Abstract
Because background matching improves concealment, prey animals have traditionally been expected to prefer parts of the habitat that match their visual appearance. However, empirical support for this is scarce. Moreover, this idea has recently been challenged by an alternative hypothesis: visual complexity of the background impedes prey detection, and hence prey could instead prefer complex parts of the habitat. We used the least killifish to test, with and without predation threat, for the importance of the visual similarity between the fish and the background, and the level of visual complexity of the background. We observed their choice between backgrounds patterned with elements based on the longitudinal black stripe of the fish. Predation risk was important under some circumstances, and induced a preference for a background of matching horizontal stripes compared with mismatching vertical stripes. Interestingly, females under predation threat showed a preference for a complex background of randomly oriented and overlapping stripes compared with matching stripes, whereas males did not discriminate between these two. Additionally, males showed a preference for matching stripes compared with complex shapes, whereas females did not discriminate between these backgrounds. We conclude that matching is important in the choice for safe habitat, but some aspects of visual complexity may override or act together with background matching.
Keywords: habitat choice, prey coloration, crypsis, background matching, complexity, predation
1. Introduction
A prey animal can use body colours and patterns that visually resemble its surrounding habitat to conceal itself [1–4]. This particular strategy of crypsis is commonly referred to as background matching [4–6]. Background matching prey coloration and its adaptive features have been recognized by biologists for a long time [7]. The related idea that prey animals can decrease their probability of being detected through behavioural features was already discussed by Alfred Russel Wallace. Wallace [8] described the Indian leafwing (Kallima paralekta), which looks astonishingly similar to a dead leaf, and wrote that ‘this resemblance, close as it is, would be of little use if the habits of the insect did not accord with it’ (p. 44). He then continued to describe his observations, and confirmed his assumption about the insect using behaviour to enhance its concealing features: ‘they were never seen to settle on a flower or a green leaf, but were many times lost sight of in a bush or tree of dead leaves’ ([8], p. 44).
It has been shown experimentally that background matching effectively reduces predation risk imposed by predators, for example, in fishes [9–11] and birds [12–14]. Preference for backgrounds that reduce the risk of detection has thus been suggested to be an important and wide spread strategy among prey animals to decrease their predation risk [3,15,16]. It is also a common assumption that prey animals have been selected to actively prefer visually matching backgrounds. However, considering the popularity of this idea [12,17–20], surprisingly few experimental studies testing it exist [21–24]. Moreover, several studies have not found support for such behaviour [25–27]. Thus, even though the idea that prey are being selected to prefer matching backgrounds has become such a common assumption, support for the idea is not as strong as is often believed.
Interestingly, the notion that preference for matching backgrounds minimizes the risk of detection has recently been challenged by an alternative hypothesis. It has been proposed that the visual complexity of the background could be an additional factor that hampers detection of prey [28]. For instance, increased visual background complexity increases prey search time by blue tits (Cyanistes caeruleus) [29,30]. Dimitrova & Merilaita [29,30] argue that the increased amount of visual information in the background may make it harder for the predators to receive necessary visual information about the prey. Experiments in visual psychology lend support to this notion [31–33]. Consequently, it could pay off for a prey to prefer visually complex backgrounds to simpler ones in order to decrease predation risk [28,29]. So far, the relative importance of preferences for matching and complex backgrounds, and how they contribute to background choice in prey, have not been addressed.
Here, we have investigated these two hypotheses—preference for visually matching and preference for visually complex backgrounds—by observing the background choice of the least killifish Heterandria formosa (Girard, 1859). We used artificial backgrounds, which enabled us to easily manipulate their visual features, while other conditions were kept equal. We first demonstrated that such backgrounds can be used to induce background choice, and then we investigated whether the least killifish showed any preference between four different background patterns that were matching (i.e. matched the most prominent feature of their body pattern) or mismatching, or visually more complex, in order to establish whether matching, visual complexity or both are important to cover seeking prey. We also manipulated predation risk to investigate whether the observed behavioural responses could be an anti-predator adaptation.
2. Material and methods
(a). Study species
The least killifish, with a standard length of up to 3.5 cm, is the smallest member of the Poeciliidae family. The least killifish live in slowly moving freshwater streams or ponds, but also occur in brackish waters in southeastern parts of the USA. We chose the least killifish as our study animal because of their simple colour pattern, which consists of a light beige colour and a black longitudinal stripe on the side both in males and in females (figure 1). In their natural habitat, H. formosa commonly occur in heterogeneous, submerged vegetation beds, where their stripes appear to have a concealing function [34]. Thus, the least killifish is a suitable species for this study, and their pattern enabled us to easily create artificial backgrounds with black elements that approximately matched the lateral stripe of the fish. All least killifishes used in this experiment were laboratory-reared descendants (one to two generations) from a wild population in Otter Creek, Florida, USA.
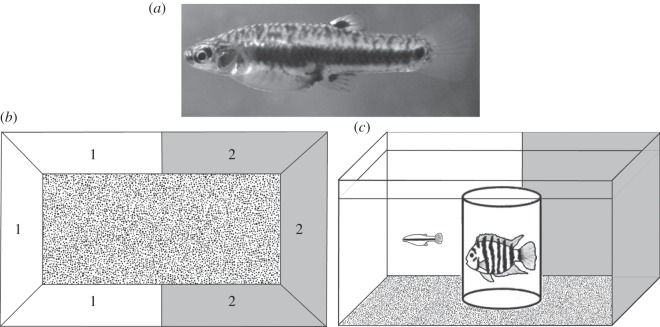
Figure 1.
(a) Photo of the study species, the least killifish Heterandria formosa. (b) Schematic of the experimental set-up shown from above, where each side of the experimental aquaria (labelled 1 and 2) were covered with one of the four experimental backgrounds. (c) The experimental setup from the side, with one test individual (least killifish) and the simulated predation threat (convict cichlid) in the transparent cylinder-shaped holding tank located in the middle of the experimental aquarium.
To investigate whether background choice in the least killifish was related to the level of predation risk, we used convict cichlids (Amatitlania siquia) to simulate predation threat. Convict cichlids are substantially larger than the least killifish, and can span a total length of up to 12 cm. The convict cichlids were laboratory-reared descendants from a wild population in Lake Xiloá, Nicaragua, Central America.
The least killifishes were kept in 200 l holding tanks (length 100 × width 50 × height 40 cm), about 200 fish in each. The cichlids were kept in similar tanks, about 30 fish in each. Water temperature was 24–28°C, and the light : dark rhythm was 16 : 8 h. The least killifishes were fed one to three times daily ad libitum with live newly hatched brine shrimp (Artemia spp.) or commercial flake food. Cichlids were fed one to two times daily ad libitum with frozen red chironomids (Chironomidae spp.) or commercial cichlid pellets. The experiments were conducted in summer and autumn 2009 in the aquatic laboratory at Åbo Akademi University in Turku, Finland.
(b). Demonstration of behavioural response to artificial backgrounds
To test whether the background choice in the least killifish could be induced by manipulating the appearance of artificial backgrounds, we first presented them with a choice between a black and a white background in 12 l aquaria (width 30 × depth 20 × height 20 cm). We covered one half of an aquarium (the short side, half of the bottom and half of the long side) with black, and the other half with white water-resistant paper (‘Rite in the Rain’, J. L. Darling Corporation, Tacoma, WA), leaving only the long side in the front uncovered for observations. Water depth was 15 cm, and water temperature was 25°C. Aquarium lights were located 25 cm above the aquaria. One killifish was placed in the middle of an aquarium. We then scored the position (black or white half) of the fish once every 5 min for one hour, resulting in 12 observations per individual. A total of 24 individuals (both males and females) were tested.
(c). Experiments 1–3: experimental stimuli
We investigated the importance of background pattern on background choice of the least killifish with three experiments. All the backgrounds were made with a purpose-written program using the software Matlab v. R2008b (The MathWorks, Inc., Natick, MA), and then printed with a laser printer (HP LaserJet P4015x with 1200 dpi resolution) on the water-resistant paper. To reproduce the black stripes of the least killifish in the background, we first anaesthetized 22 randomly chosen individuals with 2-phenoxyethanol, and then photographed and measured the maximum width and length of their stripes by using the software ImageJ (US NIH, Bethesda, MD). The average length (±s.d.) was 12.5 mm (±3.2 mm), and the width covaried with length (linear regression: F1,20 = 47.14, p < 0.00001, adjusted r2 = 0.69, y = 0.096x – 0.01). The stripes for the backgrounds were produced by randomly sampling the length from a normal distribution with the mean and s.d. of the length of stripes of the fish, and by assigning each stripe the width given by the regression equation. Even though females are larger than males in this species, the proportion between stripe length and body length did not differ between sexes (Mann–Whitney U-test: W = 195, nfemales = 19, nmales = 19, p = 0.69).
We produced four differently patterned backgrounds for experiments 1–3. Importantly, all of them had identical black-to-white ratio, consisting of 22 per cent black. In each aquarium, the two backgrounds covered exactly 50 per cent each of the sides (figure 1). In every experiment, we varied the sides of the two background types between replicates to exclude the influence of any other factor than the background.
The bottom of each aquarium was covered with a pattern that consisted of black, randomly distributed squares and did not differ between the two halves (figure 1). The sizes of the squares followed the variation in width of the fishes' own stripes, and the black-to-white ratio was the same as on the sides of the aquaria. To prevent any disturbance from outside, we covered the area in front of the aquaria with black plastic that had small viewing holes (one for each aquarium) to allow observations.
(d). Experiments 1–3: experimental procedure
We ran each of experiments 1–3 both with and without simulated predation threat. For the predation treatment, we put a convict cichlid in a cylindrical container made of transparent plastic and mesh (diameter 10 × height 15 cm), and placed it in the middle of the aquarium (figure 1). This was performed 1 h prior to the start of each trial to allow the cichlid to calm down. We switched the cichlid against a new one between every predation trial. Otherwise, these experiments were carried out exactly the same way as the corresponding experiments without a predator.
In the beginning of each replicate, a randomly chosen killifish was placed in the middle of the experimental aquarium. If there was a cichlid in the middle, the least killifish was placed in front of the predator cage. After the fish had been allowed to calm down for 2 min, the observation started and the location of the fish was recorded in real time with the event-recording software J-watcher (v. 1.0; http://galliform.psy.mq.edu.au/jwatcher). Each replicate lasted for 15 min, and the time spent on each side was continuously recorded throughout the observation time. An equal number of females and males were used in each treatment group, and we used each individual (n = 220) only once.
(i). Experiment 1: horizontal versus vertical stripes
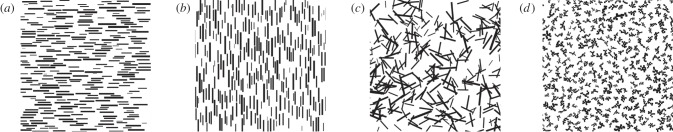
The background representing the closest match to the pattern of the fish consisted of stripes that had similar shape, size and the typical horizontal orientation of the stripe of the swimming fish. Hereafter, the matching background will be referred to as horizontal orientation (HO; figure 2a). In experiment 1, the HO background was presented together with a background that consisted of similar stripes but with a mismatching, vertical orientation (referred to as VO; figure 2b). A total of 60 individuals (30 females and 30 males) were used in this experiment, and half of them had a predator present.
Figure 2.
Samples from each of the four experimental backgrounds used in experiment 1–3: (a) HO, matching orientation background; (b) VO, mismatching orientation background; (c) CO, complex orientation background; and (d) CS, complex pattern shape.
(ii). Experiment 2: matching versus complex stripe orientation
In experiment 2, the fish were presented with a choice between the HO background and a background consisting of the size- and shape-matching stripes, but now with added complexity through random orientation and by allowing overlap between the stripes (referred to as CO for complex orientation; figure 2c). The random orientation and overlap of the stripes decreased the match between the fish and the CO background. The overlap also increased the complexity (i.e. the perimeter-to-√area ratio [30]) of the shape of the elements. A total of 80 individuals (40 males and 40 females) were used in this experiment, and half of them had a predator present.
(iii). Experiment 3: matching versus complex pattern shape
In the third experiment, the HO background was presented together with a background consisting of non-overlapping elements with increased complexity of shape compared with the stripes (hereafter CS for complex shape; figure 2d). These elements were produced by using the area of the stripes, but in a way that increased the perimeter-to-√area ratio of the shape. Each shape was produced from squares, with sides being equal to the height of a randomly chosen stripe on the HO background. Identical squares were added in random but adjoining positions, until the area was equal to the area of the stripe. The total number of these shapes equalled to the number of stripes on the HO background. We used 80 individuals (40 males and 40 females) in this experiment, and half of them had a predator present.
(e). Statistical analyses
We used the proportion of time spent in the HO side as the dependent variable (data are available in the electronic supplementary material, appendix, table S1). All our data conformed to the assumptions of parametric tests, and we used a two-way analysis of variance (ANOVA) to test for the effects of predation threat (predator absent or present), the sex of the fish and their interaction. Furthermore, to test whether the background choice deviated from the expected value of 50 per cent time in both halves of the aquarium, we used two-tailed, one-sample t-tests. All analyses were conducted using the statistical software R for Windows, v. 2.9.2 [35].
3. Results
(a). Demonstration of behavioural response to artificial backgrounds
When testing the experimental set-up with black and white backgrounds, the least killifish showed a strong preference for the black background (one-sample t-test: t23 = 17.72, p < 0.0001). They were observed in the black half of the aquaria in 86.5 ± 10 per cent (mean ± s.d.) of the observations. This result shows that the artificial, printed backgrounds induce background choice in the least killifish.
(b). Experiment 1: horizontal versus vertical stripes
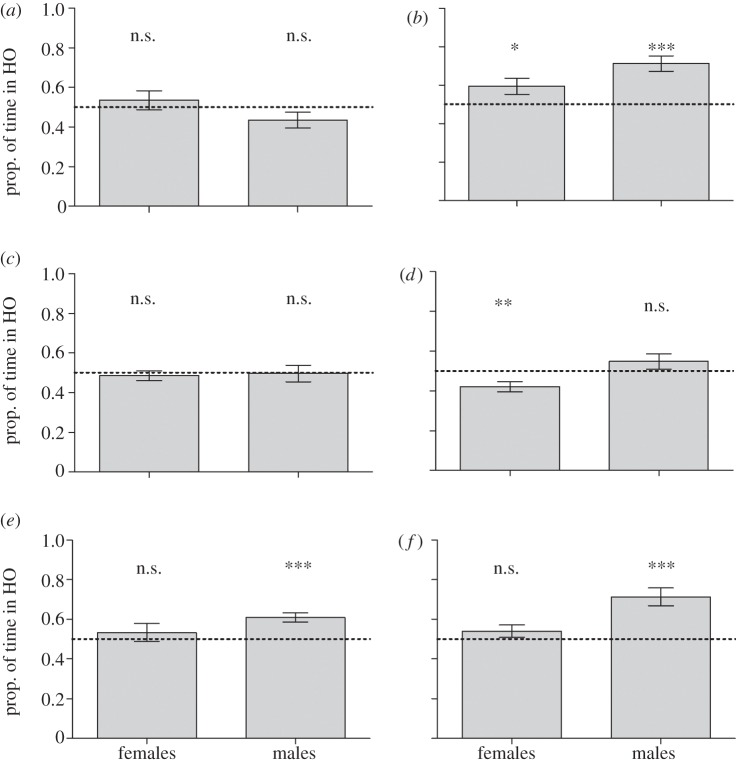
In the first background pattern choice experiment, we tested the fishes' choice between the HO and the VO background. We found a significant effect of simulated predation threat on the least killifishes' background choice (table 1); the fish spent more time on the HO side in the presence of a predator. Sex and the interaction between sex and predation did not influence the background choice of least killifishes (table 1). When testing for background preference, our results showed that in the absence of a predator, neither males (t = 1.65, d.f. = 14, p = 0.12) nor females (t = 0.76, d.f. = 14, p = 0.46) showed any background preference (figure 3a). When under simulated predation threat, however, both males (t = 5.38, d.f. = 14, p < 0.001) and females (t = 2.28, d.f. = 14, p = 0.038) preferred the HO background before the VO background (figure 3b).
Table 1.
Results from the two-factor ANOVAs showing the influence of sex, predation and their interaction on time spent on all pairs of tested backgrounds in experiments 1–3: (i) matching (HO) versus mismatching background (VO), (ii) matching (HO) versus complex orientation (CO) and (iii) matching (HO) versus complex shape (CS). **p < 0.01, *p < 0.05.
| source | d.f. | F | p |
|---|---|---|---|
| (i) experiment 1: horizontal (HO) versus vertical orientation (VO) | |||
| sex | 1 | 2.96 | 0.091 |
| predation | 1 | 5.91 | 0.018* |
| interaction | 1 | 1.10 | 0.30 |
| residuals | 56 | ||
| (ii) experiment 2: horizontal (HO) versus complex orientation (CO) | |||
| sex | 1 | 4.34 | 0.040* |
| predation | 1 | 0.04 | 0.83 |
| interaction | 1 | 3.00 | 0.087 |
| residuals | 76 | ||
| (iii) experiment 3: horizontal (HO) versus complex shape (CS) | |||
| sex | 1 | 11.08 | 0.0013** |
| predation | 1 | 2.08 | 0.15 |
| interaction | 1 | 1.72 | 0.19 |
| residuals | 76 | ||
Figure 3.
Mean (±s.e.) time spent on each background by both males and females (a,c,e) without predation threat and (b,d,f) with simulated predation threat. (a,b) n = 15 for HO versus VO treatment, (c,d) n = 20 for HO versus CO treatment, and (e,f) n = 20 for HO versus CS treatment. The dashed line indicates the 50% expectation (i.e. no choice). For each treatment, we tested whether time spent in HO deviated from the 50% expectation using the t-test (***p < 0.001; **p < 0.01; *p < 0.05; n.s. denotes not significant).
(c). Experiment 2: matching versus complex stripe orientation
In our second experiment, the fish were presented with a choice between the HO and the more complex CO background. Females spent more time on the CO side than males did (table 1). The effect of predation was not significant. The interaction between sex and predation was close to significance (table 1). The background preference test revealed that in the absence of a predator neither females (t = −0.60, d.f. = 19, p = 0.55) nor males (t = −0.07, d.f. = 19, p = 0.94) showed any preference for either background (figure 3c). In the presence of a predator, females preferred the CO background (t = −3.03, d.f. = 19, p = 0.0068, figure 3d), whereas males showed no preference for either side (t = 1.42, d.f. = 19, p = 0.17, figure 3d).
(d). Experiment 3: matching versus complex pattern shape
In the third experiment, where the fish were presented with a choice between the HO background and the background consisting of more complex pattern shape (CS), males spent more time on the HO background than females (table 1). The presence of a predator and the interaction between sex and predation did not affect the time the fish spent on each side (table 1). Regardless of predator presence, males spent significantly more time in the HO background than in the CS background (no predator: t = 4.76, d.f. = 19, p < 0.001; predator present: t = 4.72, d.f. = 19, p < 0.001; figure 3e,f), whereas females did not show a preference for either background (no predator: t = 0.76, d.f. = 19, p = 0.46; predator present: t = 1.22, d.f. = 19, p = 0.24; figure 3e,f).
4. Discussion
Our results clearly show that the visual features of backgrounds can have an important effect on the habitat choice of fish, and this is at least partly related to predator avoidance. The test with the black and the white background demonstrated that habitat choice can be induced in the least killifish by varying only the visual appearance of artificial backgrounds. The least killifish showed a strong preference for the black over the white background. This result is also in accordance with several previous studies showing preference for dark backgrounds in various taxa, such as fishes [36], isopods [37] and amphibians [27]. Moreover, although many species of fish can rapidly adjust the brightness of their body coloration according to the brightness level of the environment [38], a recent study [34] shows that the least killifish does not have such ability. Also this fact may contribute to the strength of the behavioural response that we observed here.
The main aim of our study was to investigate the importance of the visual complexity of the background and the resemblance between prey patterning and background in background choice of prey. Our results from experiments 1–3, in which we carefully controlled for the mean intensity (i.e. black-to-white ratio) of the backgrounds, demonstrate the importance of background pattern. Interestingly, significant background preference was mainly observed in the presence of a predator, whereas in the absence of a predator the fish used the two backgrounds equally much. This strongly suggests that these observed preferences represent an anti-predator adaptation. Moreover, because the least killifish in our study were laboratory-reared, and the backgrounds were artificial and novel to the fish, these preferences could not be explained by any previously learned association between the appearance of a habitat and predation risk or resource availability, but is instead likely to be an innate response to an observed risk.
When presented with a choice between the HO and the VO backgrounds in experiment 1, in the presence of a predator, both males and females showed a strong preference for the background pattern that matched the typical horizontal stripe of the fish. One might argue that the preference for horizontal stripes could reflect a preference to stay in a school to dilute the risk of being targeted by a predator when under threat (assuming that the stripe functions as an intraspecific signal), but we do not find this explanation likely. Although least killifishes tend to aggregate near water margins, they are not considered a truly schooling species as the fish are not seen moving around in a coordinate way [39] (Kai Lindström and Colette St Mary 2012, personal communication). Moreover, if a preference for horizontal stripes would reflect a preference to stay in a school, we would then have expected the fish under predation threat to consistently prefer the same, horizontally striped background also in experiments 2 and 3, and this was not the case. Instead, we think that the result of experiment 1 reflects a preference for habitats that yield protection through improved background matching [12,40]. The result of experiment 1 suggests that the least killifish might be able to assess and respond to some cues of visual similarity between their body pattern and the background, and that those cues serve as indicators for habitat safety, because any preference observed mainly occurred while under simulated predation risk.
In experiment 2, the fish were offered a choice between the HO background composed of orientation-matching stripes and the CO background composed of a more complex, less regular background pattern in which the stripes overlapped and varied in orientation. Females preferred the complex background under predation threat, whereas males showed no difference between these two backgrounds. For males, this could be due to two obvious reasons: either they found both backgrounds equally protective, or they found them equally unprotective. The first alternative seems more likely because males displayed a strong preference for the matching background in experiment 1.
In the third experiment, the fish were presented with a choice between the HO background and the CS background consisting of irregular and variable shapes. Males showed a preference for the matching background. This might indicate that the males experience that this background deviated too much from the males' own pattern and that the level or type of complexity was not protective enough. Females, in contrast, did not show a preference between these two, but appear to have experienced them as equally protective.
As mentioned in §1, both a visual match in the appearance of prey colour pattern and background, and visual complexity of the background are known to decrease predation risk [9,12,29,30,41]. Collectively, our results show that in the least killifish, background matching is an important aspect of background choice, but also some aspects of complexity are important and may even override background matching. Females in experiment 2 showed a strong preference for the CO background when under predation threat, whereas they did not discriminate between the HO and the CS background in experiment 3. This may be because the complex background of experiment 2 better corresponds with the aspects of complexity that the least killifish prefer or because the stripes constituting that complex pattern might allow the fish to simultaneously benefit from matching and complexity. In natural habitats, visual complexity is probably often a less specific requirement, and therefore more likely to come across than a visual match between a specific pattern and background. Also, for an animal to choose visually matching backgrounds, they need either to make a comparison between themselves and the background to find matching backgrounds [22], or to have an innate preference for some background matching properties. Background preference has previously been studied particularly in colour polymorphic species, because preference for matching backgrounds could contribute to maintenance of polymorphism [42]. However, in polymorphic species, genetically determined preference might be problematic because recombination may tear down the genetic coupling between a particular appearance (colour morph) and the optimal preference corresponding to it [43]. For these reasons, visual complexity could in many habitats serve as an easier and more straightforward cue to decrease predation risk even in polymorphic species.
Our experiments revealed some differences in the background preferences between males and females. This might indicate that the least killifish adopt different background choice, or habitat use strategies between sexes, as previously suggested in many other species [26,37,44,45]. Because female least killifishes are larger than males and probably have higher resource needs owing to higher growth rate and egg production [46], it is possible that to acquire the necessary resources they need to use the habitat differently compared with males. This would suggest that opportunity costs for background matching (e.g. lost opportunity to feed in profitable foraging patches that would provide poor background matching [16]) could be higher for females than males. Thus, even if both background options (matching and complex backgrounds) effectively decreased predation risk, females might still benefit from preferring the more complex background if it expands the range of microhabitats where they can forage. This would reduce some of the opportunity costs that come with a limited amount of suitable backgrounds owing to background matching. Also, if males could acquire their necessary resources by using a smaller range of microhabitats than females in their natural environment, this would suggest lower opportunity costs for background matching in males and could explain why males generally showed preference for the matching background (experiments 1 and 3).
To conclude, we suggest that background matching is important in habitat choice of prey, but also that some aspects of visual complexity of the available backgrounds could serve as more straightforward and important cues in habitat choice. Future studies should investigate whether visual variability of a habitat could reveal an increased preference for complexity and decreased preference for matching backgrounds, because increased visual variability is expected to constrain background matching [47]. Aims for future studies would be to identify the cues of complexity that prey respond to, and whether the complexity needs to be combined with some aspects of the animal's own pattern to also provide some benefits from matching.
Acknowledgements
The study was performed with permission from the State Provincial Office of Southern Finland (decision STH672A).
This work has been funded by the Academy of Finland (S.M.). We are grateful to Kai Lindström for providing us with the fishes. We thank Anders Forsman and two anonymous referees for valuable comments on earlier drafts of this manuscript, and Levente Basco for assistance in the laboratory.
References
- 1.Poulton E. B. 1890. The colours of animals: their meaning and use especially considered in the case of insects. London, UK: Kegan Paul, Trench, Trubner and Co. Ltd [Google Scholar]
- 2.Thayer A. H. 1918. Camouflage. Sci. Monthly 7, 481–494 [Google Scholar]
- 3.Cott H. B. 1940. Adaptive coloration in animals. London, UK: Methuen & Co., Ltd [Google Scholar]
- 4.Endler J. A. 1978. A predator's view of animal color patterns. Evol. Biol. 11, 319–364 [Google Scholar]
- 5.Stevens M., Merilaita S. 2009. Introduction. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B 364, 423–427 10.1098/rstb.2008.0217 (doi:10.1098/rstb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merilaita S., Stevens M. 2011. Crypsis through background matching. In Animal camouflage: mechanisms and function (eds Stevens M., Merilaita S.), pp. 17–33 Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Darwin E. 1794. Zoönomia, or the laws of organic life, vol. 1. London, UK: Johnson [Google Scholar]
- 8.Wallace A. R. 1891. Natural selection and tropical nature. London, UK: Macmillan and Co [Google Scholar]
- 9.Feltmate B. W., Williams D. D. 1989. A test of crypsis and predation avoidance in the stonefly Paragnetina media (Plecoptera: Perilidae). Anim. Behav. 37, 992–999 10.1016/0003-3472(89)90143-7 (doi:10.1016/0003-3472(89)90143-7) [DOI] [Google Scholar]
- 10.Merilaita S., Lyytinen A., Mappes J. 2001. Selection for cryptic coloration in a visually heterogeneous habitat. Proc. R. Soc. Lond. B 268, 1925–1929 10.1098/rspb.2001.1747 (doi:10.1098/rspb.2001.1747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsson I. J., Källman-Eriksson K. 2008. Cryptic prey colouration increases search time in brown trout (Salmo trutta): effects of learning and body size. Behav. Ecol. Sociobiol. 62, 1613–1620 10.1007/s00265-008-0590-8 (doi:10.1007/s00265-008-0590-8) [DOI] [Google Scholar]
- 12.Kettlewell H. B. D. 1955. Recognition of appropriate backgrounds by the pale and black phases of Lepidoptera. Nature 175, 943–944 10.1038/175943a0 (doi:10.1038/175943a0) [DOI] [PubMed] [Google Scholar]
- 13.Cooper J. M., Allen J. A. 1994. Selection by wild birds on artificial dimorphic prey on varied backgrounds. Biol. J. Linn. Soc. 51, 433–466 10.1111/j.1095-8312.1994.tb00972.x (doi:10.1111/j.1095-8312.1994.tb00972.x) [DOI] [Google Scholar]
- 14.Johannesson K., Ekendahl A. 2002. Selective predation favouring cryptic individuals of marine snails (Littorina). Biol. J. Linn. Soc. 76, 137–144 10.1111/j.1095-8312.2002.tb01720.x (doi:10.1111/j.1095-8312.2002.tb01720.x) [DOI] [Google Scholar]
- 15.Godin J.-G. J. 1997. Behavioural ecology of teleost fishes. Oxford, UK: Oxford University Press [Google Scholar]
- 16.Ruxton G. D., Sherrat T. N., Speed M. P. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Popham E. J. 1943. Ecological studies of the commoner species of British corixidae. J. Anim. Ecol. 12, 124–136 10.2307/1372 (doi:10.2307/1372) [DOI] [Google Scholar]
- 18.Sargent T. D., Keiper R. R. 1969. Behavioural adaptations of cryptic moths. І. Preliminary studies on bark-like species. J. Lepidopterists’ Soc. 23, 1–9 [Google Scholar]
- 19.Kettlewell H. B. D., Conn D. L. T. 1977. Further background-choice experiments on cryptic Lepidoptera. J. Zool. Lond. 181, 371–376 10.1111/j.1469-7998.1977.tb03250.x (doi:10.1111/j.1469-7998.1977.tb03250.x) [DOI] [Google Scholar]
- 20.Caro T. 2005. Antipredator defenses in birds and mammals. London, UK: The University of Chicago Press Ltd [Google Scholar]
- 21.Boarman M., Askew R. R., Cook L. M. 1974. Experiments on resting site selection by nocturnal moths. J. Zool. Lond. 172, 343–355 10.1111/j.1469-7998.1974.tb04110.x (doi:10.1111/j.1469-7998.1974.tb04110.x) [DOI] [Google Scholar]
- 22.Gillis J. E. 1982. Substrate matching cues in the cryptic grasshopper Circotettix rabula rabula (Rehn & Hebard). Anim. Behav. 30, 113–116 10.1016/S0003-3472(82)80244-3 (doi:10.1016/S0003-3472(82)80244-3) [DOI] [Google Scholar]
- 23.Ahnesjö J., Forsman A. 2006. Differential habitat selection by pygmy grasshopper color morphs: interactive effects of temperature and predator avoidance. Evol. Ecol. 20, 235–257 10.1007/s10682-006-6178-8 (doi:10.1007/s10682-006-6178-8) [DOI] [Google Scholar]
- 24.Sandoval C. P. 1994. Differential visual predation on morphs of Timema cristinae (Phasmatodeae: Timemidae) and its consequences for host range. Biol. J. Linn. Soc. 52, 341–356 10.1111/j.1095-8312.1994.tb00996.x (doi:10.1111/j.1095-8312.1994.tb00996.x) [DOI] [Google Scholar]
- 25.Lees D. R. 1975. Resting site selection in the geometrid moth Phigalia pilosaria (Lepidoptera: Geometridae). J. Zool. Lond. 176, 341–352 10.1111/j.1469-7998.1975.tb03206.x (doi:10.1111/j.1469-7998.1975.tb03206.x) [DOI] [Google Scholar]
- 26.Merilaita S., Jormalainen V. 1997. Evolution of sex differences in michrohabitat choice and colour polymorphism in Idotea baltica. Anim. Behav. 54, 769–778 10.1006/anbe.1996.0490 (doi:10.1006/anbe.1996.0490) [DOI] [PubMed] [Google Scholar]
- 27.Garcia T. S., Sih A. 2003. Color change and color dependent behavior in response to predation risk in the salamander sister species Ambystoma barbouri and Ambystoma texanum. Oecologia 137, 131–139 10.1007/s00442-003-1314-4 (doi:10.1007/s00442-003-1314-4) [DOI] [PubMed] [Google Scholar]
- 28.Merilaita S. 2003. Visual background complexity facilitates the evolution of camouflage. Evolution 57, 1248–1254 [DOI] [PubMed] [Google Scholar]
- 29.Dimitrova M., Merilaita S. 2010. Prey concealment: visual background complexity and prey contrast distribution. Behav. Ecol. 21, 176–181 10.1093/beheco/arp174 (doi:10.1093/beheco/arp174) [DOI] [Google Scholar]
- 30.Dimitrova M., Merilaita S. 2012. Prey pattern regularity and background complexity affect detectability of background-matching prey. Behav. Ecol. 23, 384–390 10.1093/beheco/arr201 (doi:10.1093/beheco/arr201) [DOI] [Google Scholar]
- 31.Farmer E. W., Taylor R. M. 1980. Visual search through color displays: effects of target-background similarity and background uniformity. Percept. Psychophys. 27, 267–272 10.3758/BF03204265 (doi:10.3758/BF03204265) [DOI] [PubMed] [Google Scholar]
- 32.Wolfe J. M., Friedman-Hill S. R., Stewart M. I., O'Connell K. M. 1992. The role of categorization in visual search for orientation . J. Exp. Psychol. Hum. Percept. Perform. 18, 34–49 10.1037/0096-1523.18.1.34 (doi:10.1037/0096-1523.18.1.34) [DOI] [PubMed] [Google Scholar]
- 33.Gordon I. E. 1968. Interactions between items in visual search. J. Exp. Psychol. 76, 348–355 10.1037/h0025482 (doi:10.1037/h0025482) [DOI] [PubMed] [Google Scholar]
- 34.Cox S., Chandler S., Barron C., Work K. 2009. Benthic fish exhibit more plastic crypsis than non-benthic species in a freshwater spring. J. Ethol. 27, 497–505 10.1007/s10164-008-0148-2 (doi:10.1007/s10164-008-0148-2) [DOI] [Google Scholar]
- 35.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 36.Bradner J., McRobert S. P. 2001. Background colouration influences body colour segregation in mollies. J. Fish Biol. 59, 673–681 10.1006/jfbi.2001.1680 (doi:10.1006/jfbi.2001.1680) [DOI] [Google Scholar]
- 37.Merilaita S., Jormalainen V. 2000. Different roles of feeding and protection in diel microhabitat choice of sexes in Idotea baltica. Oecologia 122, 445–451 10.1007/s004420050965 (doi:10.1007/s004420050965) [DOI] [PubMed] [Google Scholar]
- 38.Clarke J. M., Schluter D. 2011. Colour plasticity and background matching in a threespine stickleback species pair. Biol. J. Linn. Soc. 102, 902–914 10.1111/j.1095-8312.2011.01623.x (doi:10.1111/j.1095-8312.2011.01623.x) [DOI] [Google Scholar]
- 39.Leips J., Travis J. 1999. The comparative expression of life-history traits and its relationship to the numerical dynamics of four populations of the least killifish. J. Anim. Ecol. 68, 595–616 10.1046/j.1365-2656.1999.00311.x (doi:10.1046/j.1365-2656.1999.00311.x) [DOI] [Google Scholar]
- 40.Marshall N. J. 2000. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. Lond. B 355, 1243–1248 10.1098/rstb.2000.0676 (doi:10.1098/rstb.2000.0676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merilaita S. 2001. Habitat heterogeneity, predation and geneflow: color polymorphism in the isopod Idotea baltica. Evol. Ecol. 15, 103–116 10.1023/A:1013814623311 (doi:10.1023/A:1013814623311) [DOI] [Google Scholar]
- 42.Garcia-Dorado A. 1986. The effect of niche preference on polymorphism protection in a heterogeneous environment. Evolution 40, 936–945 10.2307/2408754 (doi:10.2307/2408754) [DOI] [PubMed] [Google Scholar]
- 43.Gillespie J. 1974. Polymorphism in patchy environments. Am. Nat. 108, 145–151 10.1086/282894 (doi:10.1086/282894) [DOI] [Google Scholar]
- 44.Shine R. 1986. Sexual differences in morphology and niche utilization in an aquatic snake, Acrochordus arafurae. Oecologia 69, 260–267 10.1007/BF00377632 (doi:10.1007/BF00377632) [DOI] [PubMed] [Google Scholar]
- 45.Asakura A. 1995. Sexual differences in life history and resource utilization by the hermit crab. Ecology 76, 2295–2313 10.2307/1941703 (doi:10.2307/1941703) [DOI] [Google Scholar]
- 46.Alcock J. 2005. Animal behavior: an evolutionary approach, 8th edn. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 47.Merilaita S., Tuomi J., Jormalainen V. 1999. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 67, 151–161 10.1111/j.1095-8312.1999.tb01858.x (doi:10.1111/j.1095-8312.1999.tb01858.x) [DOI] [Google Scholar]