Abstract
Fast dynamic control of skin coloration is rare in the animal kingdom, whether it be pigmentary or structural. Iridescent structural coloration results when nanoscale structures disrupt incident light and selectively reflect specific colours. Unlike animals with fixed iridescent coloration (e.g. butterflies), squid iridophores (i.e. aggregations of iridescent cells in the skin) produce dynamically tuneable structural coloration, as exogenous application of acetylcholine (ACh) changes the colour and brightness output. Previous efforts to stimulate iridophores neurally or to identify the source of endogenous ACh were unsuccessful, leaving researchers to question the activation mechanism. We developed a novel neurophysiological preparation in the squid Doryteuthis pealeii and demonstrated that electrical stimulation of neurons in the skin shifts the spectral peak of the reflected light to shorter wavelengths (greater than 145 nm) and increases the peak reflectance (greater than 245%) of innervated iridophores. We show ACh is released within the iridophore layer and that extensive nerve branching is seen within the iridophore. The dynamic colour shift is significantly faster (17 s) than the peak reflectance increase (32 s), revealing two distinct mechanisms. Responses from a structurally altered preparation indicate that the reflectin protein condensation mechanism explains peak reflectance change, while an undiscovered mechanism causes the fast colour shift.
Keywords: structural coloration, neural stimulation, skin patterning
1. Introduction
Animal coloration is pigmentary or structural; light is either absorbed by pigments or disrupted through wavelength interference caused by nanoscale structures [1–3]. These nanostructures reflect light such that the observed colour is dependent on the viewing angle, also known as iridescence [3–5]. Iridescence plays an important role in animal communication and camouflage as it provides the body with the ability to vibrantly reflect any colour, something pigments cannot match in intensity or colour range [6,7]. For example, the blue colours in invertebrates, birds, fishes, lizards and amphibians are almost always iridescence based [6–10]. Some species can alter their structural coloration as a result of various environmental stimuli while others possess physiological control over their iridescence. For example, tree swallows (Tachycineta bicolor) can alter their iridescent blue-green feathers based on relative humidity [11], the tree lizard (Urosaurus ornatus) can change its iridescent underbelly with changes in temperature and the tortoise beetle (Charidotella egregia) will actively change its structural coloration from gold to red when provoked [12]. In contrast, the iridescent cells of many fish and frog species are responsive to exogenous application of neuromodulators, taking seconds to minutes to respond [13–19]. Furthermore, electrical stimulation of nerves activates iridescence in fishes [20–22].
Neural control of iridescence in an invertebrate species has not been demonstrated to our knowledge, but squid are good candidates. Squid produce some of the most extensive and rapid skin colour changes in the animal kingdom [23] and aggregations of iridocytes in their skin—termed iridophores—change colour upon exogenous application of acetylcholine (ACh) [24–27]. Using silver staining and ACh esterase staining, nerves were identified in proximity to iridophores in the arms of Doryteuthis pealeii [28], but electrical stimulation of the skin, pallial and visceral nerves in the squid Lolliguncula brevis did not elicit iridophore activation [23], and extensive microscopy failed to locate iridophore synapses [27]. Thus, although neural control of squid iridophores has been suggested [27,29], this remained to be demonstrated.
We investigated the iridophore control mechanism with a new skin preparation. We show that electrically stimulating a subset of skin nerves results in a robust and reproducible activation of iridophores in the fin and dorsal mantle of squid (D. pealeii). We used immunohistochemistry to label neuronal branches within these active iridophores. To investigate the temporal dynamics of iridophore activation under natural stimulation (i.e. neural release of ACh), we measured the colour (wavelength) and intensity (reflectance) at high resolution, and show that the temporal dynamics of these two properties differ. Moreover, stimulation of structurally compromised skin results in a normal reflectance change, but the colour change is dramatically reduced. Together, our results suggest that the iridophore response is driven by two mechanisms. The implications of these new findings are discussed.
2. Material and methods
(a). Animals
Adult Atlantic longfin squid (D. pealeii, aka. Loligo pealeii; figure 1a) were collected by brief trawling runs from the coastal waters near Woods Hole, Massachusetts. From large holding tank populations, individuals that showed no evidence of skin damage in at least one fin were transferred to separate holding tanks for males and females, respectively. Each squid was fed daily with small live fish (Fundulus spp.), and animals were kept for up to two weeks before being used for experiments.
Figure 1.
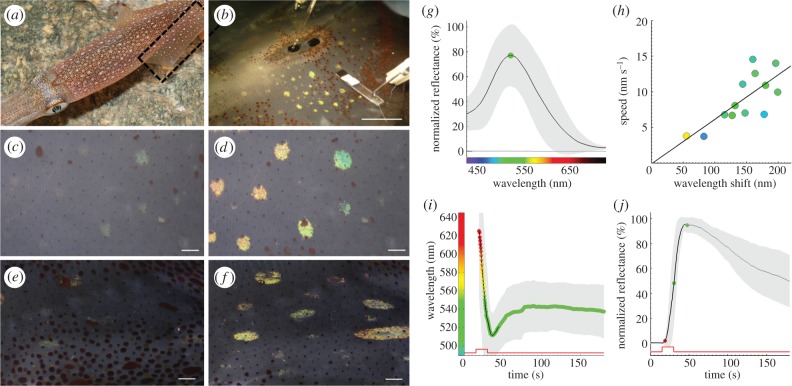
Neurally activated iridescence in squid iridophores. (a) Doryteuthis pealeii have conspicuous pigmentary chromatophores and underlying structurally coloured iridophores. Box shows the tissue location for fin preparations. (b) ‘Intact fin’ preparation yields a green iridophore phenotype when a nearby nerve branch is stimulated (5 V, 300 µs pulses at 10 Hz for 15 s) through a proximal suction pipette. (c) Fin iridophores at rest have low peak reflectance. (d) After electrical stimulation of the fin nerve, a field of colour-shifted iridophores is clearly visible (see also electronic supplementary material, movie S1). (e) Dorsal mantle iridophores, often larger than fin iridophores, at rest are barely visible. (f) After electrical stimulation of dorsal nerve branches (15 V, 300 µs pulses at 10 Hz for 15 s), dorsal mantle iridophores also respond with a large shift in peak colour and peak reflectance similar to those in the fin. (g) Mean reflectance spectra of fin iridophores after stimulation. (h) Fin iridophores that shifted the most in peak reflected wavelength also did so faster (they shifted at higher nm s–1; colours inside circles represent peak of the iridophore spectra at the farthest wavelength shift). (i,j) Timing of the peak wavelength shift and peak reflectance increase, respectively. The step in the red trace indicates the stimulation timing. Grey, standard deviation. n = 4 animals and 13 iridophores with one to three repeat measures from each iridophore. Scale bars: (b) 1 cm, (c–f) 1 mm.
(b). Dissection and electrophysiology
(i). Set-up
The chamber was filled with Tris-buffered (20 mM; pH 7.8) natural seawater (NSW), which was re-circulated and held at a constant temperature (15°C) using a refrigerated water chiller. The dissection tray was viewed with a stereo dissection trinocular microscope on a boom stand positioned above the fin to allow dissection, video imaging and spectrometry. Light for dissection, imaging and measuring spectra was provided by a halogen fibre-optic light source (Schott).
(ii). Tissue preparation
Squid were anaesthetized by immersion in seawater containing a sublethal concentration of ethanol (3%), and then killed by decapitation and decerebration. The ventral mantle muscle was partly cut away and the internal organs removed. Only fins that had no evidence of skin damage were used in preparations. The preparation was placed dorsal side down in a large perfusion chamber and pinned down near the periphery of the fin and remaining mantle. We readily observed nerves that ran in tracts from the large fin nerve, and branched subsequently to either terminate within iridophores or pass through them (see the electronic supplementary material, figure S1). One intact and three reduced preparations were developed for this research.
(iii). ‘Intact’ fin preparation
A small window (0.5 × 1 cm) of ventral skin and fin muscle near the base of the fin was removed by surgical dissection, leaving the innermost layer of the dorsal skin exposed, to enable the location of nerves. Nerves were tightly interwoven with connective tissue and travelled throughout the iridophore layer, which required very precise and careful dissection to remove enough length of nerve for electrical stimulation. Once the nerve was dissected away from the connective tissue, the dorsal skin was opened fully to allow access from the dorsal surface. The preparation was inverted and pinned with ventral surface facing down (see the electronic supplementary material, figure S2a). Immediately prior to stimulation, nerves were cut and sucked into glass pipettes that formed a tight seal around the nerve bundle. Custom pipettes with suitable internal diameters were made by manually cutting and polishing with a Sutter ceramic tile.
(iv). Three reduced preparations
Similar to the intact fin, (i) tissue above and below the iridophore layer was removed (figure 2d,e; electronic supplementary material, figure S2b); (ii) tissue above the iridophore layer was removed (figure 3a,b; electronic supplementary material, figure S2c); and (iii) tissue below the iridophore layer was removed (figure 3c; electronic supplementary material, figure S2d).
Figure 2.
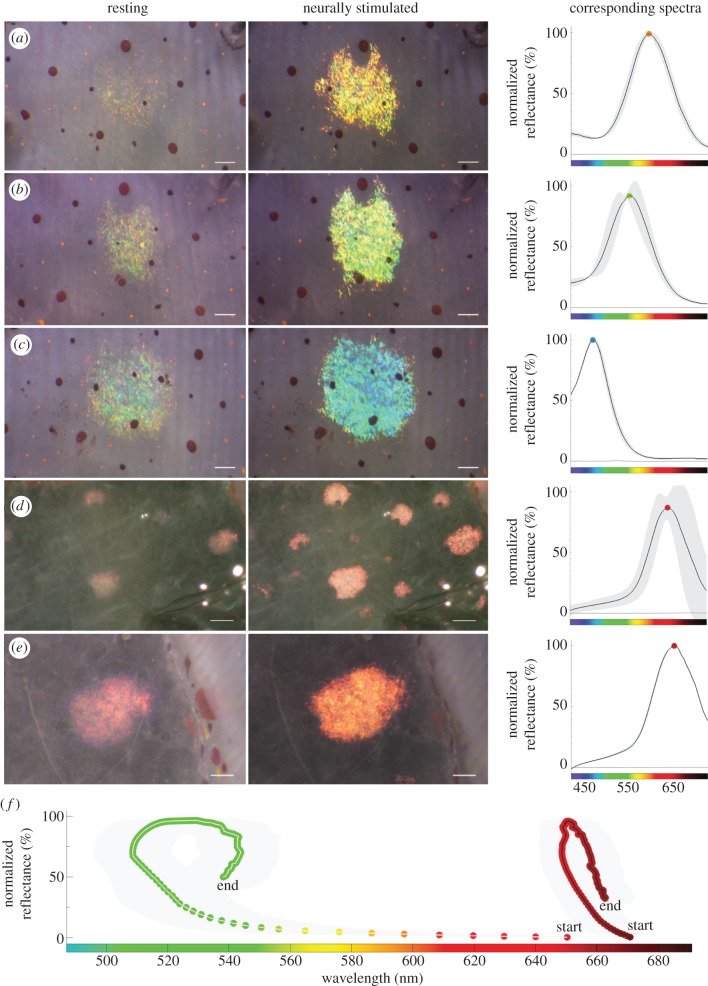
Neurally tuneable structural coloration. (a–c) Examples of single iridophore coloration before and after stimulation to a single nerve. Mean maximum response spectra shown on the right (two or three measurements). (d) A preparation as in figure 1b, with subsequent removal of the layers below and above the iridophore layer, such that only the nerves, iridophores and connective tissue remain. In this preparation, neural stimulation (before and after 15 V, 300 µs pulses at 10 Hz for 15 s shown) activates iridophores, but the responses now show a red phenotype (n = 3 animals and 5 iridophores, with three repeat measures from each iridophore; see also electronic supplementary material, movie S2). (e) Representative example of a single red iridophore response to electrical stimulation with mean maximum response spectra (three measurements). Note that automatic brightness correction was applied between acquisitions for images in (a–c) and (e). (f) Plot of maximal wavelength versus maximal normalized reflectance. The points are at approximately 0.3 s intervals. The green (left) curve shows the mean iridophore response from intact skin (same data as figure 1g–j), while the red (right) curve shows the mean iridophore response from the open skin preparation shown in figure 2d. Dots display the wavelength of maximum peak reflectance at each sampled time point. Grey, standard deviation. Scale bars: (a–c,e) 200 µm, (d) 1 mm.
Figure 3.
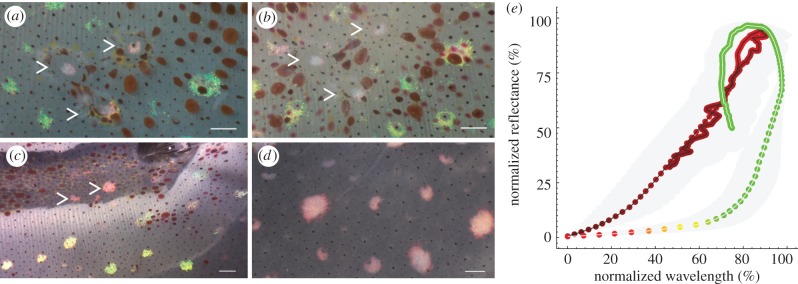
Structural coloration and peak reflectance have separate mechanisms. (a) Neurally stimulated squid skin (15 V, 300 µs pulses at 10 Hz for 15 s), with three dissected openings (epidermis and chromatophores removed). Only the iridophores beneath the altered tissue show a red phenotype. (b) Application of 200 mM ACh to the same preparation does not recover the green phenotype. (c) Neurally stimulating tissue where the fin muscle and ventral skin (both below the iridophore layer) had been removed results in a red phenotype (see also electronic supplementary material, movie S3). (d) Application of 200 mM ACh to the type of preparation shown in (c) did not recover the green phenotype. (In the picture shown, the ventral surface is facing the viewer.) In (a–c), arrowheads indicate activated iridophores below or above removed layers. (e) Normalization of the peak reflectance change and colour shift for the red phenotype (red circles) and green phenotype (red–green circles) shows that the peak reflectance and colour shift changes are proportional for most of the red phenotype response, and that the decay phase also follows the same constant. However, the peak reflectance versus colour shift responses in the green phenotype are not proportional, and the rise and decay phases show different dynamics. Dots display the wavelength of maximum peak reflectance at each sampled time point. (a–d) Scale bars, 1 mm.
To stimulate iridophores reliably and periodically over several hours, we stimulated with 5 V pulses. Each pulse was 300 µs in duration and was repeated at a frequency of 10 Hz over a period of 15 s unless otherwise stated. Upon neural stimulation, both chromatophores and iridophores responded. The iridophore-activated field included part of the chromatophore-activated field and areas immediately anterior. By increasing the stimulation frequency (0.5–80 Hz) and/or voltage (1–50 V), a higher number of iridophores with higher reflectances could be observed, but this trend eventually saturated.
(c). Videography and spectrometry
Once a nerve was connected to the stimulator (model 2100, A-M Systems), we coordinated electrical stimulation with videography or spectrometry (through the trinocular port of the stereo microscope), using a Power Lab data acquisition unit (PL3504) and Lab Chart software (AD Instruments) to trigger equipment, including a blue light-emitting diode (LED) light pulse (observable in the video or spectra measurements). High-definition video was collected at 30 frames per second with a Canon EOS 5D Mark II digital camera in manual mode. Prior to spectra collection, a diffuse reflectance standard (model WS-1, Ocean Optics) was used to calibrate the spectrometer (model QE65000, Ocean Optics) for the halogen light source (Schott). Light was directed from above, within 5° of vertical, using a dual fibre-optic light guide. Spectral and reflectance measurements were collected via a 1 mm optical fibre assembly coupled from the microscope to a spectrometer every 0.3 s over 3–8 min, and data were logged with SpectraSuite to the computer's hard disk. During each spectral measurement, video was recorded simultaneously by using a Zeiss eye piece adaptor and an HD video recorder (model HDR-XR520V, Sony) to collect representative images for each spectral change. Note that automatic brightness correction was applied during the Sony HDR-XR520V acquisition.
To avoid chromatophore activity affecting the quantification of iridophore responses, we selected for spectral and reflectance measurements only iridophores that were anterior to the chromatophore receptive field (where chromatophores did not respond to electrical stimulation). To analyse spectra data, collected over several minutes, we used Matlab (Mathworks) to normalize and subtract the background level of reflectance, and then smooth the data with a Savitzky–Golay filter to more easily visualize and compare responses between iridophores and preparations. We plotted the mean and standard deviation for the normalized, background-subtracted and filtered spectra from iridophores for the baseline and maximum reflectance. To determine spectral dynamics, background reflectance was subtracted and the wavelength at peak reflectance found for each measurement over time.
(d). Whole-mount immunohistochemistry and confocal imaging
After stimulation and simultaneous videography or spectrometry, we forward-filled some of the large nerve bundles with 3 per cent Lucifer yellow (LY). The nerve was re-cut about 1–2 mm shorter, and then sucked immediately into a tight-fitting glass pipette, and the electrode rapidly backfilled with 3 per cent LY in 200 mM lithium chloride using a microfil needle. After 20 min, the LY dye had diffused down the nerve sufficiently to be seen with a fluorescence dissection microscope. We filled the nerve for a minimum of 10 h with the re-circulating bath in operation. The injected tissue was fixed for 12 h in 4 per cent paraformaldehyde in Tris-buffered NSW, then triple rinsed and stored in Tris NSW at 4°C.
Tissue was processed as described by Gonzalez-Bellido & Wardill [30]. Briefly, the tissue was dehydrated and rehydrated in a series of steps to remove lipids and any trapped air, and then permeated with collagenase (0.5 mg ml–1) and hyaluronidase (300 µg ml–1) to open the nerve sheaths. An anti-LY antibody conjugated to NeurAvidin was used in conjunction with biotin conjugated to Dylight 633 to shift the excitation into the red and away from tissue autofluorescence. Tissue was then cleared in thiodiethanol (TDE) and mounted inside a hole in a metal slide that was contained with #0 cover glass to increase the working distance of the objective. Automated imaging was undertaken with a Zeiss 780 confocal microscope, collecting multiple tiled z-stacks with auto-brightness correction to deal with changes in structural complexity with increasing depth. Image z-stacks were stitched with the ‘Grid/Collection stitching’ plug-in within Fiji software, v. 1.46p [31]. Tracing of neurons was completed with the freely available software Vaa3D, v. 2 [32].
3. Results
(a). Neural stimulation changes structural coloration
Electrically stimulating skin nerves in the fin produced dramatic changes in colour and peak reflectance of squid iridophores (figure 1a–d; electronic supplementary material, movie S1). Dorsal mantle iridophores, which are larger in size than fin iridophores, could also be stimulated using similar methods (figure 1e,f). Upon activation, fin iridophores shifted their average spectral peak of reflected light from red (624 ± 42 nm) to green (511 ± 26 nm), with an average maximum shift of 145 ± 43 nm (figure 1g–i), while the average maximum peak reflectance increased by 245 ± 120% (figure 1j). As the average peak colour was green, we termed this the ‘green phenotype’ and the error reported throughout the article as ±1 s.d. The total colour shift differed between iridophores within the innervated field (figure 2a–c; cf. figure 1d). Iridophores that shifted colour the fastest also shifted wavelength the farthest (figure 1h).
We found that iridophores were also neurally activated when all skin layers above and below the iridophore layer were removed (figure 2d,e; electronic supplementary material, movie S2), confirming that neurons release ACh within the iridophore layer in D. pealeii. However, although the average peak reflectance change in these iridophores was large (359 ± 144%), the average shift in the spectral peak of reflected light was small (30 ± 10 nm), changing from dark red (672 ± 15 nm) to pink (646 ± 14 nm), as opposed to the green phenotype (figure 2f). This red–pink shift is termed the ‘red phenotype’ throughout this paper.
To elucidate whether dissection caused the red phenotype, structural damage was induced by removing patches of the epidermis, hyaline and chromatophore layers (see electronic supplementary material, figure S2 for explanation). Neural stimulation of this preparation yielded the red phenotype only in the iridophores underneath the open patches (figure 3a). Applying ACh in physiological excess (200 mM) did not recover these red phenotypes to a green phenotype (figure 3b). However, when the optical pathway to the iridophores was left intact and only the layers below the iridophores were removed, the red phenotype was also observed upon stimulation (figure 3c; electronic supplementary material, movie S5). Exogenous application of 200 mM ACh to a similar preparation, but now with the ventral surface facing the viewer, did not recover the green phenotype (figure 3d).
(b). Dynamics of the red and green iridophore phenotypes
For appropriate comparison of the red and green phenotype dynamics, we normalized their responses. In the red phenotype, peak reflectance change and colour shift were proportional, and the rise and decay dynamics were identical (figure 3e). In contrast, the peak reflectance and colour shift changes were not proportional in iridophores that shifted from red to green, and the rise and decay dynamics showed an anticlockwise hysteresis curve (figure 3e). Furthermore, the times required to reach half the maximum colour shift (8 ± 3 s) and to reach half the peak reflectance (15 ± 2 s) differed significantly (paired t-test, n = 13 iridophores, p = 1.0e − 007).
(c). Structurally coloured iridophores are associated with neural processes
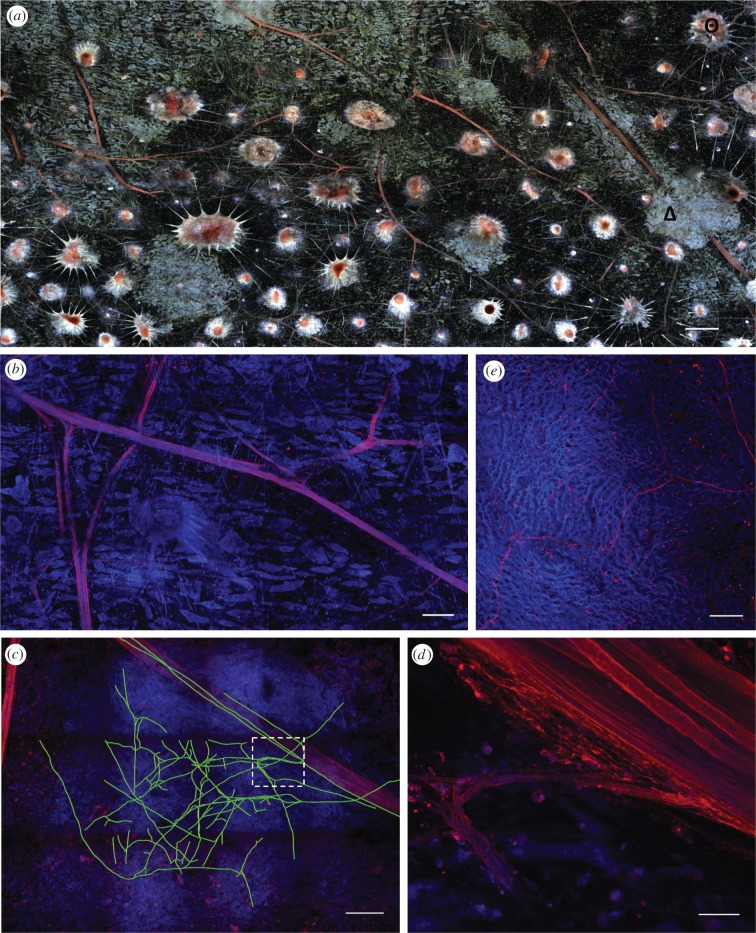
Using a whole-mount technique to track nerves throughout the skin [30], we found that LY had travelled extensively between axons (figure 4a), presumably via gap junctions. Within the large nerves that travel beneath the iridophore layer, some axons forked, creating a stereotypical Y-shape with similar-sized divisions (figure 4b). The branches of these nerves travelled towards the dorsal skin surface, through the iridophore layer and then seemed to terminate in the chromatophore layer, presumably innervating them. Often, several axons exited the nerve immediately adjacent to iridophores (figure 4d), and we traced a highly branched network that was contained exclusively within the bounds of an iridophore (figure 4c). To obtain a higher signal, we carried out immunohistochemistry with acetylated alpha-tubulin antibodies, known to label neural structures in other cephalopods such as the pygmy cuttlefish [33]. This antibody labelled very fine neural structures that seem to terminate between the iridocyte cells within the iridophores but originated in the large nerve branches (figure 4e).
Figure 4.
Iridophores are associated with neural processes. (a) Nerves in red (filled with Lucifer yellow, tagged with Dylight 633, excited with 633 nm) can be easily traced among the distinctive chromatophores (Θ) and iridophores (Δ) (imaged as autofluorescence using 405 and 514 nm laser lines) that they innervate. Confocal maximum-intensity projection image from squid fin skin, cleared with TDE, whole-mounted and scanned (50 µm of tissue; 40 × 15 tile Z-scan). (b) Lucifer yellow (red) travelled along axons, which displayed forked branches. In blue, scattered iridocytes, which are unresponsive to ACh, can be seen (imaged as autofluorescence using 405 nm). (c) Tracing (green lines) of several small axons (red signal) that diverged from the main nerve and extended minute processes within an iridophore (seen as an area of increased blue fluorescence). Only branches that could be identified reliably were traced in this tissue volume, which is shown as a sum of all the optical sections. The white box is shown at a higher magnification in (d) where axons depart from the large nerve bundle (top right part of the image). (e) Thin (0.5–1 µm) neuronal processes (in red) deep within the iridophore are labelled with acetylated alpha-tubulin antibody. Fine iridophore structure (in blue) is seen owing to tissue autofluorescence. Maximum-intensity projection image (16 µm thick). Scale bars: (a) 500 µm, (b) 150 µm, (c) 200 µm, (d) 17 µm, (e) 25 µm.
Access to all the images and raw data for this paper can be found at http://hdl.handle.net/1912/5277 in the Woods Hole Open Access Server (WHOAS). This material consists of images and data that support the four figures in this paper.
4. Discussion
(a). Neural stimulation changes structural coloration
We demonstrate that skin structural coloration can be controlled by neurons that descend from the squid central nervous system. Electrically stimulating nerves in the fin or dorsal mantle produced dramatic changes in colour and peak reflectance of squid iridophores (figure 1), despite previous failed attempts in the squid L. brevis [23] and the squid Alloteuthis subulata [25]. Upon activation, fin iridophores shifted their average spectral peak of reflected light from red (624 nm) to a green phenotype (511 nm).
Given that the iridophore colour shift in various squid species is related to the amount of ACh applied in vitro as bath application [23–27,34], our results suggest that the specific colour of each iridophore as well as speed of change is controlled by the nervous system, similar to spatial chromatophore patterning that occurs in the dermal layer just above. Previous studies with silver staining in L. brevis did not find neurons within the iridophore layer [23]; thus, two possible mechanisms of activation were proposed: (i) that ACh might be released from neurons in the nearby chromatophore layer in a cholinergic non-synaptic diffusion system similar to starfish tube feet [35]; or (ii) that ACh is delivered via the circulatory system, thus acting as a hormone. Both proposed mechanisms in L. brevis would account for the slow appearance (minutes) of iridescence in that species. However, loliginid squid such as Doryteuthis and Loligo are known to behaviourally express iridescence much faster (several seconds) [23], so different mechanisms of activation might be expected.
We found that iridophores were also neurally activated when all skin layers above and below the iridophore layer were removed (figure 2d,e), confirming that neurons release ACh within the iridophore layer in D. pealeii. However, although the average peak reflectance change in these iridophores was large (359%), the average shift in spectral peak of reflected light was small (30 nm), remaining predominantly red in colour. This red phenotype has been observed in similar preparations of squid and cuttlefish tissue [23,26,36]. We hypothesized three possible causes for this: (i) change in haemolymph chemistry owing to interaction with the bathing solution, (ii) disruption of the optical properties of light interacting with the iridophore owing to damage of the hyaline layer, or (iii) structural damage to muscles or connective tissue that support the iridophores.
Cephalopods have an open circulatory system in the skin [37]. Therefore, over time, an opening in the skin allows diffusion of the bathing media across it. After sufficient time, changes owing to the bathing media should affect the whole fin tissue. However, when stimulating after 4 h of bathing, we still saw a sharp delineation between the area directly exposed to the bathing media and that which was intact (figure 3c). Thus, it is unlikely that this red phenotype is caused by changes in the haemolymph composition.
The red phenotype is not likely to arise from altered properties of the optical pathway because this phenotype results from structurally damaging the layers located ventrally or dorsally to the iridophores. Instead, it seems most plausible that the iridophore anchoring system (muscular or connective) was disrupted, affecting the iridophore structure, hence its coloration. This interpretation concurs with the suggestion that muscles found in the vicinity of iridophores may partially control reflectance in D. pealeii [38] and D. plei [29]. In L. brevis, muscle cells were noted in extracted portions of the iridophore layer and seen, in transmission electron micrographs, in proximity to iridophores [23,27]. The function of such muscles is unknown. However, during and after neural stimulation in the intact fin, we observed that within some iridophores, groups of iridocytes rapidly flickered. Such spontaneous ‘flicker’ was independent of adjacent chromatophore activity, lasted 400 ms and resulted in a dramatic reflectance change (see the electronic supplementary material, figure S3 and movie S4). This observation further suggests that a muscular mechanism may also play a role in the iridophore response, but whether the red phenotype results from muscle damage remains to be shown.
(b). Dynamics of the red and green iridophore phenotypes
Currently, changes in iridophore peak reflectance and colour are thought to be driven exclusively by the condensation of proteins named reflectins [39]. Initial condensation reflects light and increases peak reflectance, thereby increasing the visibility of the iridophore. Further condensation is accompanied by decreased platelet thickness and increased inter-platelet spacing, thus shifting the peak iridophore colour to shorter wavelengths and increased peak reflectance [24,25,27,40]. Such reports proposed that ACh activates the colour and peak reflectance changes in parallel, taking more than a minute to complete in bath application. However, precise quantification of the iridophore speed of colour and peak reflectance change had not been reported, although observations of live squid indicated changes of the order of a few seconds [23]. Our preparation allowed us to investigate the timing of these two parameters with high temporal resolution. If colour spectra and peak reflectance change were the outputs of the exact same mechanism, it would be expected that changes in their output would be proportional; altering the activation mechanism would impact colour shift and peak reflectance proportionally. However, the times required to reach half the maximum colour shift (8 s) and half the peak reflectance (15 s) differed significantly, suggesting that a second mechanism was at play. Moreover, the iridophore responses from structurally compromised tissue, where the peak reflectance increase was retained but the amount of colour shift was decreased, provided additional support for two activation mechanisms. By normalizing the responses, the red phenotype had proportional rise and decay dynamics for colour versus reflectance, while the green phenotype rise and decay dynamics were not proportional (figure 3e). Hence, this difference in the proportional dynamics of colour and reflectance indicates that the reflectin phosphorylation process [26] accounts for the full peak reflectance change, but only part of the colour shift. Consequently, a second activation mechanism seems responsible for the largest and fastest part of the colour shift. Under this hypothesis, unidentified muscles within the iridophore could alter the structure of the iridocytes.
(c). Structurally coloured iridophores are associated with neural processes
Using a whole-mount technique [30], skin nerves were traced starting beneath the iridophore layer. The branches of these nerves travelled towards the dorsal skin surface, through the iridophore layer and then seemed to terminate in the chromatophore layer. We found highly branched neurites that were contained exclusively within the bounds of an iridophore in LY-filled axon preparations and in tissue labelled with a neural specific antibody. However, we did not reliably identify synaptic boutons or en passant synapses. Part of the difficulty lies in the reflective nature of iridophores, which produce scatter, making it difficult to obtain sharp images. One reason that classical synapses innervating iridophores have not been located despite considerable efforts [27,38,41,28] is that iridophore reflectance change may be caused by a volume transmission method [42], where en passant neural terminals [43] release ACh at distances greater than a synaptic cleft and diffusion allows ACh to reach its targeted receptor [35]. ACh is now shown to be an important volume transmission signal in the vertebrate brain [44,45], while en passant terminals are common in the octopus brain [46] and proposed to control chromatophore muscles [47]. Imaging the ACh release site is the focus of ongoing investigations.
(d). Ecological importance of iridophore colour control
For successful communication, a signal must be produced at the appropriate time scale. In the squid D. pealeii, we observed that the spectral change (17 ± 4 s) was faster than the reflectance change (32 ± 8 s). The electrically stimulated response was also faster than responses elicited by exogenous ACh applications to squid L. brevis and D. pealeii (i.e. 60–90 s [23,25,48]). Furthermore, the mean wavelength iridophore colour (511 ± 26 nm) at maximum stimulation approximately matches the peak wavelength sensitivity of squid rhodopsin at 493 nm [49]. This is in accordance with previous suggestions that iridophores, with their dynamic brightness, coloration and angle dependency, are likely to function prominently in intraspecific communication [23,50–53]. Blue and green iridescent signals are also likely to play a role in interspecific signals, as in secondary defence displays [54], because these colours are predominant at a wider range of depths than long wavelengths and many predatory fishes have visual capabilities in these colours. Conversely, iridophores of D. pealeii are often shown during camouflage body patterns, and their reflected colours complement the yellow, red and brown pigmented chromatophores [55].
The dramatic squid iridophore colour changes reported previously and here are somewhat perplexing because most cephalopods are colour-blind [56–58]. However, accompanying the iridophore colour shift is a change in the polarization angle [28,36,51] that is behaviourally relevant to cephalopods (see reviews [59,60]). Thus, although polarization of the iridophore's reflectance was not measured in this study, the rapid half-maximum spectral rise time described here (5–11 s) may approximate the speed of polarization shift (30° in 1 s) reported by Shashar & Hanlon [61].
(e). Conclusions
We have shown that structural coloration of squid fin and mantle iridophores can be controlled by nerves in that specific skin layer. We provide evidence that (i) nerves in the iridophore layer of the fin carry axons that control iridophore state; (ii) ACh delivery occurs within the iridophore layer; and (iii) neuronal branching exists within the iridophores of this squid species. Our results suggest that the amount of ACh delivered to each iridophore may determine the speed of colour change (ranging 17 ± 4 s) and the total shift of the spectral peak (up to 145 ± 43 nm). That is, the animal may have fine neural control over the output of the structural coloration. In addition, the largest proportion of the iridophore colour shift is dependent upon structural tissue integrity, as the maximum peak reflectance change can be evoked by neural activation in a reduced preparation with only nerves and the iridophore layer present, but the colour shift is reduced significantly owing to the absence of the other dermal layers. We propose that the iridophore response results from the summation of two mechanisms. The first mechanism, involving reflectin protein phosphorylation, is already known and explains the large change in peak reflectance. The second mechanism responsible for most of the colour shift, which is much faster than the peak reflectance change, is undetermined, but may be the result of muscular activity, either within the iridophore or in a nearby dermal layer.
Acknowledgements
We thank fellow lab members for their support and discussion of this study, and in particular Lydia Mäthger for help with spectrometry and advice, and Tom Cronin for comments on the manuscript. We thank MBL Equipment Resources, MBL Apparatus Department and Zeiss Microscopes for assistance with equipment. We thank the MBL Central Microscopy facility for providing imaging resources and the Aquatic Resources Division of MBL for supplying squid. We are very grateful for funding from ONR Basic Research Challenge grant no. N00014-10-1-0989. Additional funding was also provided by DARPA grant W911NF-10-1-0113 and AFOSR grant FA9950090346.
References
- 1.Fox H. M., Vevers G. 1960. The nature of animal colours New York, NY: Macmillan [Google Scholar]
- 2.Fox D. L. 1976. Animal biochromes and structural colours: physical, chemical, distributional and physiological features of coloured bodies in the animal world, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- 3.Land M. F. 1972. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 24, 75–106 10.1016/0079-6107(72)90004-1 (doi:10.1016/0079-6107(72)90004-1) [DOI] [PubMed] [Google Scholar]
- 4.Denton E. J., Land M. F. 1971. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. Lond. B 178, 43–61 10.1098/rspb.1971.0051 (doi:10.1098/rspb.1971.0051) [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita S., Yoshioka S., Miyazaki J. 2008. Physics of structural colors. Rep. Prog. Phys. 71, 1–30 10.1088/0034-4885/71/7/076401 (doi:10.1088/0034-4885/71/7/076401) [DOI] [Google Scholar]
- 6.Bagnara J. T., Fernandez P. J., Fujii R. 2007. On the blue coloration of vertebrates. Pigm. Cell Res. 20, 14–26 10.1111/j.1600-0749.2006.00360.x (doi:10.1111/j.1600-0749.2006.00360.x) [DOI] [PubMed] [Google Scholar]
- 7.Vukusic P. 2006. Structural colour in Lepidoptera. Curr. Biol. 16, R621–R623 10.1016/j.cub.2006.07.040 (doi:10.1016/j.cub.2006.07.040) [DOI] [PubMed] [Google Scholar]
- 8.Greenewalt C. H., Brandt W., Friel D. D. 1960. Iridescent colors of hummingbird feathers. J. Opt. Soc. Am. 50, 1005–1013 10.1364/josa.50.001005 (doi:10.1364/josa.50.001005) [DOI] [Google Scholar]
- 9.Vukusic P., Sambles J. R., Lawrence C. R. 2000. Colour mixing in wing scales of a butterfly. Nature 404, 457. 10.1038/35006561 (doi:10.1038/35006561) [DOI] [PubMed] [Google Scholar]
- 10.Kuriyama T., Miyaji K., Sugimoto M., Hasegawa M. 2006. Ultrastructure of the dermal chromatophores in a lizard (Scincidae: Plestiodon latiscutatus) with conspicuous body and tall coloration. Zool. Sci. 23, 793–799 10.2108/Zsj.23.793 (doi:10.2108/Zsj.23.793) [DOI] [PubMed] [Google Scholar]
- 11.Eliason C. M., Shawkey M. D. 2010. Rapid, reversible response of iridescent feather color to ambient humidity. Opt. Express 18, 21 284–21 292 10.1364/OE.18.021284 (doi:10.1364/OE.18.021284) [DOI] [PubMed] [Google Scholar]
- 12.Vigneron J. P., et al. 2007. Switchable reflector in the Panamanian tortoise beetle Charidotella egregia (Chrysomelidae: Cassidinae). Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 76, 031907. 10.1103/PhysRevE.76.031907 (doi:10.1103/PhysRevE.76.031907) [DOI] [PubMed] [Google Scholar]
- 13.Mäthger L. M., Land M. F., Siebeck U. E., Marshall N. J. 2003. Rapid colour changes in multilayer reflecting stripes in the paradise whiptail, Pentapodus paradiseus. J. Exp. Biol. 206, 3607–3613 10.1242/Jeb.00599 (doi:10.1242/Jeb.00599) [DOI] [PubMed] [Google Scholar]
- 14.Kasukawa H., Oshima N., Fujii R. 1987. Mechanism of light reflection in blue damselfish motile iridophore. Zool. Sci. 4, 243–257 [Google Scholar]
- 15.Oshima N., Fujii R. 1987. Motile mechanism of blue damselfish (Chrysiptera cyanea) iridophores. Cell Motil. Cytoskel. 8, 85–90 10.1002/cm.970080112 (doi:10.1002/cm.970080112) [DOI] [Google Scholar]
- 16.Fujii R., Kasukawa H., Miyaji K., Oshima N. 1989. Mechanisms of skin coloration and its changes in the blue-green damselfish, Chromis viridis. Zool. Sci. 6, 477–486 [Google Scholar]
- 17.Nagaishi H., Oshima N. 1989. Neural control of motile activity of light-sensitive iridophores in the neon tetra. Pigm. Cell Res. 2, 485–492 10.1111/j.1600-0749.1989.tb00243.x (doi:10.1111/j.1600-0749.1989.tb00243.x) [DOI] [PubMed] [Google Scholar]
- 18.Bagnara J. T., Hadley M. E., Taylor J. D. 1969. Regulation of bright-colored pigmentation of amphibians. Gen. Comp. Endocr. 2, 425–438 10.1016/0016-6480(69)90052-5 (doi:10.1016/0016-6480(69)90052-5) [DOI] [Google Scholar]
- 19.Novales R. R., Davis W. J. 1969. Cellular aspects of control of physiological color changes in amphibians. Am. Zool. 9, 479–488 [DOI] [PubMed] [Google Scholar]
- 20.Iga T., Takabatake I., Watanabe S. 1987. Nervous regulation of motile iridophores of a freshwater goby, Odontobutis obscura. Comp. Biochem. Physiol. C Comp. Pharmacol. 88, 319–324 10.1016/0742-8413(87)90128-9 (doi:10.1016/0742-8413(87)90128-9) [DOI] [Google Scholar]
- 21.Kasukawa H., Oshima N., Fujii R. 1986. Control of chromatophore movements in dermal chromatic units of blue damselfish II. The motile iridophore. Comp. Biochem. Phys. C 83, 1–7 10.1016/0742-8413(86)90003-4 (doi:10.1016/0742-8413(86)90003-4) [DOI] [PubMed] [Google Scholar]
- 22.Muske L. E., Fernald R. D. 1987. Control of a teleost social signal I. Neural basis for differential expression of a color pattern. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 160, 89–97 10.1007/BF00613444 (doi:10.1007/BF00613444) [DOI] [PubMed] [Google Scholar]
- 23.Hanlon R. T., Cooper K. M., Budelmann B. U., Pappas T. C. 1990. Physiological color change in squid iridophores I. Behavior, morphology and pharmacology in Lolliguncula brevis. Cell Tissue Res. 259, 3–14 10.1007/BF00571424 (doi:10.1007/BF00571424) [DOI] [PubMed] [Google Scholar]
- 24.Tao A. R., DeMartini D. G., Izumi M., Sweeney A. M., Holt A. L., Morse D. E. 2010. The role of protein assembly in dynamically tunable bio-optical tissues. Biomaterials 31, 793–801 10.1016/j.biomaterials.2009.10.038 (doi:10.1016/j.biomaterials.2009.10.038) [DOI] [PubMed] [Google Scholar]
- 25.Mäthger L. M., Collins T. F. T., Lima P. A. 2004. The role of muscarinic receptors and intracellular Ca2+ in the spectral reflectivity changes of squid iridophores. J. Exp. Biol. 207, 1759–1769 10.1242/jeb.00955 (doi:10.1242/jeb.00955) [DOI] [PubMed] [Google Scholar]
- 26.Izumi M., et al. 2010. Changes in reflectin protein phosphorylation are associated with dynamic iridescence in squid. J. R. Soc. Interface 7, 549–560 10.1098/rsif.2009.0299 (doi:10.1098/rsif.2009.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper K. M., Hanlon R. T., Budelmann B. U. 1990. Physiological color change in squid iridophores II. Ultrastructural mechanisms in Lolliguncula brevis. Cell Tissue Res. 259, 15–24 10.1007/BF00571425 (doi:10.1007/BF00571425) [DOI] [PubMed] [Google Scholar]
- 28.Shashar N., Borst D. T., Ament S. A., Saidel W. M., Smolowitz R. M., Hanlon R. T. 2001. Polarization reflecting iridophores in the arms of the squid Loligo pealeii. Biol. Bull. 201, 267–268 10.2307/1543358 (doi:10.2307/1543358) [DOI] [PubMed] [Google Scholar]
- 29.Hanlon R. T. 1982. The functional organization of chromatophores and iridescent cells in the body patterning of Loligo plei (Cephalopoda, Myopsida). Malacologia 23, 89–119 [Google Scholar]
- 30.Gonzalez-Bellido P. T., Wardill T. J. In press Labeling and confocal imaging of neurons in thick invertebrate tissue samples. Cold Spring Harb. Protoc. (doi:10.1101/pdb.prot069625) [DOI] [PubMed] [Google Scholar]
- 31.Preibisch S., Saalfeld S., Tomancak P. 2009. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 10.1093/bioinformatics/btp184 (doi:10.1093/bioinformatics/btp184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng H. C., Ruan Z. C., Long F. H., Simpson J. H., Myers E. W. 2010. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets. Nat. Biotechnol. 28, 348–353 10.1038/Nbt.1612 (doi:10.1038/Nbt.1612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shigeno S., Yamamoto M. 2002. Organization of the nervous system in the pygmy cuttlefish, Idiosepius paradoxus Ortmann (Idiosepiidae, Cephalopoda). J. Morphol. 254, 65–80 10.1002/Jmor.10020 (doi:10.1002/Jmor.10020) [DOI] [PubMed] [Google Scholar]
- 34.Cooper K. M., Hanlon R. T. 1986. Correlation of iridescence with changes in iridophore platelet ultrastructure in the squid Lolliguncula brevis. J. Exp. Biol. 121, 451–455 [DOI] [PubMed] [Google Scholar]
- 35.Florey E., Cahill M. A. 1980. Cholinergic motor control of sea urchin tube feet: evidence for chemical transmission without synapses. J. Exp. Biol. 88, 281–292 [DOI] [PubMed] [Google Scholar]
- 36.Chiou T. H., Mäthger L. M., Hanlon R. T., Cronin T. W. 2007. Spectral and spatial properties of polarized light reflections from the arms of squid (Loligo pealeii) and cuttlefish (Sepia officinalis L.). J. Exp. Biol. 210, 3624–3635 10.1242/Jeb.006932 (doi:10.1242/Jeb.006932) [DOI] [PubMed] [Google Scholar]
- 37.Madan J. J., Wells M. J. 1997. A ‘hyaline’ layer in the skin of squids. J. Mar. Biol. Assoc. UK 77, 1247–1250 10.1017/S0025315400038790 (doi:10.1017/S0025315400038790) [DOI] [Google Scholar]
- 38.Mirow S. 1972. Skin color in the squids Loligo pealii and Loligo opalescens. II. Iridophores. Z. Zellforsch Mik. Ana. 125, 176–190 10.1007/BF00306787 (doi:10.1007/BF00306787) [DOI] [PubMed] [Google Scholar]
- 39.Crookes W. J., Ding L. L., Huang Q. L., Kimbell J. R., Horwitz J., McFall-Ngai M. J. 2004. Reflectins: the unusual proteins of squid reflective tissues. Science 303, 235–238 10.1126/science.1091288 (doi:10.1126/science.1091288) [DOI] [PubMed] [Google Scholar]
- 40.Sutherland R. L., Mäthger L. M., Hanlon R. T., Urbas A. M., Stone M. O. 2008. Cephalopod coloration model. I. Squid chromatophores and iridophores. J. Opt. Soc. Am. A 25, 588–599 10.1364/JOSAA.25.000588 (doi:10.1364/JOSAA.25.000588) [DOI] [PubMed] [Google Scholar]
- 41.Cloney R. A., Brocco S. L. 1983. Chromatophore organs, reflector cells, iridocytes and leucophores in cephalopods. Am. Zool. 23, 581–592 [Google Scholar]
- 42.Zoli M., Agnati L. F. 1996. Wiring and volume transmission in the central nervous system: the concept of closed and open synapses. Prog. Neurobiol. 49, 363–380 10.1016/0301-0082(96)00020-2 (doi:10.1016/0301-0082(96)00020-2) [DOI] [PubMed] [Google Scholar]
- 43.Sheard P. W., Duxson M. J. 1997. The transient existence of ‘en passant’ nerve terminals in normal embryonic rat skeletal muscle. Dev. Brain Res. 98, 259–264 10.1016/S0165-3806(96)00184-8 (doi:10.1016/S0165-3806(96)00184-8) [DOI] [PubMed] [Google Scholar]
- 44.Sarter M., Parikh V., Howe W. M. 2009. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci. 10, 383–390 10.1038/nm2635 (doi:10.1038/nm2635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamasaki M., Matsui M., Watanabe M. 2010. Preferential localization of muscarinic M(1) receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J. Neurosci. 30, 4408–4418 10.1523/Jneurosci.5719-09.2010 (doi:10.1523/Jneurosci.5719-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray E. G., Young J. Z. 1964. Electron microscopy of synaptic structure of octopus brain. J. Cell Biol. 21, 87–103 10.1083/jcb.21.1.87 (doi:10.1083/jcb.21.1.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubas F. 1987. Innervation of chromatophore muscle-fibers in the octopus Eledone cirrhosa. Cell Tissue Res. 248, 675–682 10.1007/BF00216498 (doi:10.1007/BF00216498) [DOI] [Google Scholar]
- 48.Mäthger L. M., Hanlon R. T. 2007. Malleable skin coloration in cephalopods: selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tissue Res. 329, 179–186 10.1007/s00441-007-0384-8 (doi:10.1007/s00441-007-0384-8) [DOI] [PubMed] [Google Scholar]
- 49.Hubbard R., St George R. C. C. 1958. The rhodopsin system of the squid. J. Gen. Physiol. 41, 501–528 10.1085/jgp.41.3.501 (doi:10.1085/jgp.41.3.501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shashar N., Rutledge P. S., Cronin T. W. 1996. Polarization vision in cuttlefish—a concealed communication channel? J. Exp. Biol. 199, 2077–2084 [DOI] [PubMed] [Google Scholar]
- 51.Mäthger L. M., Shashar N., Hanlon R. T. 2009. Do cephalopods communicate using polarized light reflections from their skin? J. Exp. Biol. 212, 2133–2140 10.1242/Jeb.020800 (doi:10.1242/Jeb.020800) [DOI] [PubMed] [Google Scholar]
- 52.Mäthger L. M., Denton E. J. 2001. Reflective properties of iridophores and fluorescent ‘eyespots’ in the loliginid squid Alloteuthis subulata and Loligo vulgaris. J. Exp. Biol. 204, 2103–2118 [DOI] [PubMed] [Google Scholar]
- 53.Mäthger L. M., Denton E. J., Marshall N. J., Hanlon R. T. 2009. Mechanisms and behavioural functions of structural coloration in cephalopods. J. R. Soc. Interface 6, S149–S163 10.1098/rsif.2008.0366.focus (doi:10.1098/rsif.2008.0366.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanlon R. T., Messenger J. B. 1996. Cephalopod behavior. Cambridge, UK: Cambridge University Press [Google Scholar]
- 55.Hanlon R. T., Maxwell M. R., Shashar N., Loew E. R., Boyle K. L. 1999. An ethogram of body patterning behavior in the biomedically and commercially valuable squid Loligo pealei off Cape Cod, Massachusetts. Biol. Bull. 197, 49–62 10.2307/1542996 (doi:10.2307/1542996) [DOI] [PubMed] [Google Scholar]
- 56.Brown P. K., Brown P. S. 1958. Visual pigments of the octopus and cuttlefish. Nature 182, 1288–1290 10.1038/1821288a0 (doi:10.1038/1821288a0) [DOI] [PubMed] [Google Scholar]
- 57.Marshall N. J., Messenger J. B. 1996. Colour-blind camouflage. Nature 382, 408–409 10.1038/382408b0 (doi:10.1038/382408b0)8684479 [DOI] [Google Scholar]
- 58.Mäthger L. M., Barbosa A., Miner S., Hanlon R. T. 2006. Color blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vis. Res. 46, 1746–1753 10.1016/j.visres.2005.09.035 (doi:10.1016/j.visres.2005.09.035) [DOI] [PubMed] [Google Scholar]
- 59.Cronin T. W., Shashar N., Caldwell R. L., Marshall J., Cheroske A. G., Chiou T. H. 2003. Polarization vision and its role in biological signaling. Integr. Comp. Biol. 43, 549–558 10.1093/icb/43.4.549 (doi:10.1093/icb/43.4.549) [DOI] [PubMed] [Google Scholar]
- 60.Roberts N. W., Porter M. L., Cronin T. W. 2011. The molecular basis of mechanisms underlying polarization vision. Phil. Trans. R. Soc. B 366, 627–637 10.1098/rstb.2010.0206 (doi:10.1098/rstb.2010.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shashar N., Hanlon R. T. 1997. Squids (Loligo pealei and Euprymna scolopes) can exhibit polarized light patterns produced by their skin. Biol. Bull. 193, 207–208 [DOI] [PubMed] [Google Scholar]