Abstract
Reef-building corals form bio-diverse marine ecosystems of high societal and economic value, but are in significant decline globally due, in part, to rapid climatic changes. As immunity is a predictor of coral disease and thermal stress susceptibility, a comprehensive understanding of this new field will likely provide a mechanistic explanation for ecological-scale trends in reef declines. Recently, several strides within coral immunology document defence mechanisms that are consistent with those of both invertebrates and vertebrates, and which span the recognition, signalling and effector response phases of innate immunity. However, many of these studies remain discrete and unincorporated into the wider fields of invertebrate immunology or coral biology. To encourage the rapid development of coral immunology, we comprehensively synthesize the current understanding of the field in the context of general invertebrate immunology, and highlight fundamental gaps in our knowledge. We propose a framework for future research that we hope will stimulate directional studies in this emerging field and lead to the elucidation of an integrated network of coral immune mechanisms. Once established, we are optimistic that coral immunology can be effectively applied to pertinent ecological questions, improve current prediction tools and aid conservation efforts.
Keywords: invertebrate immunity, coral immunology, melanin, coagulation, TOLL-like receptors, immune cells
1. Introduction
Human-induced local impacts and climatic changes are threatening the persistence of coral reefs [1]. This is largely due to a coincident increase in occurrences of compromised coral health, such as disease and the breakdown of symbiosis with Symbiodinium algae, known as bleaching [2]. As immunity determines, the ability of an organism to resist and eliminate infection and to recover from injury, it can be used as a predictor of compromised health susceptibility [3,4]. Therefore, understanding immune mechanisms will likely enable better insight into coral declines at individual, population and ecological scales. However, coral immunology is an emergent field, with many disjointed studies and lacks a comprehensive synthesis of the current knowledge [5].
Reef-building corals (Order Scleractinia, Class Anthozoa) fall within the phylum Cnidaria, and therefore not only occupy a vital ecological niche, but also a basal position in metazoan phylogeny. Concomitantly, innate immunity is an evolutionarily ancient system, suggesting that the origins of well-documented immune mechanisms of bilaterian organisms may reside within the basal phyla [6]. However, cnidarians' anatomical simplicity and phylogenetic distance from mammals, led to assumed genomic simplicity [7], and thus they have been overlooked as immunological models. However, it has recently become apparent that anthozoans possess a genomic complexity more similar to vertebrates than model invertebrates (e.g. Drosophila melanogaster; [8]), suggesting that the field of immunology may benefit from an understanding of cnidarian immunity [9].
This review defines the current field of coral immunology by placing it within the context of the better-established field of invertebrate immunology. In doing so, we highlight key knowledge gaps and provide order to the recent surge of studies, many of which remain overlooked by the fields of coral biology and/or invertebrate immunology. In the light of the urgent need for mechanistic explanations of compromised coral health, we also develop a comprehensive framework for future coral immunological studies, emphasizing the necessity of establishing a solid foundation of fundamental knowledge, to encourage the rapid and effective expansion of this vital field.
2. Invertebrate innate immunity
Invertebrates have evolved innate immune mechanisms to resist infection and maintain tissue integrity, but lack the adaptive immune systems of vertebrates [9]. An innate immune response delivers an immediate reaction to the presence of non-self and/or compromised tissue integrity in three broad phases: recognition of a threat (detection of non-self; allorecognition), signalling pathways to activate appropriate defence mechanisms and effector responses, which are responsible for eliminating the threat ([10]; figure 1). Key effector responses that will be addressed in this review include antimicrobial peptides (AMPs), melanin synthesis, coagulation and immune cell activation. Although the innate immune phases can be employed in response to numerous immune activators, such as parasitism [13], allorecognition (see reviews by [14] and [5]) and potentially by symbiosis establishment [2], innate immunity is perhaps most commonly referred to in response to infectious disease and injury. In this review, we therefore focus on the network of immune mechanisms invertebrates employ in response to these latter two immune activators.
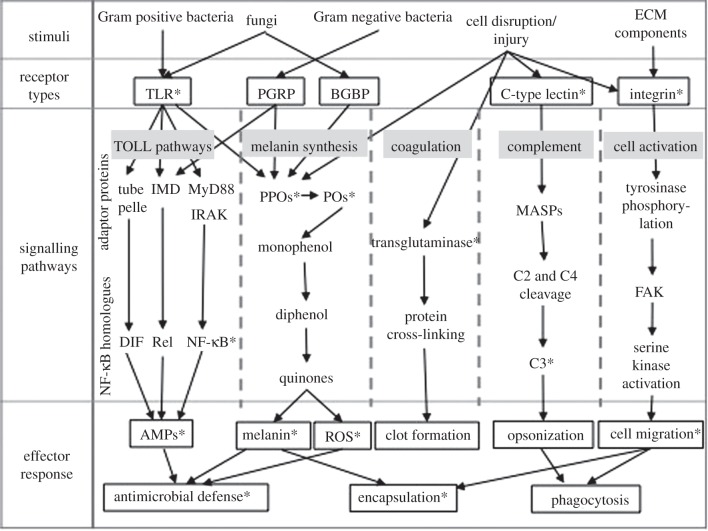
Figure 1.
A simplified overview of the main invertebrate immunity components (synthesized from [11] and [12]). Starred (*) components have been identified within anthozoans. TLR, TOLL-like receptor; PGRP, peptidoglycan recognition protein; BGBP, beta-glucan binding protein; IMD, immunodeficiency; MyD88, myeloid differentiation primary-response protein 88, PPOs, prophenoloxidases; POs, phenoloxidases; MASPs, mannose binding lectin-associated serine proteases; FAK, focal adhesion kinases; AMPs, antimicrobial peptides; ROS, reactive oxygen species.
(a). Recognition
Invertebrates employ a suite of pattern recognition receptors (PRRs) and soluble proteins to recognize a broad array of pathogenic microbes and cellular disruption ([15]; figure 1). PRRs bind to conserved pathogen-associated molecular patterns (PAMPs) [16], such as the lipopolysaccharides (LPS; [7]). Each receptor binds to one or more PAMP and is responsible for inducing appropriate signalling pathways (figure 1). Numerous PRRs have been identified within invertebrates, and several recognition-related genes have been identified within corals during microarray and transcriptional studies in response to environmental stress, e.g. tachylectin [17] and a spondin [18]. However, to date, only three have been specifically investigated as immune-related genes: TOLL-like receptors (TLRs), lectins and integrin ([19,20]; figure 1).
(i). TOLL-like receptors
TLRs are highly conserved trans-membrane proteins that recognize PAMPs and activate signalling for AMP synthesis ([21]; figure 1). TLRs consist of a leucine-rich repeat (LRR) outer domain, which determines the PAMPs they bind to [22], and cytoplasmic components with a TOLL/interleukin-1receptor homology (TIR) domain (e.g. adaptor protein MyD88) that activate downstream signalling pathways [15,21]).
Consistent with their high evolutionary conservation, TLRs have been identified across the invertebrates, for example, within Porifera [23], Nematoda [21], Echinodermata and Arthropoda (see review [24]). Within Cnidaria, complete TLRs (with both TIR domains and LRRs) have only been characterized within two species, one within the anemone Nematostella vectensis [20] and five TLRs in the coral Acropora digitifera [25]. However, N. vectensis has five additional TIR domains [20,25,26], while Hydra sp. possesses two [27,28] and Acropora millepora has one within an investigated transcriptome [20]. Although only identified within two cnidarians, the presence of TLRs needs to be confirmed within a representative suite of species and their role within coral immunity needs to be corroborated with functional studies, which are conspicuously absent [20]. While searches of available transcriptome and genome databases would provide a starting point, elucidation of coral TLR stimuli and the resultant responses would represent a great leap towards a full understanding of the coral immune network.
(ii). Lectins
C-type lectins belong to a superfamily of carbohydrate-binding proteins that are dissolved within the extracellular matrix (ECM) or are cell surface receptors [29] and are important to a diversity of innate immune functions [11]. Lectins activate signalling pathways that lead to cell adhesion and opsonization [29], induce the melanin-synthesis (phenoloxidase, PO) cascade [30] and AMP production [31].
Many C-type lectins, such as the mannose-binding lectin (MBL), have been identified within numerous invertebrates, including the Nematode Caenorhabditis elegans and Arthropods, e.g. D. melanogaster (reviewed by [11]). C-type lectins have also been identified within the cnidarians N. vectensis [32], Hydra sp. [33] and corals, including Montastraea faveolata, A. millepora [34], Oculina varicose [35] and Porites astreoides [18]. In addition to its identification within A. millepora, the mannose-binding-like lectin gene, Millectin, showed increased expression when exposed to a bacterial challenge and Symbiodinium endosymbionts [19]. This represents one of the few studies confirming cnidarian immune gene homologue function, and suggests an involvement in symbiosis. In order to provide further insight into the role of this lectin within the coral immunity network, downstream pathway activation and the resultant effector responses need to be investigated.
(iii). Integrins
Integrins are trans-membrane alpha beta heterodimers that mediate interactions between cells and the ECM [36]. In addition to fundamental roles in development, integrins are involved in multiple immunological cellular processes, such as cell migration and differentiation, as well as fibrillar matrix formation and signal transduction [37].
Integrins are found throughout the invertebrates, including within Arthropoda; D. melanogaster [38] and Porifera; Suberites domuncula [39]. Within Cnidaria, three alpha and four beta integrin subunits have been identified in N. vectensis, indicating at least three complete integrins [8,32]. Hydra magnipapillata has at least one alpha and beta integrin present [28] and two beta subunits have been identified in the hard coral A. millepora [20,40,41]. Although the diversity of cnidarian integrins is more complex than first thought [41], functional studies need to be conducted. The presence and diversity of integrins also needs to be confirmed within a broader suite of coral species.
(b). Signalling pathways
(i). Antimicrobial peptide synthesis pathways
AMPs rapidly kill a broad spectrum of microbes and are produced by all multi-cellular organisms [42]. Most organisms have multiple AMPs that are produced by different pathways, e.g. TOLL or IMD pathway ([12]; figure 1). Insect AMP synthesis signalling pathways are activated via the TIR adaptor proteins and comprise a suite of components, including a protein complex family of transcription factors known as nuclear factor kappa B (NF-κB)/Rel and their corresponding inhibitors ([21]; figure 1). In addition to insects and other higher invertebrates (e.g. Mollusca; [43]), TOLL pathway components have been found within ancestral metazoans, including Porifera [44]. Within the cnidarian N. vectensis, multiple TOLL pathway components have been identified, including an NF-κB inhibitor (IκB), NF-κB, a mitogen-activated protein kinase (MAPK) and a nucleotide-binding domain (reviewed by [21]). Consistent with the lack of a complete TLR within H. magnipapillata, only one pathway component was identified, potentially suggesting gene loss [20]. Three TOLL pathway components were located with an A. millepora transcriptome [20]. These studies demonstrate the presence of this pathway within non-bilaterian animals for the first time, confounding hypotheses that immune-related TOLL evolved after the divergence of Nematoda [21]. However, the role of cnidarian TOLL pathway components in immunity cannot yet be confirmed as functional studies are lacking.
(ii). Proteolytic cascades
Melanin-synthesis pathways. The melanin-synthesis pathways, synonymous with the prophenoloxidase (PPO) system, are responsible for cytotoxic defence and the melanization (encapsulation) of foreign bodies (reviewed by [45]; figure 1). Melanin synthesis is triggered by pathogens, their derived products and upon injury, leading to cleavage of PPO into PO. Melanin synthesis is then the rapid outcome of a cascade involving the catalysis of monophenol hydroxylation and diphenol oxidation by various POs, as well as autocatalytic reactions ([45]; figure 1). PPOs, and POs, exist in various isoforms that represent different components of, potentially several, melanin-synthesis pathways, which likely have differing functions [46,47]. While the tyrosinase-type melanin synthesis pathway has been the most intensively investigated with regard to invertebrate immunity [48,49], an invertebrate laccase-type pathway has also been documented [50]. However, aside from a likely role in cuticle formation, roles of the laccase-type pathway remain to be elucidated [46,50].
Although traditionally studied within the Arthropoda [46,51], melanin-synthesis pathways are present across the invertebrates, including Mollusca [50] and Annelida [52]. A melanin-synthesis pathway was the first classic invertebrate immune response to be investigated within corals, with PO and PPO activities documented in two hard coral species, A. millepora and Porites sp. [13], and the gorgonian sea fan Gorgonia ventalina [53,54]. Increased melanin-synthesis pathway activities in compromised [13], infected [53] and thermally stressed tissue [55] were also demonstrated. Subsequently, PO activity was documented within 22 healthy Indo-Pacific hard and soft corals and, in conjunction with other immunity parameters, can be used to predict coral families' susceptibility to both disease and bleaching [4]. Significantly, functional studies [55] and the potential use of melanin-synthesis activity as a predictive tool demonstrate the role of this pathway not only in coral resistance to infection but also to bleaching.
Further demonstrating the importance of melanin-synthesis within corals, POs and PPOs indicating the presence of both laccase and tyrosinase-type melanin pathways have been identified within suites of Indo-Pacific and Caribbean corals [56,57]. However, field sampling of diseased A. hyacinthus colonies has raised currently unresolvable questions of the role of baseline immunity in resisting disease versus immunocompetence in pathogen elimination [58]. Similarly, the variable responses of PPOs to LPS and thermal treatments among three Caribbean coral species indicates that they likely use this innate immunity component distinctly [59], reinforcing the need for multi-species approaches to coral immunology studies.
Coagulation cascades. Coagulation ensures the rapid re-establishment of tissue integrity after injury [60] to prevent fluid loss and infection [45]. Transglutaminases are essential coagulation enzymes and function by forming a gel upon interaction with plasma proteins (reviewed by [45]). Transglutaminases have been identified within molluscs [61] and a suite of arthropods (reviewed by [62]). Coagulation remains poorly investigated within anthozoans, with transglutaminase activity only documented within one reef-building coral Porites cylindrica [57]. Therefore, coral transglutaminases need to be conclusively identified within a suite of corals and their role within coagulation functionally tested.
(iii). Immune cell activation
Immune cells communicate via chemical signalling within both their internal and external environments [36] and are primarily responsible for phagocytosis and encapsulation (figure 1). Immune cell activity cross-links with multiple effector responses, and thus immune cells have numerous activators, including the lectin complement network and integrin signalling [12].
The complement system forms a bridge between innate and adaptive immunity and results in opsonization (figure 1), whereby phagocytosis is enhanced via host tagging of pathogens [63]. In invertebrates, the complement system can be activated by the lectin and alternative pathways, which both culminate in the activation of a2-macroglobin protein C3 [11]. The lectin pathway (figure 1) is triggered by C-type lectin receptors, e.g. MBL, which leads to the cleavage of pathway components C4 and C2 to form C3 [11,63]. The alternative pathway does not involve receptors, but is activated by C3 binding to micro-organisms [11,63]. Components of the complement system, such as C3 and homologues that contain a thiloester protein (TEP), have been located within a diverse range of invertebrates, e.g. echinoderms and arthropods (reviewed by [64]). C3 has been identified within several anthozoans; Swiftia exserta [65], N. vectensis [32] and the hard corals A. millepora [20,66] and Porites lobata [18], and is thus indicative of lectin-mediated cellular immune functions within corals. However, although C3 genes have been included in expression studies of thermal stress [18,67], functional studies targeting the role of C3 in coral immunity are lacking.
Integrins signal both mechanically via cell anchorage and chemically by outside-in signalling into the cell [36]. Integrin signalling induces the activation of focal adhesion kinases (FAKs; figure 1), a central component of cell migration and cell-cycling [68]. FAK associates with other signalling molecules [68,69], causing phosphorylation and signalling, which leads to a diverse array of immune cell-related effector responses ([68]; figure 1). Though integrin signalling pathway components have been identified within many invertebrates, including arthropods [70] and echinoderms [71], they remain unidentified within cnidarians. The lack of information on cnidarian integrin pathway components, and thus cell signalling, represents a significant gap in our current knowledge of coral immunity.
(c). Effector responses
(i). Antimicrobial peptide activity
AMPs are located within granular immune cells and on epithelial layers [42] and act by disrupting microbial cell membranes [72]. A suite of AMPs is usually associated with a single organism, such as the seven types described for Drosophila [73]. AMPs have been identified in a wide variety of invertebrate phyla, including within the Porifera (Discodermia sp.), Arthropoda, e.g. Penaeus vannamei and Echinodermata, e.g. Stronglyocentrotus droebachiensis and the Cnidiarian jellyfish Aurelia aurita (reviewed by [74]). Within H. magnipapillata, three AMPs have been identified, despite the proposed loss of representative TOLL pathway components [20], and are produced in response to a variety of pathogens, including proteobacteria and E. coli [27,75]. Conversely, no AMPs have been documented within the anemone N. vectensis, despite the suite of TOLL pathway components being located within the genome [20], though this is likely an artefact of incomplete investigation, rather than an actual absence of AMPs. One AMP (Damicornin) was recently identified in the hard coral Pocillopora damicornis, and demonstrated activity in response to both Gram-positive bacteria and fungi [76].
Although AMPs have only been confirmed in one anthozoan (P. damicornis), bactericidal activity has been documented for numerous anthozoans [55,59,77]. In addition to AMPs, these bactericidal activities may be due to mucus-associated antibiotic-producing microbes, small peptides and secondary metabolites (reviewed by [78]). The origin of coral bactericidal activity needs to be determined in order to accurately interpret results of inoculation studies where, for example, bactericidal activity of coral tissue decreased upon exposure to a pathogen elicitor [59]. Similarly, further studies are needed to elucidate the suite of AMPs that are potentially synthesized by hard corals, and the pathogens they eliminate.
(ii). Melanin synthesis
The products of melanin-synthesis pathways (melanin and cytotoxic intermediates; figure 1) are central to invertebrate immunity [46]. Melanin creates a physical barrier, encapsulating invading organisms, protecting healthy tissue and providing structural support during tissue repair [46]. Cytotoxic by-products, including reactive oxygen species (ROS), assist in antimicrobial defence by immobilizing and killing pathogens [46,79], but are also capable of causing self-harm [45,80].
Melanin-associated encapsulation and structural support usually involves the degranulation of immune cells within which the melanin-synthesis pathway is active [81], such as crystal cells of insects [48] and haemocytes of crustaceans [82]. For anthozoans, melanization was first documented within the sea fan G. ventalina as a barrier against a fungal infection [54], and Mullen et al. [83] described the amoebocytes involved. In the same species, aggregations of amoebocytes were documented around fungal infections, and their granular content was confirmed to be melanin [53]. Melanin-containing granular cells were subsequently identified in hard corals (Porites spp.), and their higher densities in compromised and infected tissue supports a role in immunity [13,84]. Subsequently, melanin-containing cells have been found within a suite of healthy Indo-Pacific corals (Scleractinia and Alcyonacea), suggesting their likely ubiquity among anthozoans [4]. A recent study confirmed the role of these cells in an injury response, via degranulation to form a rudimentary clot and subsequent aggregation and/or proliferation to provide structural reinforcement [85].
Cytotoxic aspects of melanin-synthesis are less explored within corals and would provide a complimentary avenue for future studies. Similarly, the possible ecological implications of the variation in abundance of melanin-containing granular cells [4,13,59,85] and their likely involvement in bleaching resistance [4] warrants further exploration.
(iii). Clot formation
The effector response associated with the activation of the coagulation pathway is, primarily, the formation of an insoluble clot [62]. Clot formation is an excellent example of cross-linking among immune pathways, with clots formed by transglutaminase activity that become melanized for stability [62] and involves the aggregation of immune cells [81].
Clot formation has been investigated within many invertebrates, including Arthropods such as Drosophila [81]. Within cnidarians, while observations of tissue regeneration [86,87] suggest that clot formation must initially occur, clotting has only been confirmed within one hard coral, P. cylindrica [85]. While this clot formation coincided with melanin and immune cell activity, its initiation by transglutaminase was not documented [85] and thus needs to be confirmed.
(iv). Immune cells
Invertebrate immune cells become activated in response to foreign organisms and injury [88] to phagocytose and/or encapsulate pathogens and re-establish tissue integrity. Immune cells with phagocytic potential have been identified across the invertebrates, e.g. within Arthropoda [81] and Echinodermata [89]. During wound repair, after clot formation, specialized immune cells infiltrate the wound site and phagocytose cell debris and foreign organisms [90]. Infiltrated cells then proliferate, forming granulation tissue, which consists of multiple cell types, collagen and a basic ECM, and provides a platform for re-epithelialization [81,89]. Although there are differences among species, these cellular phases of wound healing have been documented within numerous invertebrates [81,91].
Within the Cnidaria, cells with phagocytic potential were identified in the sea anemone Actinia equina and the soft coral S. exserta over 10 years ago [92,93]. An investigation of the gorgonian Plexaurella fusifera provided preliminary insights into the processes and cells involved in anthozoan wound healing [94]. However, within hard corals, investigations of immune cells have been hindered by the assumption that they are small and confined to the acellular mesogleoa. Despite this hindrance, as described earlier, melanin-containing granular cells (granular amoebocytes) have been characterized within a host of anthozoans [4,13,55,85]. Additionally, the immune cells and phases involved in wound healing were recently documented for the hard coral P. cylindrica [85]. However, the full suite of coral immune cells and their functional repertoire, particularly in response to pathogenic invasion, has yet to be understood.
3. Coral-specific immune complexities
Coral obligate endosymbionts, Symbiodinium, provide valuable energetic resources. However, how this fundamental relationship is established, maintained and broken down, remains to be conclusively resolved, but likely involves immune mechanisms of both partners [2]. While some recent studies hint at a relationship between host immunity and symbiosis [4,19,55,66], this has yet to be directly investigated. Significantly, immune involvement in symbiosis suggests that understanding immune mechanisms will likely provide insight into questions pertaining to coral bleaching.
An additional aspect of coral biology that remains unresolved is the function of fluorescent proteins (FPs). Nonetheless, FPs are often used as a measure of thermal stress due to their rapid responses to environmental changes [95,96]. Many hypotheses have been proposed for the role of these eye-catching proteins, for example, in Symbiodinium photoprotection [97], and more recently as components of an immune response, potentially with antioxidant function. This latter hypothesis stemmed from the higher abundance of FPs in injured [13] and infected coral tissue [84] and from evidence that they scavenge oxygen radicals more efficiently than other proteins [98]. While these recent findings do not conclusively elucidate FP function, they support the hypothesis that FPs are part of an immune response, which frequently induces oxidative stress [80]. The potential role of FPs in immunity should therefore be considered as the field of coral immunology expands, and will hopefully lead to the elucidation of their biological function.
4. A framework for developing the field of coral immunology
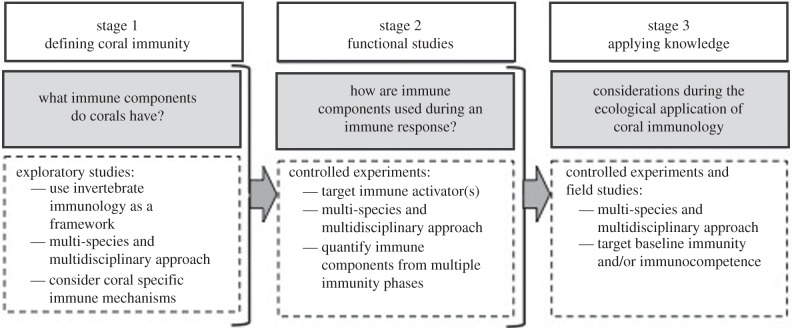
The progression of the field of coral immunology has recently been criticized for its paucity and lack of functional studies [5]. While the gaps in our current knowledge support these statements, coral immunology is an emerging field and thus such deficiencies are currently to be expected. As a detailed knowledge of fundamental coral biology is essential for accurately answering pressing climate-related questions, elucidating coral immune mechanisms and their functions, should be a research priority. We therefore propose a three-stage framework for comprehensively developing the field of coral immunology (figure 2) by progressively building on layers of knowledge so that pertinent questions on coral declines can be accurately addressed.
Figure 2.
A proposed framework for the development of coral immunology.
The first stage of the framework aims to define coral immunity by elucidating the suite of immune mechanisms anthozoans use. We propose that this aim should be addressed by establishing the presence or the absence of common invertebrate immune mechanisms within corals, using a multi-species and multidisciplinary approach to target key gaps in our current knowledge, such as immune cell characterization. The expected imminent proliferation of new, and exhaustive searches of current coral transcriptome and genome databases will undoubtedly expedite this stage. Additionally, potential coral-specific immune mechanisms should also be considered, e.g. FPs, perhaps in conjunction with stage 2 of the framework.
The aim of stage 2 is to confirm the role of the identified mechanisms (stage 1) within coral immunity by conducting controlled experiments (figure 2). As controlled experiments will determine immune component function and biological relevance, stage 2 is crucial to the development of coral immunology and should be executed and published with stage 1 [13,53]. During the design of the controlled experiments, the type of immune activator (e.g. injury or parasitic infection) to be used needs to be considered, as the biological relevance of different immune mechanisms will likely vary among them. Ideally, a multi-species and multidisciplinary approach should also be taken to maximize the information output and to account for species-specific differences. Additionally, quantifying immune mechanisms from more than one immune phase will increase the likelihood of elucidating interrelationships among components, and investigating multiple immune pathways will help to unravel cross-linkages within the immune network.
Once stages 1 and 2 have been addressed, the resultant information can be used to effectively tackle ecological scale and climate-related questions (stage 3) by providing clearer interpretations of findings. Ecological aspects of immunology can begin to be addressed, such as variations within and among populations, species and over time, and the role of baseline immunity in resisting disease versus the immunocompetence that fights it. Current coral immunology is only just at the cusp of these ecological applications with the melanin-synthesis pathway [4,59].
5. Conclusions
Corals possess components of the established innate immune phases and key invertebrate immune pathways. However, the gaps in the current knowledge are numerous, with the most striking being the lack of functional studies of proposed coral immune components. The proposed framework of future research offers a logical and systematic approach that will enable the rapid and effective development of the field, and additionally enhance coral biological and general immunological knowledge. Moreover, as immunology likely underpins health-related coral declines, its inclusion into coral biology and ecology will enhance our ecological understanding and predictions of how climatic changes will likely shape our future reefs.
Acknowledgements
J. C. Bythell, B. L. Willis, P. De Wit and R. Puschendorf for valuable comments on manuscript drafts.
References
- 1.Hoegh-Guldberg O., Bruno J. F. 2010. The impact of climate change on the World's marine ecosystems. Science 328, 1523–1528 10.1126/science.1189930 (doi:10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 2.Weis V. M., Allemand D. 2009. What determines coral health? Science 324, 1153–1155 10.1126/science.1172540 (doi:10.1126/science.1172540) [DOI] [PubMed] [Google Scholar]
- 3.Butt D., Raftos D. 2008. Phenoloxidase-associated cellular defence in the Sydney rock oyster, Saccostrea glomerata, provides resistance against QX disease infections. Dev. Comp. Immunol. 32, 299–306 10.1016/j.dci.2007.06.006 (doi:10.1016/j.dci.2007.06.006) [DOI] [PubMed] [Google Scholar]
- 4.Palmer C. V., Bythell J. C., Willis B. L. 2010. Immunity parameters of reef corals underpin bleaching and disease susceptibility. FASEB J. 24, 1935–1946 10.1096/fj.09-152447 (doi:10.1096/fj.09-152447) [DOI] [PubMed] [Google Scholar]
- 5.Rinkevich B. 2011. The ‘immunology trap’ of anthozoans. Invert. Survival J. 8, 153–161 [Google Scholar]
- 6.Beutler B. 2004. Innate immunity: an overview. Mol. Immunol. 40, 845–859 10.1016/j.molimm.2003.10.005 (doi:10.1016/j.molimm.2003.10.005) [DOI] [PubMed] [Google Scholar]
- 7.Loker E. S., Adema C. M., Zhang S. M., Kepler T. B. 2004. Invertebrate immune systems—not homogeneous, not simple, not well understood. Immunol. Rev. 198, 10–24 10.1111/j.0105-2896.2004.0117.x (doi:10.1111/j.0105-2896.2004.0117.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putnam N. H., et al. 2007. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 10.1126/science.1139158 (doi:10.1126/science.1139158) [DOI] [PubMed] [Google Scholar]
- 9.Cooper E. L. 2010. Evolution of immune systems from self/not self to danger to artificial immune systems (AIS). Phys. Life Rev. 7, 55–78 10.1016/j.plrev.2009.12.001 (doi:10.1016/j.plrev.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann J. A., Kafatos F. C., Janeway C. A., Ezekowitz R. A. B. 1999. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 10.1126/science.284.5418.1313 (doi:10.1126/science.284.5418.1313) [DOI] [PubMed] [Google Scholar]
- 11.Fujita T., Matsushita M., Endo Y. 2004. The lectin–complement pathway—its role in innate immunity and evolution. Immunol. Rev. 198, 185–202 10.1111/j.0105-2896.2004.0123.x (doi:10.1111/j.0105-2896.2004.0123.x) [DOI] [PubMed] [Google Scholar]
- 12.Iwanaga S., Lee B. L. 2005. Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 38, 128–150 10.5483/BMBRep.2005.38.2.128 (doi:10.5483/BMBRep.2005.38.2.128) [DOI] [PubMed] [Google Scholar]
- 13.Palmer C. V., Mydlarz L. D., Willis B. L. 2008. Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proc. R. Soc. B 275, 2687–2693 10.1098/rspb.2008.0335 (doi:10.1098/rspb.2008.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed K. C., Muller E. M., van Woesik R. 2010. Coral immunology and resistance to disease. Dis. Aqu. Org. 90, 85–92 10.3354/dao02213 (doi:10.3354/dao02213) [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R., Janeway C. A. 2002. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 10.1126/science.1068883 (doi:10.1126/science.1068883) [DOI] [PubMed] [Google Scholar]
- 16.Cooper E. L., Kvell K., Englemann P., Nemeth P. 2006. Still waiting for the toll? Immunol. Lett. 104, 18–28 10.1016/j.imlet.2005.11.012 (doi:10.1016/j.imlet.2005.11.012) [DOI] [PubMed] [Google Scholar]
- 17.Desalvo M. K., Voolstra C. R., Sunagawa S., Schwarz J. A., Stillman J. H., Coffroth M. A., Szmant A. M., Medina M. 2008. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol. Ecol. 17, 3952–3971 10.1111/j.1365-294X.2008.03879.x (doi:10.1111/j.1365-294X.2008.03879.x) [DOI] [PubMed] [Google Scholar]
- 18.Kenkel C., et al. 2011. Diagnostic gene expression markers of acute heat-light stress in Porites spp. PLoS ONE 6, e26914. 10.1371/journal.pone.0026914 (doi:10.1371/journal.pone.0026914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvennefors E. C. E., Leggat W., Kerr C. C., Ainsworth T. D., Hoegh-Guldberg O., Barnes A. C. 2010. Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Dev. Comp. Immunol. 34, 1219–1229 10.1016/j.dci.2010.06.016 (doi:10.1016/j.dci.2010.06.016) [DOI] [PubMed] [Google Scholar]
- 20.Miller D. J., Hemmrich G., Ball E. E., Hayward D. C., Khalturin K., Funayama N., Agata K., Bosch T. C. G. 2007. The innate immune repertoire in Cnidaria—ancestral complexity and stochastic gene loss. Genome Biol. 8, R59. 10.1186/gb-2007-8-4-r59 (doi:10.1186/gb-2007-8-4-r59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irazoqui J. E., Urbach J. M., Ausubel F. M. 2010. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 10, 47–58 10.1038/nri2689 (doi:10.1038/nri2689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrandon D., Imler J. L., Hetru C., Hoffmann J. A. 2007. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 10.1038/nri2194 (doi:10.1038/nri2194) [DOI] [PubMed] [Google Scholar]
- 23.Wiens M., Korzhev M., Perovic-Ottstadt S., Luthringer B., Brandt D., Klein S., Muller W. E. G. 2007. Toll-like receptors are part of the innate immune defense system of sponges (Demospongiae: Porifera). Mol. Biol. Evol. 24, 792–804 10.1093/molbev/msl208 (doi:10.1093/molbev/msl208) [DOI] [PubMed] [Google Scholar]
- 24.Leulier F., Lemaitre B. 2008. Toll-like receptors: taking an evolutionary approach. Nat. Rev. Genet. 9, 165–178 10.1038/nrg2303 (doi:10.1038/nrg2303) [DOI] [PubMed] [Google Scholar]
- 25.Shinzato C., et al. 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476, 320–323 10.1038/nature10249 (doi:10.1038/nature10249) [DOI] [PubMed] [Google Scholar]
- 26.Sullivan J. C., Kalaitzidis D., DGilmore T. D., Finnerty J. R. 2007. Rel homology domain-containing transcription factors in the cnidarian Nematostella vectensis. Devel. Gen. Evol. 217, 63–72 10.1007/s00427-006-0111-6 (doi:10.1007/s00427-006-0111-6) [DOI] [PubMed] [Google Scholar]
- 27.Bosch T. C. G., et al. 2009. Uncovering the evolutionary history of innate immunity: the simple metazoan Hydra uses epithelial cells for host defence. Dev. Comp. Immunol. 33, 559–569 10.1016/j.dci.2008.10.004 (doi:10.1016/j.dci.2008.10.004) [DOI] [PubMed] [Google Scholar]
- 28.Chapman J. A., et al. 2010. The dynamic genome of Hydra. Nature 464, 592–596 10.1038/nature08830 (doi:10.1038/nature08830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo Y., Nakazawa N., Iwaki D., Takahashi M., Matsushita M., Fujita T. 2010. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J. Innate Immunity 2, 33–42 10.1159/000227805 (doi:10.1159/000227805) [DOI] [PubMed] [Google Scholar]
- 30.Ling E. J., Yu X. Q. 2006. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. 30, 289–299 10.1016/j.dci.2005.05.005 (doi:10.1016/j.dci.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 31.Olafsen J. A., Fletcher T. C., Grant P. T. 1992. Agglutinin activity in Pacific oyster (Crassostrea gigas) hemolymph following in vivo Vibrio anguillarum challenge. Dev. Comp. Immunol. 16, 123–138 10.1016/0145-305X(92)90013-3 (doi:10.1016/0145-305X(92)90013-3) [DOI] [PubMed] [Google Scholar]
- 32.Reitzel A. M., Sullivan J. C., Traylor-Knowles N., Finnerty J. R. 2008. Genomic survey of candidate stress-response genes in the esturine anemone Nematostella vectensis. Biol. Bull. 214, 233–254 10.2307/25470666 (doi:10.2307/25470666) [DOI] [PubMed] [Google Scholar]
- 33.Hanai K., Kitajima M. A. 1983. The effects of lectins on the feeding response in Hydra japonica. Comp. Biochem. Physiol. A 76, 283–287 10.1016/0300-9629(83)90328-6 (doi:10.1016/0300-9629(83)90328-6) [DOI] [Google Scholar]
- 34.Schwarz R. S., Bosch T. C. G., Cadavid L. F. 2008. Evolution of polydom-like molecules: identification and characterization of cnidarian polydom (Cnpolydom) in the basal metazoan Hydractinia. Dev. Comp. Immunol. 32, 141–151 10.1016/j.dci.2008.03.007 (doi:10.1016/j.dci.2008.03.007) [DOI] [PubMed] [Google Scholar]
- 35.Hayes M. L., Eytan R. I., Hellberg M. E. 2010. High amino acid diversity and positive selection at a putative coral immunity gene (tachylectin-2). BMC Evol. Biol. 10, 150. 10.1186/1471-2148-10-150 (doi:10.1186/1471-2148-10-150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson M. S., Lu N., Denessiouk K., Heino J., Gullberg D. 2009. Integrins during evolution: evolutionary trees and model organisms. Biochim. Biophys. Acta Biomembr. 1788, 779–789 10.1016/j.bbamem.2008.12.013 (doi:10.1016/j.bbamem.2008.12.013) [DOI] [PubMed] [Google Scholar]
- 37.Takada Y., Ye X., Simon S. 2007. The integrins. Genome Biol. 8, 215. 10.1186/gb-2007-8-5-215 (doi:10.1186/gb-2007-8-5-215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmblad T., Thörnqvist P.-O., Söderhäll K., Johansson M. W. 1997. Identification and cloning of an integrin β subunit from hemocytes of the freshwater crayfish Pacifastacus lenisculus. J. Exp. Zool. 277, 255–261 (doi:10.1002/(SICI)1097-010X(19970215)277:3<255::AID-JEZ6>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 39.Wimmer W., Perovic S., Kruse M., Schroder H. C., Krasko A., Batel R., Muller W. E. G. 1999. Origin of the integrin-mediated signal transduction—functional studies with cell cultures from the sponge Suberites domuncula. Euro. J. Biochem. 260, 156–165 10.1046/j.1432-1327.1999.00146.x (doi:10.1046/j.1432-1327.1999.00146.x) [DOI] [PubMed] [Google Scholar]
- 40.Brower D. L., Brower S. M., Hayward D. C., Ball E. E. 1997. Molecular evolution of integrins: genes encoding integrin beta subunits from a coral and a sponge. Proc. Natl Acad. Sci. USA 94, 9182–9187 10.1073/pnas.94.17.9182 (doi:10.1073/pnas.94.17.9182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knack B. A., Iguchi A., Shinzato C., Hayward D. C., Ball E. E., Miller D. J. 2008. Unexpected diversity of cnidarian integrins: expression during coral gastrulation. BMC Evol. Biol. 8, 136. 10.1186/1471-2148-8-136 (doi:10.1186/1471-2148-8-136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 10.1038/415389a (doi:10.1038/415389a) [DOI] [PubMed] [Google Scholar]
- 43.Zhang L. L., Li L., Zhang G. F. 2011. A Crassostrea gigas toll-like receptor and comparative analysis of TLR pathway in invertebrates. Fish Shellfish Immunol. 30, 653–660 10.1016/j.fsi.2010.12.023 (doi:10.1016/j.fsi.2010.12.023) [DOI] [PubMed] [Google Scholar]
- 44.Gauthier M., Degnan B. M. 2008. The transcription factor NF-κB in the demosponge Amphimedon queenslandica: insights on the evolutionary origin of the Rel homology domain. Dev. Gen. Evol. 218, 23–32 10.1007/s00427-007-0197-5 (doi:10.1007/s00427-007-0197-5) [DOI] [PubMed] [Google Scholar]
- 45.Cerenius L., Kawabata S. I., Lee B. L., Nonaka M., Soderhall K. 2010. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem. Sci. 35, 575–583 10.1016/j.tibs.2010.04.006 (doi:10.1016/j.tibs.2010.04.006) [DOI] [PubMed] [Google Scholar]
- 46.Cerenius L., Lee B. L., Söderhäll K. 2008. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271 10.1016/j.it.2008.02.009 (doi:10.1016/j.it.2008.02.009) [DOI] [PubMed] [Google Scholar]
- 47.Sugumaran M. 2002. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 15, 2–9 10.1034/j.1600-0749.2002.00056.x (doi:10.1034/j.1600-0749.2002.00056.x) [DOI] [PubMed] [Google Scholar]
- 48.Bidla G., Hauling T., Dushay M. S., Theopold U. 2008. Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J. Innate Immun. 1, 301–308 10.1159/000168009 (doi:10.1159/000168009) [DOI] [PubMed] [Google Scholar]
- 49.Seppala O., Jokela J. 2011. Immune defence under extreme ambient temperature. Biol. Lett. 7, 119–122 10.1098/rsbl.2010.0459 (doi:10.1098/rsbl.2010.0459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luna-Acosta A., Rosenfeld E., Amari M., Fruitier-Arnaudin I., Bustamante P., Thomas-Guyon H. 2010. First evidence of laccase activity in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 28, 719–726 10.1016/j.fsi.2010.01.008 (doi:10.1016/j.fsi.2010.01.008) [DOI] [PubMed] [Google Scholar]
- 51.Söderhäll K., Cerenius L. 1998. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 10, 23–28 10.1016/S0952-7915(98)80026-5 (doi:10.1016/S0952-7915(98)80026-5) [DOI] [PubMed] [Google Scholar]
- 52.Prochazkova P., Silerova M., Stijlemans B., Dieu M., Halada P., Joskova R., Beschin A., De Baetselier P., Bilej M. 2006. Evidence for proteins involved in prophenoloxidase cascade Eisenia fetida earthworms. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 176, 581–587 10.1007/s00360-006-0081-z (doi:10.1007/s00360-006-0081-z) [DOI] [PubMed] [Google Scholar]
- 53.Mydlarz L. D., Holthouse S. F., Peters E. C., Harvell C. D. 2008. Celluar responses in sea fan corals: granular amoebocytes react to pathogen and climate stressors. PLoS ONE 3, e1811. 10.1371/journal.pone.0001811 (doi:10.1371/journal.pone.0001811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petes L. E., Harvell C. D., Peters E. C., Webb M. A. H., Mullen K. M. 2003. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar. Ecol. Prog. Ser. 264, 167–171 10.3354/meps264167 (doi:10.3354/meps264167) [DOI] [Google Scholar]
- 55.Mydlarz L. D., Couch C. S., Weil E., Smith G., Harvell C. D. 2009. Immune defenses of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event. Dis. Aquat. Org. 87, 67–78 10.3354/dao02088 (doi:10.3354/dao02088) [DOI] [PubMed] [Google Scholar]
- 56.Mydlarz L. D., Palmer C. V. 2011. The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp. Biochem. Physiol. A. 159, 372–378 10.1016/j.cbpa.2011.03.029 (doi:10.1016/j.cbpa.2011.03.029) [DOI] [PubMed] [Google Scholar]
- 57.Palmer C. V., Bythell J. C., Willis B. L. 2012. Enzyme activity demonstrates multiple pathways of innate immunity in Indo-Pacific corals. Proc. R. Soc. B 279, 3879–3887 10.1098/rspb.2011.2487 (doi:10.1098/rspb.2011.2487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer C. V., Bythell J. C., Willis B. L. 2011. A comparative study of phenoloxidase activity in diseased and bleached colonies of the coral Acropora millepora. Dev. Comp. Immunol. 10, 1098–1101 10.1016/j.dci.2011.04.001 (doi:10.1016/j.dci.2011.04.001) [DOI] [PubMed] [Google Scholar]
- 59.Palmer C. V., McGinty E. S., Cummings D., Bartels E., Mydlarz L. D. 2011. Patterns of coral ecological immunology: variation in the responses of Caribbean corals to elevated temperature and a pathogen elicitor. J. Exp. Biol. 15, 4240–4249 10.1242/jeb.061267 (doi:10.1242/jeb.061267) [DOI] [PubMed] [Google Scholar]
- 60.Loof T. G., Schmidt O., Herwald H., Theopold U. 2011. Coagulation systems of invertebrates and vertebrates and their roles in innate immunity: the same side of two coins?. J. Innate Immun. 3, 34–40 10.1159/000321641 (doi:10.1159/000321641) [DOI] [PubMed] [Google Scholar]
- 61.Nozawa H., Seki N. 2001. Purification of transglutaminase from scallop striated adductor muscle and NaCl-induced inactivation. Fish. Sci. 67, 493–499 10.1046/j.1444-2906.2001.00263.x (doi:10.1046/j.1444-2906.2001.00263.x) [DOI] [Google Scholar]
- 62.Theopold U., Schmidt O., Soderhall K., Dushay M. S. 2004. Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 25, 289–294 10.1016/j.it.2004.03.004 (doi:10.1016/j.it.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 63.Endo Y., Takahashi M., Fujita T. 2006. Lectin complement system and pattern recognition. Immunobiology 211, 283–293 10.1016/j.imbio.2006.01.003 (doi:10.1016/j.imbio.2006.01.003) [DOI] [PubMed] [Google Scholar]
- 64.Mayflyan K. R., Kang Y. H., Dodds A. W., Sim R. B. 2008. The complement system in innate immunity. Nucleic Acids Mol. Biol. 21, 219–236 10.1007/978-3-540-73930-2_10 (doi:10.1007/978-3-540-73930-2_10) [DOI] [Google Scholar]
- 65.Dishaw L. J., Smith S. L., Bigger C. H. 2005. Characterization of a C3-like cDNA in a coral: phylogenetic implications. Immunogenetics 57, 535–548 10.1007/s00251-005-0005-1 (doi:10.1007/s00251-005-0005-1) [DOI] [PubMed] [Google Scholar]
- 66.Kvennefors E. C. E., Leggat W., Hoegh-Guldberg O., Degnan B. M., Barnes A. C. 2008. An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Dev. Comp. Immunol. 32, 1582–1592 10.1016/j.dci.2008.05.010 (doi:10.1016/j.dci.2008.05.010) [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Lanetty M., Harii S., Hoegh-Guldberg O. 2009. Early molecular response of coral larvae to hyperthermal stress. Mol. Ecol. 18, 5101–5114 10.1111/j.1365-294X.2009.04419.x (doi:10.1111/j.1365-294X.2009.04419.x) [DOI] [PubMed] [Google Scholar]
- 68.Schwartz M. A. 2001. Integrin signaling revisited. Trends in Cell Biology 11, 466–470 10.1016/S0962-8924(01)02152-3 (doi:10.1016/S0962-8924(01)02152-3) [DOI] [PubMed] [Google Scholar]
- 69.Plows L. D., Cook R. T., Davies A. J., Walker A. J. 2006. Integrin engagement modulates the phosphorylation of focal adhesion kinase, phagocytosis, and cell spreading in molluscan defence cells. Biochim. Biophys. Acta-Mol. Cell Res. 1763, 779–786 10.1016/j.bbamcr.2006.04.008 (doi:10.1016/j.bbamcr.2006.04.008) [DOI] [PubMed] [Google Scholar]
- 70.Metheniti A., Paraskevopoulou N., Lambropoulou M., Marmaras V. J. 2001. Involvement of FAK/Src complex in the processes of Escherichia coli phagocytosis by insect hemocytes. FEBS Lett. 496, 55–59 10.1016/S0014-5793(01)02405-X (doi:10.1016/S0014-5793(01)02405-X) [DOI] [PubMed] [Google Scholar]
- 71.Garcia M. G., Toney S. J., Hille M. B. 2004. Focal adhesion kinase (FAK) expression and phosphorylation in sea urchin embryos. Gene Exp. Patterns 4, 223–234 10.1016/j.modgep.2003.08.005 (doi:10.1016/j.modgep.2003.08.005) [DOI] [PubMed] [Google Scholar]
- 72.Shai Y. 2002. Mode of action of membrane active antimicrobial peptides. Biopolymers 66, 236–48 10.1002/bip.10260 (doi:10.1002/bip.10260) [DOI] [PubMed] [Google Scholar]
- 73.Lemaitre B., Hoffmann J. 2007. The host defense of Drosophila melanogaster. Ann. Rev. Immunol. 25, 697–743 10.1146/annurev.immunol.25.022106.141615 (doi:10.1146/annurev.immunol.25.022106.141615) [DOI] [PubMed] [Google Scholar]
- 74.Otero-Gonzalez A. J., Magalhaes B. S., Garcia-Villarino M., Lopez-Abarrategui C., Sousa D. A., Dias S. C., Franco O. L. 2010. Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J. 24, 1320–1334 10.1096/fj.09-143388 (doi:10.1096/fj.09-143388) [DOI] [PubMed] [Google Scholar]
- 75.Augustin R., Fraune S., Bosch T. C. G. 2010. How hydra senses and destroys microbes. Semin. Immunol. 22, 54–58 10.1016/j.smim.2009.11.002 (doi:10.1016/j.smim.2009.11.002) [DOI] [PubMed] [Google Scholar]
- 76.Vidal-Dupiol J., et al. 2011. Innate immune responses of a scleractinian coral to vibriosis. J. Biol. Chem. 286, 22 688–22 698 (doi:10.1074/jbc.M110.216358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koh E. G. L. 1997. Do scleractinian corals engage in chemical warfare against microbes? J. Chem. Ecol. 23, 379–398 10.1023/B:JOEC.0000006366.58633.f4 (doi:10.1023/B:JOEC.0000006366.58633.f4) [DOI] [Google Scholar]
- 78.Dunn S. 2009. Immunorecognition and immunoreceptors in the Cnidaria. Invert. Surviv. J. 6, 7–14 [Google Scholar]
- 79.Zhao P. C., Li J. J., Wang Y., Jiang H. B. 2007. Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem. Mol. Biol. 37, 952–959 10.1016/j.ibmb.2007.05.001 (doi:10.1016/j.ibmb.2007.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sadd B. M., Siva-Jothy M. T. 2006. Self-harm caused by an insect's innate immunity. Proc. R. Soc. B 273, 2571–2574 10.1098/rspb.2006.3574 (doi:10.1098/rspb.2006.3574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galko M. J., Krasnow M. A. 2004. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2, 1114–1126 10.1371/journal.pbio.0020239 (doi:10.1371/journal.pbio.0020239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Söderhäll K., Smith V. J. 1986. The prophenoloxidase activating system as a recognition and defence system in arthropods. In Hemocytic and humoral immunity in arthropods (ed. Gupta A. P.), pp. 251–286 New York, NY: Wiley [Google Scholar]
- 83.Mullen K., Peters E. C., Harvell C. D. 2004. Coral resistence to disease. In Coral health and disease (eds Rosenberg E., Loya Y.), pp. 377–399 New York, NY: Springer [Google Scholar]
- 84.Palmer C. V., Roth M. S., Gates R. D. 2009. Red fluorescent protein responsible for pigmentation in trematode-infected Porites compressa tissues. Biol. Bull. 215, 68–74 [DOI] [PubMed] [Google Scholar]
- 85.Palmer C. V., Traylor-Knowles N. G., Willis B. L., Bythell J. C. 2011. Corals use similar immune cells and wound-healing processes as those of higher organisms. PLoS ONE 6, e23992. 10.1371/journal.pone.0023992 (doi:10.1371/journal.pone.0023992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meesters E. H., Noordeloos M., Bak R. P. M. 1994. Damage and regeneration: links to growth in the reef-building coral Montastrea annularis. Mar. Ecol. Prog. Ser. 112, 119–128 10.3354/meps112119 (doi:10.3354/meps112119) [DOI] [Google Scholar]
- 87.Titlyanov E. A., Titlyanova T. V., Yakovleva I. M., Nakano Y., Bhagooli R. 2005. Regeneration of artificial injuries on scleractinian corals and coral/algal competition for newly formed substrate. J. Exp. Mar. Biol. Ecol. 323, 27–42 10.1016/j.jembe.2005.02.015 (doi:10.1016/j.jembe.2005.02.015) [DOI] [Google Scholar]
- 88.Zuk M., Stoehr A. M. 2002. Immune defense and host life history. Am. Nat. 160, S9–S22 10.1086/342131 (doi:10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 89.Biressi A., Zou T., Dupont S., Dahlberg C., Di Benedetto C., Bonasoro F., Thorndyke M., Carnevali M. D. C. 2010. Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology 129, 1–19 10.1007/s00435-009-0095-7 (doi:10.1007/s00435-009-0095-7) [DOI] [Google Scholar]
- 90.Martin P., Leibovich S. J. 2005. Inflammatory cells during wound, repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 10.1016/j.tcb.2005.09.002 (doi:10.1016/j.tcb.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 91.Ancona Lunette G. D. 2005. Wound repair in the marine worm Sipunculus nudus (Sipunculidae). Invert. Survival J. 2, 124–131 [Google Scholar]
- 92.Hutton D. M. C., Smith V. J. 1996. Antibacterial properties of isolated amoebocytes from the sea anemone Actinia equina. Biol. Bull. 191, 441–451 10.2307/1543017 (doi:10.2307/1543017) [DOI] [PubMed] [Google Scholar]
- 93.Olano C. T., Bigger C. H. 2000. Phagocytic activities of the gorgonian coral Swiftia exserta. J. Invert. Pathol. 76, 176–184 10.1006/jipa.2000.4974 (doi:10.1006/jipa.2000.4974) [DOI] [PubMed] [Google Scholar]
- 94.Meszaros A., Bigger C. 1999. Qualitative and quantitative study of wound healing processes in the coelenterate, Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences. J. Invert. Pathol. 73, 321–331 10.1006/jipa.1999.4851 (doi:10.1006/jipa.1999.4851) [DOI] [PubMed] [Google Scholar]
- 95.Smith-Keune C., Dove S. 2008. Gene expression of a green fluorescent protein homolog as a host-specific biomarker of heat stress within a reef-building coral. Mar. Biotechnol. 10, 166–180 10.1007/s10126-007-9049-6 (doi:10.1007/s10126-007-9049-6) [DOI] [PubMed] [Google Scholar]
- 96.Yuyama I., Harii S., Hidaka M. 2012. Algal symbiont type affects gene expression in juveniles of the coral Acropora tenuis exposed to thermal stress. Mar. Environ. Res. 76, 41–47 10.1016/j.marenvres.2011.09.004 (doi:10.1016/j.marenvres.2011.09.004) [DOI] [PubMed] [Google Scholar]
- 97.Salih A., Larkum A. W. D., Cox C. 2001. Photoprotection from photoinhibition of symbiotic algae in corals by fluorescent pigments. Photosynth. Res. 69, 221–222 [Google Scholar]
- 98.Palmer C. V., Modi C. K., Mydlarz L. D. 2009. Coral fluorescent proteins as antioxidants. PLoS ONE 4, e7298. 10.1371/journal.pone.0007298 (doi:10.1371/journal.pone.0007298) [DOI] [PMC free article] [PubMed] [Google Scholar]