. Nucleic Acids Res. 33, 1834–1847 10.1093/nar/gki324 (doi:10.1093/nar/gki324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. 2004. Rational siRNA design for RNA interference. Nat. Biotechnol. 22, 326–330 10.1038/nbt936 (doi:10.1038/nbt936) [DOI] [PubMed] [Google Scholar]