Abstract
Males and females have different routes to successful reproduction, resulting in sex differences in lifespan and age-specific allocation of reproductive effort. The trade-off between current and future reproduction is often resolved differently by males and females, and both sexes can be constrained in their ability to reach their sex-specific optima owing to intralocus sexual conflict. Such genetic antagonism may have profound implications for evolution, but its role in ageing and lifespan remains unresolved. We provide direct experimental evidence that males live longer and females live shorter than necessary to maximize their relative fitness in Callosobruchus maculatus seed beetles. Using artificial selection in a genetically heterogeneous population, we created replicate long-life lines where males lived on average 27 per cent longer than in short-life lines. As predicted by theory, subsequent assays revealed that upward selection on male lifespan decreased relative male fitness but increased relative female fitness compared with downward selection. Thus, we demonstrate that lifespan-extending genes can help one sex while harming the other. Our results show that sexual antagonism constrains adaptive life-history evolution, support a novel way of maintaining genetic variation for lifespan and argue for better integration of sex effects into applied research programmes aimed at lifespan extension.
Keywords: ageing, life-history evolution, sexual conflict, sex-specific selection
1. Introduction
Sex differences in reproductive strategies are ultimately rooted in anisogamy [1] and the resulting Bateman's principle [2,3], which states that females are typically limited by the number of eggs they can produce, while males are limited by the number of mates they can fertilize. Theory maintains that sex-specific selection will optimize the fundamental trade-off between lifespan and reproduction differently for males and females [4–7], leading to ubiquitous dimorphism in lifespan and ageing rates across the animal kingdom [8–11], including humans [12]. However, recent advances in evolutionary biology strongly suggest that such optimization can be hard to attain in sexually reproducing organisms owing to genetic conflicts within the genome.
While the sexes share most of their genome, they represent dramatically different environments for the expression of alleles affecting lifespan. Because the intersexual genetic correlation between homologous traits is often high [13], sexually antagonistic selection on different loci can perpetually impede the sexes from reaching their phenotypic optima [14,15]. This evolutionary ‘tug-of-war’ is known as intralocus sexual conflict (ISC) and has been shown to play a key role in male–female coevolution [16–19]. Crucially, recent studies have demonstrated high levels of sexually antagonistic selection in sexually dimorphic traits [20], indicating that ISC is not easily resolved by the evolution of sexual dimorphism. Taken together, these findings provide a strong theoretical basis for the role of ISC in preventing the sexes from reaching their fitness optima in lifespan and ageing rates [6,7,21]. Nevertheless, quantitative genetic studies of ISC over lifespan and ageing have provided conflicting results [22,23] and this theory has never been supported experimentally [24], despite its fundamental importance for evolutionary and biomedical research.
Here we show that males live longer and females live shorter than their sex-specific optima, using artificial selection in a large genetically heterogeneous population of the seed beetle Callosobruchus maculatus. This species is an excellent model system for this analysis because it exhibits large sexual dimorphism in lifespan and ageing rates [25–27], and there is strong evidence for sexually divergent selection on these traits [11]. It is likely that the trade-offs between reproduction and somatic maintenance are resolved differently by the sexes, such that male C. maculatus maximize their fitness by high reproductive investment early in life (‘live-fast-die-young’ strategy), while females optimize their reproductive output by conserving energy for future reproduction [11,28].
To test this hypothesis, we first selected directly on male lifespan for five generations to create replicate ‘long-life’ lines where males lived on average 27 per cent longer than ‘short-life’ lines. For each generation, offspring were selected from the 25 per cent of males that lived the longest (upward selection for ‘long-life’ lines) or shortest (downward selection for ‘short-life’ lines). Using these lines, we then tested two key predictions: (i) females from upward-selected lines should live longer than females from downward-selected lines because of a positive intersexual genetic correlation for lifespan; and (ii) males from upward-selected lines should have lower fitness than males from downward-selected lines, while the opposite should be true for females. Our results corroborate both predictions, thereby providing direct experimental evidence that sexes are prevented from reaching their fitness optima for lifespan.
2. Methods
(a). Study system
We derived our selection lines from a heterogeneous south Indian stock population (‘SI USA’) of C. maculatus obtained from C. W. Fox at the University of Kentucky, USA. The population originated from infested mung beans (Vigna radiata) collected from Tirunelveli, India, in 1979 [29]. At the time of the experiment, this population had been kept in our laboratory for more than 80 generations. Both prior to and during the experiment, the beetles were cultured on mung beans and kept in climate chambers at 30°C, 50 per cent relative humidity and a 14 L : 10 D cycle. Females lay their eggs on the surface of the bean. Once the larvae hatch, they bore into the surface of the bean and complete their development there, emerging as fully functional adults after 23–27 days. Callosobruchus maculatus are facultatively aphagous capital breeders and this population has always been maintained at aphagy (i.e. the beetles obtain all of the resources needed for survival and reproduction as adults during their larval stage) [30]. One of the main strengths of this system is that the laboratory environment represents a close approximation of the natural conditions for this beetle [31].
(b). Selection procedure
First, we randomly selected 200 fertilized females from the base population. To reduce larval competition, we provided the females with sufficient laying substrate to ensure that they laid one egg per bean. When their young hatched, we randomly paired 800 virgin males with 800 virgin females, divided into four groups of 200 pairs each. After pairing, every day for 3 days, the females were swapped between different dishes, such that each male had the opportunity to cohabit and mate with a total of three different females. This was done to reduce the variance caused by differences in female condition or behaviour on male lifespan. However, after the first day, the fertilized eggs were set aside so that only the offspring from the first pairing were used in subsequent generations. This was to ensure that all of the offspring were sired by that male and not from sperm carried over from previous males. Within each group of 200 pairs, we selected two male and two female offspring from the 30 males that lived the longest, and paired them randomly to create ‘long-life’ lines (n = 4). In the same fashion, we selected offspring from the 30 males that lived the shortest to create ‘short-life’ lines (n = 4). In the F2 generation, from each of these eight lines, we selected four male and four female offspring from the 15 males that lived the longest (for long-life lines) and the 15 males that lived the shortest (for short-life lines), for a total of 60 pairs per line. We continued the same selection process (four male and four female offspring from the 15 top and bottom males) for an additional four generations, for a total of five generations (figure 1). We note that lifespan appears to go down in generations two and three in both selection regimes. Such fluctuations between generations are very common in selection experiments and may result from slight variation in ambient conditions. Since climatic conditions are tightly controlled in our system, we suggest that such fluctuations may result from slight variation in host seeds from one generation to the next.
Figure 1.
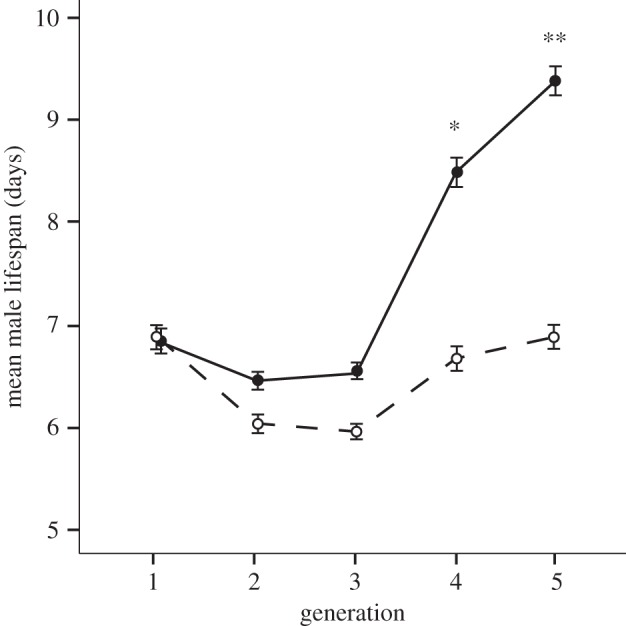
Mean lifespan ± s.e. of male seed beetles from short-life (dashed line with open circles) and long-life (solid line with filled circles) selection lines across five generations of selection. Starting in generation 4, males from long-life lines lived significantly longer than males from short-life lines (analyses of variance: F1,6 = 11.930, p = 0.014; raw data: mean ± s.e. = 8.49 ± 0.45 days for long-life versus 6.67 ± 0.34 days for short-life). This difference increased by generation 5 (analyses of variance: F1,6 = 19.335, p = 0.005; raw data: mean ± s.e. = 9.38 ± 0.19 days for long-life versus 6.88 ± 0.49 days for short-life). A single asterisk indicates significance at p < 0.05 and two asterisks indicate significance at p < 0.01.
During each generation of the experiment, fertilized eggs were isolated in individual virgin chambers (aerated plastic containers with a separate well for each individual) prior to hatching. Adults were approximately 1 day old when paired. Sib mating was excluded to maximize outbreeding. Paired individuals were placed in 60 mm Petri dishes filled with 75 mung beans. Since these females could lay up to 65 eggs per day (E. C. Berg 2010, unpublished data), we provided enough beans so that only one egg would be laid on each bean, thus eliminating the confounding effects of larval competition. To reduce variation in male mating opportunities owing to potential female mortality, all females were removed on day 4. Males were monitored daily, and the date of death was recorded.
In addition to the selection lines, beetles from the base population were maintained for use in the fitness assays. These beetles were kept in jars with 250 g mung beans, and allowed to mate and lay eggs at will. When the young hatched, on day 25, approximately 200 individuals were randomly selected and placed in a new jar with beans, and the cycle continued.
(c). Fitness and lifespan assays
Once divergent lines were established, we conducted longevity and lifetime reproductive success (LRS) assays for males and females from all experimental lines. Within each line, assays were conducted using the offspring of randomly selected individuals from the fifth generation of artificially selected beetles. These beetles were paired with individuals from the base population from which the selection lines were originally derived. All beetles were approximately 1 day old when paired. We performed three assays: (i) male fitness assay, (ii) male longevity assay, and (iii) female fitness and longevity assay. Male fitness and male longevity assays were done separately to ensure that male longevity was scored in exactly the same way as during the selection process. For female fitness and longevity assays, this was not necessary (see later).
Male fitness was measured as total number of offspring surviving to adulthood produced by focal males from experimental lines in competition with other males. This inclusive measurement incorporates all possible sources of variation in male reproductive success, including pre-copulatory and post-copulatory sexual selection, as well as viability selection. Focal males compete with background males who are sterile but active and mating with females, who then produce sterile eggs. This method has been successfully used in previous studies [32,33]. This is a particularly good way to measure fitness because males are assayed in the environment in which they evolved for at least hundreds (but most probably thousands) of generations prior to our study (i.e. the environment that represents their recent evolutionary history).
Specifically, for the male fitness assay, we created 20 replicate subpopulations for each population. For each subpopulation, we randomly selected two males from the selection line and placed them in 90 mm Petri dishes with three females from the base population, four tester sterile males from the base population and 450 mung beans. The ratio of focal versus background (base population) males, as well as sex ratio, was skewed in order to create a more competitive environment for focal males, which is consistent with their protandrous behaviour [28]. After hatch, virgin males from the base population were sterilized by irradiation with the dosage of 100 Gy using a caesium-137 source at the Division of Biomedical Radiation Sciences, Uppsala University. These sterile males were included to provide a competitive mating environment for the two focal males. All nine individuals were left in the Petri dish until they died and the offspring hatched (day 36 after pairing). Dishes were immediately frozen to kill the beetles, and hatched offspring were then counted.
In the male longevity assay, one male from each selection line (n = 30 males per line) was paired with one female from the base population and placed in a 60 mm Petri dish with 150 beans. For 3 days, females were swapped among dishes, exactly as during the artificial selection experiment. Females were removed on day 4, and male survival was monitored daily.
Finally, in the female fitness and longevity assay, both LRS and lifespan were recorded simultaneously in the same female. One female from each selection line (n = 30 females per line) was placed in a 60 mm Petri dish with a single male from the base population and 150 beans, which is sufficient to allow for laying only one egg per bean. After pairing, every day for 3 days, each male was moved to a different dish (within each line) to allow each female to mate with a total of three different males. Similar to the selection experiment, this was done to minimize differences in male quality or behaviour across females. Males were removed on day 4. Females were monitored daily to measure longevity. We scored the realized fecundity—the total number of eggs laid by a female during her lifetime—as a measure of LRS, which is the standard measure of female reproductive performance in this system.
(d). Statistical analyses
All data were analysed using SPSS 19.0 (SPSS, Inc., Chicago, IL) and JMP 9.0 (SAS Institute, Inc., Cary, NC). Two-tailed t-tests were used to test for differences in male and female longevity among long- and short-life selection treatments. All longevity data were power- and ln-transformed prior to analysis (ln[lifespan]0.3), and Levene's tests for equality of variances were performed for each test. The data used in the figures, however, are presented as untransformed values. Means are given ±1 s.e. throughout. LRS data were analysed using a general linear mixed model that included sex, selection treatment and the sex × selection treatment interaction as fixed factors, and replicate population as a random factor nested within selection treatment. Prior to inclusion in the model, LRS data were variance-standardized within sex by subtracting the mean fitness value from individual raw values and then dividing the difference by the standard deviation [34,35]. We note that the interaction is also significant when we do not standardize the data at all.
3. Results and discussion
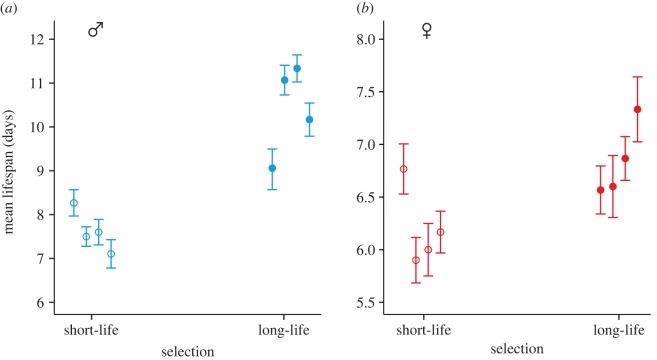
We measured longevity of males and females from all eight lines (n = 30 per sex per line) by pairing the focal beetles with the opposite-sex beetles from the tester base population and monitoring the beetles daily. There was a significant positive intersexual correlation for lifespan across the eight lines (Pearson's r = 0.759, p = 0.029; figure 2). As expected, males from long-life lines lived significantly longer than males from short-life lines (ANOVA on power-transformed ln-lifespan: F1,6 = 27.139, p = 0.002; raw data: mean ± s.e. = 10.4 ± 0.52 days for long-life versus 7.6 ± 0.24 days for short-life; figure 3a). Females from long-life lines also lived longer than females from short-life lines, although the increase in lifespan was relatively smaller than in males (ANOVA on power-transformed ln-lifespan values: F1,6 = 6.008, p = 0.0488; raw data: mean ± s.e. = 6.8 ± 0.18 days for long-life versus 6.2 ± 0.19 days for short-life; figure 3b). This result demonstrates that the genetic architecture underlying lifespan is partly shared by the sexes, and that selection on male lifespan produced a correlated genetic response in female lifespan, supporting the first of our predictions.
Figure 2.
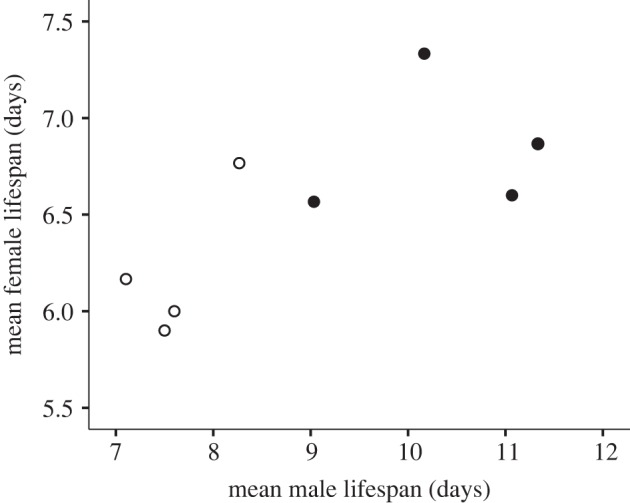
Intersexual correlation (r = 0.759, p = 0.029) for lifespan across all four upward (filled circles) and four downward (open circles) selection lines. Lifespan assays were conducted after five generations of selection on male lifespan only.
Figure 3.
Mean lifespan ± s.e. of (a) males (blue) and (b) females (red) from the short-life (open circles) and long-life (closed circles) selection lines, after five generations of selection. While selection was applied to males only, in both sexes beetles from long-life lines lived longer than beetles from short-life lines.
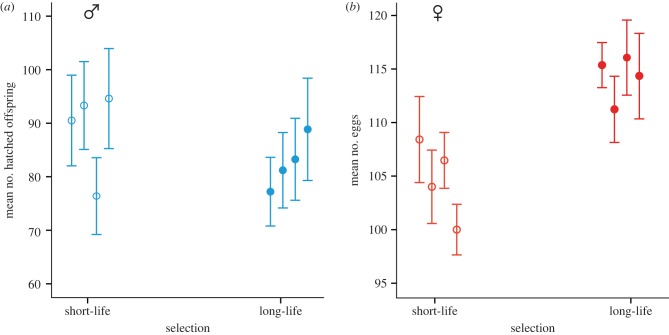
Next, we measured fitness of both males and females from all eight lines. In the male assays, focal males were competing with tester males for access to females from the base population, and the total number of offspring was counted as a measure of LRS. Sterile males were included to increase reproductive competition [32,36], providing a more accurate measure of focal male fitness in this protandrous system [11,28]. In the female assays, we paired focal females from selected lines with males from the base population and recorded realized fecundity as LRS [11,36]. To compare LRS of the sexes across selection regimes in the same model, we used the variance standardization approach, which measures the change in trait value in the units of standard deviation for this trait. We note that the results are qualitatively identical if we do not use any standardization at all. As predicted, there was a significant sex × selection interaction for lifetime offspring production (F1,6 = 8.041, p = 0.029; figure 4). One male line in this dataset fell outside the 95 per cent CI; with this outlier excluded the interaction is even stronger (F1,6 = 24.371, p = 0.002). Short-life males had higher reproductive success than long-life males (ANOVA for standardized LRS, outlier excluded: F1,5 = 11.179, p = 0.020, mean = 92.8 ± 1.21 versus 82.6 ± 2.43 offspring per replicate vial; figure 5a). In sharp contrast, females from long-life lines had higher reproductive success than females from short-life lines (ANOVA for standardized LRS: F1,6 = 21.493, p = 0.004; raw data: mean ± s.e. = 114.3 ± 1.07 eggs per female for long-life versus 104.7 ± 1.77 eggs per female for short-life; figure 5b).
Figure 4.
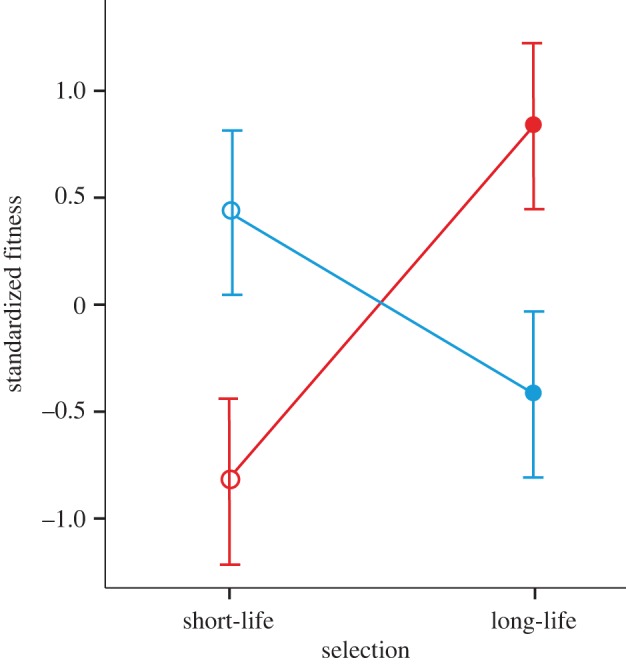
Sex × selection interaction for relative fitness ± s.e. across short-life (open circles) and long-life (closed circles) selection regimes. Fitness was estimated as LRS per sex per population and then variance-standardized within each sex. Female relative fitness (red) was higher in long-life lines, while male relative fitness (blue) was higher in short-life lines.
Figure 5.
(a) Average LRS (number of hatched offspring) ± s.e. for males from four short-life (open circles) and four long-life (closed circles) selection lines. Total number of hatched young was counted for 20 replicates per line after five generations of selection on male lifespan. One short line fell outside the 95% CI. When this line was excluded, reproductive success differed significantly between selection treatments (see text), although the difference was not significant when all lines were included (analyses of variance on standardized values: F1,6 = 1.553, p = 0.259). (b) Average fecundity (number of eggs laid) ± s.e. of females from four short-life (open circles) and four long-life (closed circles) selection lines. Total number of eggs laid was counted for 30 females per line after five generations of selection on male lifespan, with long-life females laying significantly more eggs.
Our study of seed beetles provides the first direct experimental evidence that ISC can constrain males and females from reaching their sex-specific optimal lifespan. Male and female lifespans were positively correlated, indicating that the underlying genetic architecture for lifespan is at least partly shared by both sexes, and there was a strong sex × selection interaction for LRS between the short- and long-life selection treatments, demonstrating that the optimal balance between reproduction and lifespan is indeed different for males and females. We show that both of our original predictions were supported by the data: (i) females from upward-selected lines lived longer than females from downward-selected lines; and (ii) upward selection decreased male LRS and increased female LRS. Thus, we succeeded in pulling the sexes either away from or towards their sex-specific fitness optima by selecting on a putative sexually antagonistic trait in one sex only.
Seed beetles are capital breeders from arid environments that eclose from the beans with all of the resources, including water, necessary for a lifetime of somatic maintenance, as well as egg-laying or sperm production [11,27]. Males constantly search for mates, attempt to mount resistant females [32] and may invest up to 10 per cent of their body weight in a single copulation [37]. Reproductive effort in male seed beetles is so costly that duration of a single copulation is phenotypically and genetically correlated with reduced survival [38]. Furthermore, males have to be persistent in order to achieve matings with previously mated females [32], which is expensive in terms of energy and water. The costs of mate search and courtship in this system are also very high—males that are kept in single-sex groups do not copulate but court other males, and the costs of these behaviours are comparable with the full costs of reproduction [39]. Males that are successful in mating and sperm competition will thus spend more of their resources in courtship and copulation than unsuccessful ones and will pay a longevity cost [40]. The cost of reproduction is reflected in the earlier observation that inbred C. maculatus males from this population suffer 47 per cent reduction in their lifetime reproductive performance (LRS) but live longer than outbred males [11]. In contrast, inbreeding reduces both LRS and lifespan in females [11]. Our experiment provides a clear explanation for these paradoxical results: male fitness is inversely related to lifespan in this system.
Taken together, these results suggest that C. maculatus adopts a ‘live-fast-die-young’ strategy [6,41], as predicted by the antagonistic pleiotropy theory of ageing [4], and that high genetic quality is associated with short lifespan in males [11,24]. This leads to a clear prediction that selection for short lifespan should result in higher-quality males, the result that was obtained previously in black field crickets [24] and now replicated in this study. The key novel finding of our study, however, is that selection on males led to opposing effects on female fitness, indicating strong ISC over lifespan.
Several proximate mechanisms may contribute to the reduced longevity of reproductively successful males. In C. maculatus, females vigorously kick males with their hind legs during mating [42] and this behaviour reduces the copulation duration [43]. Ejaculate size increases with copulation duration and contributes to male reproductive success because it increases female fecundity [44]. Males that are able to copulate for longer and/or produce larger ejaculates would increase their reproductive fitness but suffer a longevity cost [38]. Selection for male longevity might lead to selection on other traits, such as locomotory behaviour, metabolic rate and metabolic efficiency. Low rates of metabolism have been previously associated with long life in different taxa [45,46], including invertebrates [47,48], but overall the empirical support for this hypothesis is weak at best [46,49–51]. Alternatively, selection for improved bioenergetic efficiency could increase lifespan, potentially at the cost of reproduction [52]. Furthermore, reduction in locomotory activity in long-life beetles could increase their lifespan by preserving water and energy, but would probably reduce their reproductive success. We suggest that studying the correlated evolution of physiological, morphological and behavioural traits, as well as the patterns of genome-wide gene expression in these lines, will be likely to give clues to the observed patterns of sexual antagonism. However, while these proximate mechanisms may contribute to varying degrees to the patterns observed in our study, the key ultimate reason for why short-life males have higher reproductive success than long-life males in our experiment is probably that long-life males are of lower genetic quality [11].
The sexes commonly differ not only in how long they live and when they start to senesce, but also in how they react to environmental interventions aimed at prolonging their lifespan or decelerating the onset of ageing [53,54]. Both evolutionary causes and medical implications of sex differences in lifespan must be key targets of contemporary research on the biology of ageing, but the reasons for differences in response to lifespan-extending treatments are virtually unknown [55,56]. Here we demonstrated that selection for increased lifespan can result in opposing effects on fitness in a model organism. Our findings suggest that sexually antagonistic selection can maintain genetic variation for lifespan in populations but will also complicate a search for feasible life-extending treatments. Treatments mimicking the effects of genes responsible for lifespan extension observed in our study would have detrimental effects on male fitness. While the magnitude and direction of sex-specific selection are likely to differ considerably across species, we expect some degree of sexual antagonism in all sexually reproducing organisms, including humans, necessitating investigation into the proximate causes of sex-specific effects.
Acknowledgements
We are grateful to Jessica Abbott, Göran Arnqvist, Russell Bonduriansky, Damian Dowling, Urban Friberg, Paolo Innocenti, Ted Morrow and Felix Zajitschek for fruitful discussions and comments on this paper. The study was supported by the Swedish Research Council and European Research Council Starting grant 2010 to A.A.M. and the Carl Tryggers Stiftelse and Zoologiska Stiftelsen to E.C.B.
References
- 1.Parker G. A., Baker R. R., Smith V. G. F. 1972. The origin and evolution of gamete dimorphism and the male–female phenomenon. J. Theor. Biol. 36, 529–553 10.1016/0022-5193(72)90007-0 (doi:10.1016/0022-5193(72)90007-0) [DOI] [PubMed] [Google Scholar]
- 2.Bateman A. J. 1948. Intra-sexual selection in Drosophila melanogaster. Heredity 2, 277–287 10.1038/hdy.1948.18 (doi:10.1038/hdy.1948.18) [DOI] [PubMed] [Google Scholar]
- 3.Arnold S. J. 1994. Bateman principles and the measurement of sexual selection in plants and animals. Am. Nat. 144, S126–S149 10.1086/285656 (doi:10.1086/285656) [DOI] [Google Scholar]
- 4.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 5.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- 6.Bonduriansky R., Maklakov A., Zajitschek F., Brooks R. 2008. Sexual selection, sexual conflict and the evolution of ageing and lifespan. Funct. Ecol. 22, 443–453 10.1111/j.1365-2435.2008.01417.x (doi:10.1111/j.1365-2435.2008.01417.x) [DOI] [Google Scholar]
- 7.Promislow D. 2003. Mate choice, sexual conflict, and evolution of senescence. Behav. Genet. 33, 191–201 10.1023/A:1022562103669 (doi:10.1023/A:1022562103669) [DOI] [PubMed] [Google Scholar]
- 8.Promislow D. E. L. 1992. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. Lond. B 247, 203–210 10.1098/rspb.1992.0030 (doi:10.1098/rspb.1992.0030) [DOI] [Google Scholar]
- 9.Hunt J., Brooks R., Jennions M. D., Smith M. J., Bentsen C. L., Bussiere L. F. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027 10.1038/nature03084 (doi:10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 10.Clutton-Brock T. H., Isvaran K. 2007. Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B 274, 3097–3104 10.1098/rspb.2007.1138 (doi:10.1098/rspb.2007.1138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilde T., Maklakov A. A., Meisner K., la Guardia L., Friberg U. 2009. Sex differences in the genetic architecture of lifespan in a seed beetle: extreme inbreeding extends male lifespan. BMC Evol. Biol. 9, 33 10.1186/1471-2148-9-33 (doi:10.1186/1471-2148-9-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves B. M., Strand M., Lindsay A. R. 2006. A reassessment of sexual dimorphism in human senescence: theory, evidence, and causation. Am. J. Hum. Biol. 18, 161–168 10.1002/ajhb.20488 (doi:10.1002/ajhb.20488) [DOI] [PubMed] [Google Scholar]
- 13.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 10.2307/2407393 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 14.Rice W. R., Chippindale A. K. 2001. Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693 10.1046/j.1420-9101.2001.00319.x (doi:10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 15.Arnqvist G., Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press [Google Scholar]
- 16.Chippindale A. K., Gibson J. R., Rice W. R. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675 10.1073/pnas.98.4.1671 (doi:10.1073/pnas.98.4.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foerster K., Coulson T., Sheldon B. C., Pemberton J. M., Clutton-Brock T. H., Kruuk L. E. B. 2007. Sexually antagonistic genetic variation for fitness in red deer. Nature 447, U1107–U1109 10.1038/nature05912 (doi:10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- 18.Bonduriansky R., Chenoweth S. F. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288 10.1016/j.tree.2008.12.005 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 19.Fedorka K. M., Mousseau T. A. 2004. Female mating bias results in conflicting sex-specific offspring fitness. Nature 429, 65–67 10.1038/nature02492 (doi:10.1038/nature02492) [DOI] [PubMed] [Google Scholar]
- 20.Cox R. M., Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187 10.1086/595841 (doi:10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 21.Promislow D. E. L., Pletcher S. D. 2002. Advice to an aging scientist. Mech. Ageing Dev. 123, 841–850 10.1016/S0047-6374(02)00021-0 (doi:10.1016/S0047-6374(02)00021-0) [DOI] [PubMed] [Google Scholar]
- 22.Zajitschek F., Hunt J., Zajitschek S. R. K., Jennions M. D., Brooks R. 2007. No intra-locus sexual conflict over reproductive fitness or ageing in field crickets. PLoS ONE 2, e155 10.1371/journal.pone.0000155 (doi:10.1371/journal.pone.0000155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis Z., Wedell N., Hunt J. 2011. Evidence for strong intralocus sexual conflict in the Indian meal moth, Plodia interpunctella. Evolution 65, 2085–2097 10.1111/j.1558-5646.2011.01267.x (doi:10.1111/j.1558-5646.2011.01267.x) [DOI] [PubMed] [Google Scholar]
- 24.Hunt J., Jennions M. D., Spyrou N., Brooks R. 2006. Artificial selection on male longevity influences age-dependent reproductive effort in the black field cricket Teleogryllus commodus. Am. Nat. 168, E72–E86 10.1086/506918 (doi:10.1086/506918) [DOI] [PubMed] [Google Scholar]
- 25.Fox C. W., Dublin L., Pollitt S. J. 2003. Gender differences in lifespan and mortality rates in two seed beetle species. Funct. Ecol. 17, 619–626 10.1046/j.1365-2435.2003.00781.x (doi:10.1046/j.1365-2435.2003.00781.x) [DOI] [Google Scholar]
- 26.Fox C. W., Czesak M. E., Wallin W. G. 2004. Complex genetic architecture of population differences in adult lifespan of a beetle: nonadditive inheritance, gender differences, body size and a large maternal effect. J. Evol. Biol. 17, 1007–1017 10.1111/j.1420-9101.2004.00752.x (doi:10.1111/j.1420-9101.2004.00752.x) [DOI] [PubMed] [Google Scholar]
- 27.Fox C. W., Scheibly K. L., Wallin W. G., Hitchcock L. J., Stillwell R. C., Smith B. P. 2006. The genetic architecture of life span and mortality rates: gender and species differences in inbreeding load of two seed-feeding beetles. Genetics 174, 763–773 10.1534/genetics.106.060392 (doi:10.1534/genetics.106.060392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnqvist G., Tuda M. 2010. Sexual conflict and the gender load: correlated evolution between population fitness and sexual dimorphism in seed beetles. Proc. R. Soc. B 277, 1345–1352 10.1098/rspb.2009.2026 (doi:10.1098/rspb.2009.2026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell R. 1991. The traits of a biotype of Callosobruchus maculatus (F) (Coleoptera, Bruchidae) from South India. J. Stored Prod. Res. 27, 221–224 10.1016/0022-474X(91)90004-V (doi:10.1016/0022-474X(91)90004-V) [DOI] [Google Scholar]
- 30.Fox C. W. 1993. Multiple mating, lifetime fecundity and female mortality of the bruchis seed beetle, Callosobruchus maculatus (Coleoptera, Bruchidae). Funct. Ecol. 7, 203–208 10.2307/2389888 (doi:10.2307/2389888) [DOI] [Google Scholar]
- 31.Fox C. W., Bush M. L., Wallin W. G. 2003. Maternal age affects offspring lifespan of the seed beetle, Callosobruchus maculatus. Funct. Ecol. 17, 811–820 10.1111/j.1365-2435.2003.00799.x (doi:10.1111/j.1365-2435.2003.00799.x) [DOI] [Google Scholar]
- 32.Maklakov A. A., Arnqvist G. 2009. Testing for direct and indirect effects of mate choice by manipulating female choosiness. Curr. Biol. 19, 1903–1906 10.1016/j.cub.2009.08.058 (doi:10.1016/j.cub.2009.08.058) [DOI] [PubMed] [Google Scholar]
- 33.Bilde T., Foged A., Schilling N., Arnqvist G. 2009. Postmating sexual selection favors males that sire offspring with low fitness. Science 324, 1705–1706 10.1126/science.1171675 (doi:10.1126/science.1171675) [DOI] [PubMed] [Google Scholar]
- 34.Hereford J., Hansen T. F., Houle D. 2004. Comparing strengths of directional selection: how strong is strong? Evolution 58, 2133–2143 [DOI] [PubMed] [Google Scholar]
- 35.Houle D., Pelabon C., Wagner G. P., Hansen T. F. 2011. Measurement and meaning in biology. Q. Rev. Biol. 86, 3–34 10.1086/658408 (doi:10.1086/658408) [DOI] [PubMed] [Google Scholar]
- 36.Bilde T., Friberg U., Maklakov A. A., Fry J. D., Arnqvist G. 2008. The genetic architecture of fitness in a seed beetle: assessing the potential for indirect genetic benefits of female choice. BMC Evol. Biol. 8, 295 10.1186/1471-2148-8-295 (doi:10.1186/1471-2148-8-295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savalli U. M., Fox C. W. 1998. Genetic variation in paternal investment in a seed beetle. Anim. Behav. 56, 953–961 10.1006/anbe.1998.0853 (doi:10.1006/anbe.1998.0853) [DOI] [PubMed] [Google Scholar]
- 38.Brown E. A., Gay L., Vasudev R., Tregenza T., Eady P. E., Hosken D. J. 2009. Negative phenotypic and genetic associations between copulation duration and longevity in male seed beetles. Heredity 103, 340–345 10.1038/hdy.2009.80 (doi:10.1038/hdy.2009.80) [DOI] [PubMed] [Google Scholar]
- 39.Maklakov A. A., Bonduriansky R. 2009. Sex differences in survival costs of homosexual and heterosexual interactions: evidence from a fly and a beetle. Anim. Behav. 77, 1375–1379 10.1016/j.anbehav.2009.03.005 (doi:10.1016/j.anbehav.2009.03.005) [DOI] [Google Scholar]
- 40.Paukku S., Kotiaho J. S. 2005. Cost of reproduction in Callosobruchus maculatus: effects of mating on male longevity and the effect of male mating status on female longevity. J. Insect Physiol. 51, 1220–1226 10.1016/j.jinsphys.2005.06.012 (doi:10.1016/j.jinsphys.2005.06.012) [DOI] [PubMed] [Google Scholar]
- 41.Vinogradov A. E. 1998. Male reproductive strategy and decreased longevity. Acta Biotheor. 46, 157–160 10.1023/A:1001181921303 (doi:10.1023/A:1001181921303) [DOI] [PubMed] [Google Scholar]
- 42.Rönn J., Katvala M., Arnqvist G. 2007. Coevolution between harmful male genitalia and female resistance in seed beetles. Proc. Natl Acad. Sci. USA 104, 10 921–10 925 10.1073/pnas.0701170104 (doi:10.1073/pnas.0701170104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edvardsson M., Tregenza T. 2005. Why do male Callosobruchus maculatus harm their mates? Behav. Ecol. 16, 788–793 10.1093/beheco/ari055 (doi:10.1093/beheco/ari055) [DOI] [Google Scholar]
- 44.Edvardsson M., Canal D. 2006. The effects of copulation duration in the bruchid beetle Callosobruchus maculatus. Behav. Ecol. 17, 430–434 10.1093/beheco/arj045 (doi:10.1093/beheco/arj045) [DOI] [Google Scholar]
- 45.Pearl R. 1928. The rate of living. New York, NY: Alfred A. Knopf [Google Scholar]
- 46.Speakman J. R., et al. 2004. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3, 87–95 10.1111/j.1474-9728.2004.00097.x (doi:10.1111/j.1474-9728.2004.00097.x) [DOI] [PubMed] [Google Scholar]
- 47.Van Voorhies W. A., Ward S. 1999. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc. Natl Acad. Sci. USA 96, 11 399–11 403 10.1073/pnas.96.20.11399 (doi:10.1073/pnas.96.20.11399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trout W. E., Kaplan W. D. 1970. A relation between longevity, metabolic rate, and activity in shaker mutants of Drosophila melanogaster. Exp. Gerontol. 5, 83–92 10.1016/0531-5565(70)90033-1 (doi:10.1016/0531-5565(70)90033-1) [DOI] [PubMed] [Google Scholar]
- 49.Khazaeli A. A., Van Voorhies W., Curtsinger J. W. 2005. Longevity and metabolism in Drosophila melanogaster: genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics 169, 231–242 10.1534/genetics.104.030403 (doi:10.1534/genetics.104.030403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose M. R. 1991. Evolutionary biology of aging, 1st edn. New York, NY: Oxford University Press [Google Scholar]
- 51.Promislow D. E. L., Haselkorn T. S. 2002. Age-specific metabolic rates and mortality rates in the genus Drosophila. Aging Cell 1, 66–74 10.1046/j.1474-9728.2002.00009.x (doi:10.1046/j.1474-9728.2002.00009.x) [DOI] [PubMed] [Google Scholar]
- 52.Lane N. 2011. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. BioEssays 33, 860–869 10.1002/bies.201100051 (doi:10.1002/bies.201100051) [DOI] [PubMed] [Google Scholar]
- 53.Selman C., et al. 2009. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 10.1126/science.1177221 (doi:10.1126/science.1177221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison D. E., et al. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, U392–U395 10.1038/nature08221 (doi:10.1038/nature08221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaeberlein M., Kapahi P. 2009. Aging is RSKy business. Science 326, 55–56 10.1126/science.1181034 (doi:10.1126/science.1181034) [DOI] [PubMed] [Google Scholar]
- 56.Kaeberlein M., Kennedy B. K. 2009. AGEING: a midlife longevity drug? Nature 460, 331–332 10.1038/460331a (doi:10.1038/460331a) [DOI] [PubMed] [Google Scholar]