Abstract
Performance on perceptual tasks usually improves with training. However, too much consecutive training can be detrimental. Repeated within-day testing or overtraining demonstrates the detrimental effects this has on perceptual learning. Consolidation of learnt information during sleep has the power to prevent such deficits in learning. However, little is known regarding the role of wakeful consolidation in preventing the effects of overtraining. Here, we report that perceptual deterioration may result from the disruption of early wakeful consolidation processes. Three groups were tested on day 1 and again 24 h later, on a motion discrimination task. Participants who had a 1 h break between the two training sessions on the first day displayed improved accuracy on the second day (i.e. learning). Subjects who only completed the first training session on day 1 also exhibited learning. However, individuals who completed two blocks without a break (‘overtraining’) showed no improvement in accuracy on day 2. Interestingly, changes in reaction times were not susceptible to the effects of overtraining, but instead speeded up as a function of total performed trials. These data suggest that effects of overtraining might be due to disruption of wakeful consolidation processes.
Keywords: perceptual learning, wakeful consolidation, overtraining, perceptual deterioration, consolidation
1. Introduction
Learning a new skill is a process that causes rewiring of the brain, a phenomenon called neural plasticity. One form of neural plasticity is perceptual learning, which occurs when one’s sensory perception is improved following practice or training. Neural plasticity involves structural and functional changes, arising from the formation of new synapses and dendrites that carry information throughout the brain [1]. Once learning has occurred, the neural changes temporarily exist in a fragile state until stabilized, allowing for long-lasting improvements of the learned skill, even without additional practice [2,3]. This stabilization process, otherwise known as consolidation, involves the transfer of these changes from short-term into long-term forms of memory. If the information and/or neural changes are not adequately consolidated, then learning will be temporary or not occur at all. Various training conditions and subsequent sleep deprivation can interfere with consolidation, and thus the learning of perceptual skills. For example, training on an additional task while the first skill is still being consolidated [4] or repeated within-day training (overtraining) [5] can prevent learning. Investigating the conditions under which perceptual learning does not occur can help us to better understand the mechanisms of consolidation of cortical changes that produce long-term learning.
Overtraining in perceptual tasks can produce an initial improvement in performance, followed by gradual decline—an effect called perceptual deterioration [6–8]. Perceptual deterioration is thought to occur owing to the limited capacity of early visual areas, which become saturated with information during overtraining and hence fail to consolidate newly acquired changes [6–9]. Mednick et al. [7] found that perceptual deterioration was prevented by the introduction of brief naps between each training session, which suggests that consolidation during sleep ameliorates perceptual deterioration.
Sleep has been shown to play a crucial role in the consolidation of perceptual skills. For example, subjects failed to show significant improvement in performance on a texture discrimination task when they were deprived of sleep for 30 h after initial training [10,11]. Performance improved for subjects who were allowed normal sleep after training. This suggests that perceptual learning is only effectively consolidated when sleep occurs within a 30 h time window following training. Yotsumoto et al. [12] measured V1 activation using functional magnetic resonance imaging (fMRI) during sleep with and without preceding perceptual training. Enhanced blood oxygen level-dependent (BOLD) response in the training-related regions of V1 (but not in other regions of V1) was recorded after training, but only during non-rapid-eye-movement (NREM) sleep. Furthermore, performance following the post-training sleep session was highly correlated with the degree of BOLD response in V1 during sleep. This finding suggests that consolidation during sleep involves a highly specific low-level process, in which cortical changes directly correlate with the amount of visual perceptual learning. Interestingly, Karni et al. [13] demonstrated that rapid-eye-movement (REM) sleep also plays a functional role in the consolidation of perceptual learning, as deprivation of REM sleep interfered with the learning of texture discrimination. Therefore, both NREM and REM sleep seem to be important for consolidation.
Although sleep plays an important role in consolidation processes, the effectiveness of sleep in consolidating a newly learned skill has been found to depend on the amount of training. Using a texture discrimination task, Censor et al. [6] found that a small number of trials (approx. 200) produced learning that was unaffected by sleep, whereas a relatively larger number of trials (approx. 400) required sleep for learning to occur. A further increase in the number of trials abolished all learning effects, regardless of sleep. Therefore, in some cases, sleep is neither necessary (with small amounts of training) nor sufficient (with large amounts of training) for effective consolidation. In a landmark study, Seitz et al. [4] found evidence to suggest that wakeful consolidation can occur within 1 h of training on a perceptual task. Learning on a Vernier acuity task was disrupted when a different Vernier task was performed within 1 h of the first task. However, learning occurred when the second task was administered more than 1 h after the first task, suggesting the occurrence of wakeful consolidation.
Taken together, these studies point to the existence of two distinct stages of consolidation (awake and asleep), with each stage possibly containing multiple phases (e.g. NREM and REM during the sleep stage) [2]. It seems likely that there is considerable overlap between these stages, and interference at any stage of consolidation might disrupt subsequent stages, and hence learning. However, the role of wakeful consolidation in processes of overtraining and perceptual deterioration is unknown. Here, we explore the effects of overtraining using a motion discrimination task. We report that simply inserting a 1 h break in the middle of what would otherwise be an overtraining regime results in improved direction discrimination 24 h later. We propose that pausing training like this allows the processes of wakeful consolidation to engage, hence allowing learning to occur.
2. Methods
(a). Participants
Thirty-one undergraduate students participated in the experiment in exchange for course credit. All participants had normal or corrected-to-normal vision and were naive to the purpose of the study.
(b). Apparatus
The experiment was conducted in a darkened room with black walls, on a calibrated monitor (19 inch IBM P275) with a resolution of 1280 × 960 pixels, and a refresh rate of 100 Hz. The experiments were run in Matlab, using Psychophysics toolbox extensions on a Mac Pro computer [14,15]. Participants responded using the left and right arrow keys on the keyboard, to indicate whether they thought the coherent dots were moving leftward or rightward. A fixed viewing distance of 57 cm for all experiments was achieved using a chin rest, and participants were instructed to maintain fixation on a bull's-eye fixation point (visual angle of 0.5° in diameters) for the entire experiment.
(c). Stimuli
Random-dot motion stimuli [16,17] consisted of 100 dots that were randomly distributed inside a circular aperture for which the diameter was 11°. Each dot was two pixels, which was approximately 0.07°. All dots moved with a constant speed of 18.7° s–1. In each trial, a proportion of dots moved coherently (in a single direction), while the others moved in random directions. A range of six signal coherences was used: 2, 4, 10, 17, 25 and 45 per cent. On average, dot density was 2.7 dots per square degree and in order to conserve dot density, any of the signal dots that moved along a trajectory that would place them outside of the circular aperture were wrapped around to appear from the opposite side. Three uncorrelated sequences of dot movement were generated, and frames were interleaved so that the positions of the dots in frame four were correlated only with the dots in frames one and/or seven, and with none of the other frames. That is, each frame was correlated only with a frame that was either three frames backwards or forwards, and not the subsequent frame [16,17].
(d). Procedure
A brief practice phase consisting of approximately 10 trials was allowed prior to the commencement of training to familiarize subjects with the stimulus and task. In each trial, the stimulus was presented for 450 ms, followed by an inter-trial interval of 750 ms, during which only the fixation point was present. The next trial automatically started following the interval. Subjects focused on the fixation point until the stimulus appeared, and then decided which direction they thought the coherently moving dots were moving by pressing the appropriate key (left or right arrow key). Subjects were instructed to respond as accurately and quickly as possible. Responses could be made during stimulus presentation or in the inter-trial interval. If subjects did not respond during this time window that trial would be repeated. Auditory feedback was provided for every response: a high-pitched ‘beep’ for a correct response and a low-pitched ‘thud’ for an incorrect response. Each session consisted of 480 trials separated into five separate blocks of 96 trials. Following each block, the experimenter entered the room to start the next block. Within each block, there were 16 trials at each coherence level, with eight trials of each motion direction (left and right) in a randomly interleaved order.
A between-groups design was used, with subjects being randomly assigned to one of three different conditions: control, overtraining and delay. Learning was defined as between-day performance improvement (see §2e). In the normal learning control condition, subjects performed one session on day 1 and another on day 2 to establish whether learning would occur. In the overtraining condition, subjects performed two sessions on day 1 and one on day 2 to examine whether extended task performance on day 1 would interfere with learning. The delay condition was included to determine whether taking a 1 h break between the two sessions on day 1 would ameliorate any detrimental effects of overtraining. During the 1 h delay, subjects were given the option to either leave the laboratory or wait until 1 h had passed before commencing the second session.
(e). Data preparation and analysis
Accuracy data for each participant was inspected to assess monotonicity as a function of motion strength. This was done to ensure participants were performing the motion discrimination task correctly, as performance is known to monotonically increase with motion coherence [18]. If performance did not increase in a monotonic pattern for a particular block, data for that block were excluded from further analysis, along with corresponding reaction time data. Only accuracy data from monotonic blocks were analysed. Overall, five blocks in the normal learning condition, three blocks in the overtraining condition and five blocks in the delay condition met this exclusion criterion, and hence were not further analysed.
Data were also eliminated from subjects who consistently performed at near-chance levels for all levels of coherence, as this indicated that they could not perform the task even at the easiest levels of motion coherence. Two subjects were dropped from the normal learning control condition, three from the overtraining condition and one from the 1 h delay condition.
3. Results
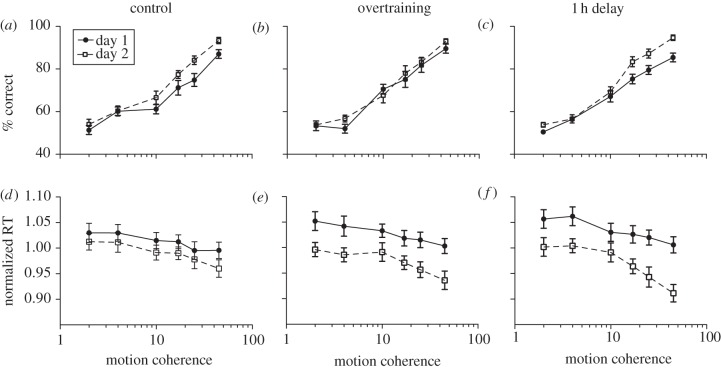
To determine whether overtraining disrupts learning, it must first be confirmed that learning can occur within our brief two-day learning paradigm. Figure 1a shows average accuracy on day 1 (solid line) and day 2 (dashed line) as a function of motion coherence. The two plots appear to diverge from 10 per cent motion coherence onwards. To quantify learning (a change in accuracy from day 1 to day 2), a comparison of cumulative Gaussian fits was conducted, which revealed a significant learning effect (F2,116 = 8.768, p < 0.001). This was supported by a repeated measures ANOVA, with which a significant main effect for time was found (day 1 versus day 2; F1,9 = 10.607, p < 0.001).
Figure 1.
Learning effects for accuracy and reaction times. (a–c) Solid lines show accuracy (% correct) for session 1 day 1, dashed lines show data for day 2. (d–f) Solid lines show normalized reaction times for session 1 day 1 dashed lines show data for day 2. (a,d) Control: subjects training for one session on day 1. (b,e) Overtraining condition: subjects training for two consecutive sessions on day 1. (c,f) Delay condition: same as overtraining condition except a 1 h delay was introduced between the two sessions on day 1. All error bars show ± s.e. of the mean.
Next, we examined data from subjects who performed twice the amount of trials on day 1 (960 trials: overtraining). Figure 1b shows data plots from session 1 on day 1 and day 2. No significant learning occurred for this group across the two days (F-test of two cumulative Gaussian fits and a repeated-measures ANOVA: both p > 0.05). This outcome dovetails nicely with perceptual learning studies that report repeated same-day testing (or overtraining) produces deterioration in performance, or perceptual deterioration, compared with less training on the first day [7–8].
Figure 1c shows the data for when the two sessions on day 1 were separated by 1 h (delay condition). Here, significant learning was found (F-test of two cumulative Gaussian fits: F2,44 = 10.28, p < 0.001; and repeated-measures ANOVA: F1,10 = 21.879, p = 0.001), demonstrating that a 1 h break between sessions (the same total number of trials as the overtraining condition) was sufficient to prevent perceptual deterioration by overtraining.
We also measured subjects' reaction times in all conditions, a common dependent measure when using random dot motion stimuli, as it allows for further investigation into the underlying mechanisms (normalized reaction times are shown in figure 1d–f). Repeated-measures ANOVAs were used to quantify any reduction in raw reaction times across the two days. For the normal learning condition, there was no significant reduction in reaction times (p > 0.05). For the overtraining condition, there was a visibly larger decrease in reaction times compared with the normal learning condition; however, this trend was non-significant (p = 0.067). In the 1 h delay condition, there was a significant reduction in reaction times (F1,10 = 5.816, p = 0.037). Changes in reaction times were not susceptible to the effects of overtraining or consolidation; faster reaction times seemed to follow the total number of experimental trials. This relationship would explain why reaction times appeared to decrease in the overtraining and delay conditions, but not the normal learning condition. This disparity between across-day changes in performance and reaction times suggests that different mechanisms might be involved in performance improvements and the reduction in reaction times. The reduction in reaction times was relatively uniform across all levels of motion coherence, evidenced by a lack of any interaction effect regarding across-day reduction in reaction times as a function of coherence (p > 0.05 for all three conditions).
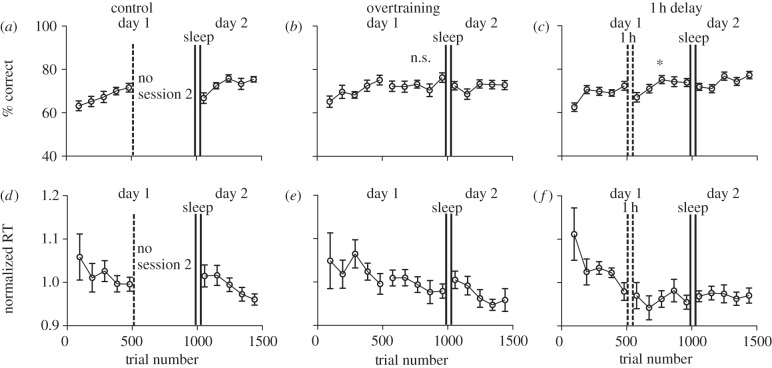
To further explore these data and to determine which parts of the procedure were responsible for the differences in accuracy, we examined learning from block to block within each session. Figure 2 shows mean accuracy (% correct, collapsed across motion coherence) as a function of trial number. A key feature of interest is the relative discrepancy between the overtraining and 1 h delay conditions—the difference between the endpoint of data for the first session and the starting point of data for the second session on day 1 of training. In other words, does the 1 h break change accuracy on session two of day 1? For the overtraining condition, a paired-samples t-test revealed a marginal, albeit significant difference between these two points (M = 75, s.d. = 7; M = 72, s.d. = 8; t10 = 2.3, p = 0.047, d = 0.38; figure 2b). For the delay condition, this difference was greater, with the starting point of the second session (M = 67, s.d. = 7) being significantly lower than the endpoint of the first session on the first day of training (M = 72, s.d. = 6; t10 = 2.63, p = 0.025, d = 0.796; figure 2c). Thus, performance was notably lower following a 1 h break, perhaps due to consolidation processes rendering information from the first session not immediately available for performance in the second session on the same day. In comparison, when subjects trained continuously for two consecutive sessions, performance did not appear to drop suddenly, indicating that task-relevant information remained accessible for performance throughout the duration of training on day 1. In addition, a repeated-measures ANOVA revealed a significant main effect of trial (F1,10 = 5.23, p = 0.002) and a significant linear trend (F1,10 = 13.012, p = 0.005) in the 1 h delay condition in the second session on day 1 (figure 2c). This shows that although performance dropped following the 1 h break, it rose steadily during the second session, demonstrating learning. In the overtraining condition, however, there was no evidence of learning during the second session on day 1, as a repeated-measures ANOVA showed no significant effect for trial nor a linear trend (p > 0.05; figure 2b).
Figure 2.
Accuracy and reaction time changes across trials. Solid vertical lines represent the period of time during which sleep occurred. (a–c) Accuracy (% correct) and (d–f) normalized reaction times as a function of trial number averaged across coherence levels. (a,d) Control: subjects performed only one session on day 1. (b,e) Overtraining condition: subjects performed two consecutive sessions on day 1. (c,f) Delay condition: subjects performed two sessions on day 1, separated by a 1 h delay (represented by dashed vertical lines). All error bars show ± s.e. of the mean. The asterisk over the second session in (c) indicates a positive linear trend, whereas the ‘n.s.’ over the second session in (b) indicates a non-significant linear trend.
Inspecting the changes in reaction time from block to block in the first session (figure 2d–f), it can be seen that the most substantial reduction during this session occurred for the 1 h delay condition (figure 2f). This may explain why the across-day reduction was significant for the 1 h delay condition (figure 1f) and not the overtraining condition (figure 2e).
4. Discussion
The current study demonstrates that learning of motion discrimination failed when subjects engaged in too much consecutive training, or overtraining. Interestingly, learning was restored with the introduction of a 1 h break. The effects of overtraining in previous studies have been understood in terms of perceptual deterioration, which occurs due to neural saturation or fatigue of early visual areas [2,3,5–9].
The current findings are the first to show that overtraining interferes with wakeful consolidation. In line with Mednick et al. [7], who found that brief naps between sessions prevented perceptual deterioration and restored learning, simply introducing a 1 h break on day 1 prevented perceptual deterioration in the present study. This suggests a consolidation mechanism that not only works to stabilize perceptual changes during sleep, but also while awake. In support, Seitz et al. [4] demonstrated that wakeful consolidation was disrupted by training on a related but different task immediately after initial training, but not if a 1 h period lapsed before onset of the second task. The authors concluded that the second task (when occurring immediately after the first) overwrote the fragile memory trace formed by training on the first task. However, the introduction of a 1 h break allowed for the memory traces formed by training on the first task to stabilize and become immune to interference from the second task. Similarly, we propose that the 1 h break in the current study allowed the memory traces formed during training in the first session to consolidate and develop immunity to saturation from overtraining.
Interestingly, performance at the beginning of the second session, directly after the 1 h break, was lower than performance without any break, suggesting that some information may not have been available. One explanation for this may be that information learnt from session 1 entered into a stage of consolidation where it was temporarily unavailable or was somewhat reformatted for more permanent storage. Nevertheless, more learning was evident within the second session following the 1 h break, whereas learning seemed to plateau more in the second session of the overtraining condition. That is, performance seemed to drop off following a 1 h break, then recover with additional training, suggesting additional learning processes were engaged during the second session, after the delay. These additional learning processes may have complimented the learning that occurred during the first session and seemed to be beneficial for subsequent performance on day 2.
Alternatively, the beneficial effects of an early period of wakeful consolidation may extend into later stages of consolidation and/or learning. It is possible that wakeful consolidation facilitates subsequent sleep consolidation and even learning processes on day 2. In contrast, a different type of learning process that occurs under fatigued conditions (i.e. when overtraining) could explain the differences between the overtraining and 1 h delay condition. For example, further practising a sport such as skiing once fatigued (and hence performing poorly) may cause the skier to learn ‘sloppy form’, which might lead to poorer skills the next day. In a similar fashion, sensory neurons that are performing perceptual calculations sub-optimally because they are fatigued could lead to learning sub-optimal routines or calculations. Such a theory puts the focus on new suboptimal learning of information rather than the consolidation of previously learnt information. This alternative theory can account for the current data and previous behavioural reports on wakeful consolidation.
Another question raised by the current findings is why overtraining does not appear to affect reaction times in the same way as it affects accuracy. In addition, the reduction in reaction times seems to be uniform across motion coherences. One possibility is that the mechanisms that drive changes in reaction times differ from those driving accuracy. More specifically, reaction time changes may be due to non-sensory processes such as motor learning that continue to improve for low motion coherences even though the sensory components do not.
In conclusion, overtraining in perceptual learning of motion discrimination can be understood in terms of neural saturation or fatigue in task-related areas, which interferes with wakeful consolidation. This interference effect can be alleviated with a short break of 1 h, suggesting that a critical phase of consolidation occurs within this 1 h period. The current findings highlight the fragility of initial memory traces formed when learning a novel perceptual or decisional task and the importance of taking breaks to allow for consolidation. It will be exciting to see what future research unveils regarding overtraining and consolidation as general principles of the nervous system. Identifying the mechanisms (psychological, neural, pharmacological, etc.) behind such processes should lead to a greater understanding of the optimal learning conditions across a range of applications.
Acknowledgements
We thank Franco Caramia for programming support and Alexandra Vlassova for helpful comments. This work was supported by an NHMRC CJ Martin Fellowship 457146 and NHMRC project grant no. APP1024800 to J.P. and UNSW faculty grants.
References
- 1.Wandell B. A., Smirnakis S. M. 2009. Plasticity and stability of visual field maps in adult primary visual cortex. Nat. Rev. Neurosci. 10, 873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sagi D. 2011. Perceptual learning in vision research. Vision Res. 51, 1552–1566 10.1016/j.visres.2010.10.019 (doi:10.1016/j.visres.2010.10.019) [DOI] [PubMed] [Google Scholar]
- 3.Sasaki Y., Nanez J. E., Watanabe T. 2010. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 11, 53–60 10.1038/nrn2737 (doi:10.1038/nrn2737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seitz A. R., Yamagishi N., Werner B., Goda N., Kawato M., Watanabe T. 2005. Task-specific disruption of perceptual learning. Proc. Natl Acad. Sci. USA 102, 14 895–14 900 10.1073/pnas.0505765102 (doi:10.1073/pnas.0505765102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mednick S. C., Arman A. C., Boynton G. M. 2005. The time course and specificity of perceptual deterioration. Proc. Natl Acad. Sci. USA 102, 3881–3885 10.1073/pnas.0407866102 (doi:10.1073/pnas.0407866102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Censor N., Karni A., Sagi D. 2006. A link between perceptual learning, adaptation and sleep. Vision Res. 46, 4071–4074 10.1016/j.visres.2006.07.022 (doi:10.1016/j.visres.2006.07.022) [DOI] [PubMed] [Google Scholar]
- 7.Mednick S. C., Nakayama K., Cantero J. L., Atienza M., Levin A. A., Pathak N., Stickgold R. 2002. The restorative effect of naps on perceptual deterioration. Nat. Neurosci. 5, 677–681 [DOI] [PubMed] [Google Scholar]
- 8.Ofen N., Moran A., Sagi D. 2007. Effects of trial repetition in texture discrimination. Vision Res 47, 1094–1102 10.1016/j.visres.2007.01.023 (doi:10.1016/j.visres.2007.01.023) [DOI] [PubMed] [Google Scholar]
- 9.Mednick S., Nakayama K., Stickgold R. 2003. Sleep-dependent learning: a nap is as good as a night. Nat. Neurosci. 6, 697–698 10.1038/nn1078 (doi:10.1038/nn1078) [DOI] [PubMed] [Google Scholar]
- 10.Stickgold R., Whidbee D., Schirmer B., Patel V., Hobson J. A. 2000. Visual discrimination task improvement: a multi-step process occurring during sleep. J. Cogn. Neurosci. 12, 246–254 10.1162/089892900562075 (doi:10.1162/089892900562075) [DOI] [PubMed] [Google Scholar]
- 11.Stickgold R., James L., Hobson J. A. 2000. Visual discrimination learning requires sleep after training. Nat. Neurosci. 3, 1237–1238 10.1038/81756 (doi:10.1038/81756) [DOI] [PubMed] [Google Scholar]
- 12.Yotsumoto Y., et al. 2009. Location-specific cortical activation changes during sleep after training for perceptual learning. Curr. Biol. 19, 1278–1282 10.1016/j.cub.2009.06.011 (doi:10.1016/j.cub.2009.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karni A., Tanne D., Rubenstein B. S., Askenasy J. J., Sagi D. 1994. Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265, 679–682 10.1126/science.8036518 (doi:10.1126/science.8036518) [DOI] [PubMed] [Google Scholar]
- 14.Pelli D. G. 1997. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 10, 437–442 10.1163/156856897X00366 (doi:10.1163/156856897X00366) [DOI] [PubMed] [Google Scholar]
- 15.Brainard D. H. 1997. The psychophysics toolbox. Spat. Vis. 10, 433–436 10.1163/156856897X00357 (doi:10.1163/156856897X00357) [DOI] [PubMed] [Google Scholar]
- 16.Roitman J. D., Shadlen M. N. 2002. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 22, 9475–9489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shadlen M. N., Newsome W. T. 2001. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 86, 1916–1936 [DOI] [PubMed] [Google Scholar]
- 18.Gold J. I., Shadlen M. N. 2007. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 10.1146/annurev.neuro.29.051605.113038 (doi:10.1146/annurev.neuro.29.051605.113038) [DOI] [PubMed] [Google Scholar]