Abstract
Background
Thyroid enlargement and thyroid nodules are common in the general population. This review concerns their proper diagnostic assessment and treatment.
Methods
We selectively reviewed the literature from 1990 to 2012 and evaluated original articles and reviews retrieved from the PubMed database, as well as the recommendations of the following specialty societies: the German Societies of Endocrinology and Nuclear Medicine (Deutsche Gesellschaft für Endokrinologie, Deutsche Gesellschaft für Nuklearmedizin), the German Working Group for Endocrine Surgery (Chirurgische Arbeitsgemeinschaft Endokrinologie, CAEK), the European Thyroid Association, and the American Thyroid Association.
Results
There have been very few randomized trials concerning the diagnosis and treatment of goiter. Nodular goiter can be managed by watchful waiting, drug treatment (initially with levothyroxine and iodide), radioactive iodine therapy, or surgery.
Conclusion
Many patients with nodules need no treatment at all. Treatment is indicated, however, if the patient is symptomatic and/or has an autonomously functioning (“hot”) nodule, or if cancer is suspected. Potentially cancerous nodules must be operated on. If euthyroid nodular goiter is to be treated with the main goal of size reduction, either surgery or radioactive iodine therapy can be used. Drug treatment is an option for small nodules or goiters, but iatrogenic hyperthyroidism must be avoided at all costs. The type of follow-up that is required depends on the chosen treatment.
Goiter is defined as a thyroid gland that is larger than the upper limit of normal for the patient’s age and sex: 18 mL for women, 25 mL for men. Goiter is a physical finding, not an illness in itself. It has many causes and can take on many shapes (Table 1). Moreover, it can be associated with a euthyroid, hyperthyroid, or hypothyroid metabolic state. In this review, we discuss two entities: euthyroid diffuse goiter and nodular goiter (with one or more nodules). Little scientific evidence is available to date on the diagnosis and treatment of diffuse and nodular goiter. We therefore present and discuss the relevant recommendations of thyroid specialty societies from Germany and abroad.
Table 1. Causes of thyroid enlargement (goiter).
| Disease entity | Thyroid function | Remarks |
| Diffuse goiter | euthyroid | |
| Uni- or multinodular goiter | euthyroid-hyperthyroid | in regions of iodine deficiency, may coexist with autoimmune thyroiditis |
| Thyroid cancer | euthyroid | may be present within a nodular goiter and/or autoimmune thyroiditis |
| Autoimmune thyroiditis | euthyroid, hypo- or hyperthyroid | thyrocyte destruction in Hashimoto’s thyroiditis, TSH-R-AB-mediated thyrocyte stimulation in Graves’ disease |
| De Quervain’s thyroiditis | euthyroid, hypo- or hyperthyroid | typically, tenderness of the thyroid; fever; poor general state |
| Riedel’s thyroiditis | euthyroid | |
| Thyroid cyst | euthyroid | |
| Impaired thyroxine synthesis | hypothyroid-euthyroid | TSH-triggered multinodular goiter |
| Thyroxine resistance | euthyroid | End-organ-dependent function |
| Acromegaly | euthyroid | IGF-1-dependent |
| Drugs (lithium, thyrostatic drugs) | euthyroid-hypothyroid | |
| TSH-oma | hyperthyroid | TSH-dependent |
IGF, insulin-like growth factor; TSH, thyroid-stimulating hormone; TSH-oma,TSH-secreting pituitary adenoma; TSH-R-AB, TSH-receptor antibodies
Learning objectives
The purpose of this article is to enable readers to
recognize the importance of an etiological diagnostic work-up for diffuse or nodular goiter,
understand the use of particular diagnostic tests as part of this work-up, and
gain an overview of the current scientific evidence regarding the treatment options for diffuse and nodular goiter.
Definition.
Goiter is defined as a thyroid gland whose volume exceeds 18 mL (for a woman) or 25 mL (for a man).
The epidemiology and clinical features of diffuse and nodular goiter
Thyroid enlargement and thyroid nodules are common in the general population. In the first phase of the Study of Health in Pomerania (SHIP), whose results were published in 2003, 35.9% of the 3941 probands not previously known to have thyroid disease had a goiter, and 20.2% had thyroid nodules (1). In the Papillon study, in which 96 278 working adults without known thyroid disease were examined, 9.7% had a goiter, and 23.3% had thyroid nodules. The prevalence of these two conditions is closely correlated with the dietary intake of iodine. In the recently published 5-year follow-up study of the SHIP cohort, both conditions were found to have become rarer as iodine intake increased (e1).
The pathogenesis of diffuse and nodular goiter is shown graphically in Figures 1 and 2 (3, 5, e2).
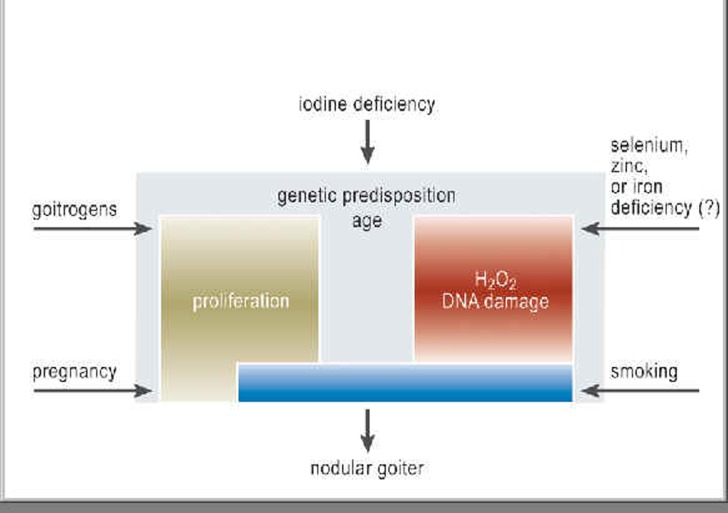
Figure 1.
The multifactorial origin of goiter: The most important known, preventable cause of thyroid enlargement is iodine deficiency. The prevalence of goiter is directly related to iodine deficiency and tends to decrease when the iodine intake of a population is increased. The risk factors for goiter include intrinsic biological factors (which account for the five- to tenfold higher prevalence of goiter among women) as well as cigarette smoking, naturally occurring goitrogens, selenium or zinc deficiency, and emotional stress.
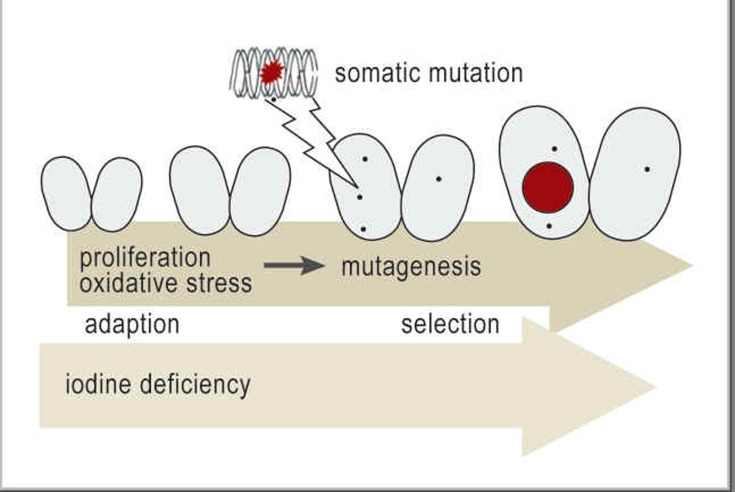
Figure 2.
Hypothetical model of the pathogenesis of nodular goiter: Nodular goiters are very heterogeneous in their functional, structural, and molecular genetic features (4). It is currently thought that, in persons with both a genetic predisposition and an iodine deficiency, a “mutagenic environment” arises (increased cellular proliferation and formation of free radicals) that promotes the appearance of somatic mutations in thyrocytes. A clonal thyroid tumor forms when the genetic defect is not repaired and the mutation is of a type that gives the proliferating cells a selective advantage
Goiter is asymptomatic in most cases (4); it may be suspected if the patient has noticed a change in collar size or has recently stopped wearing necklaces or turtleneck sweaters. Thyroid enlargement to a size of 40 mL or more is generally visible (4). Mechanical compression of the trachea and/or esophagus was found to be present in 30% to 85% of the patients in a surgical case series, even in some who were apparently asymptomatic (4). The location of a goiter and the dynamics of its growth determine whether, and how, it will produce symptoms: Retrosternal goiter often causes dysphagia, while retrotracheal goiter often causes dyspnea (4).
The diagnostic evaluation of diffuse and nodular goiter
When goiter is suspected, or the thyroid gland is visibly enlarged, a basic diagnostic evaluation is indicated, consisting of the following tests (5– 7):
targeted history-taking and physical examination with attention to the cause of goiter, potentially abnormal thyroid function, and any symptoms or signs of mechanical compression;
thyroid ultrasonography to confirm enlargement, to distinguish diffuse from uni- or multinodular goiter, and to reveal possible other causes of goiter (Table 1);
measurement of thyroid-stimulating hormone (TSH) for the evaluation of thyroid function.
If the clinical or ultrasonographic examination reveals nodular goiter with one or more nodules, two further questions must be answered:
Is autonomous thyroid function present (i.e., is the nodule “hot”)?
Is (are) the nodule(s) benign or malignant?
Diagnostic evaluation.
History-taking and physical examination with attention to the cause of goiter
Thyroid ultrasonography
Measurement of thyroid-stimulating hormone (TSH) for the evaluation of thyroid function
Aside from history-taking and physical examination, the tests that can help answer these questions include laboratory testing, functional studies (scintigraphy), imaging studies (ultrasonography and, in special cases, computerized tomography without contrast medium or magnetic resonance tomography), and fine-needle biopsy (FNB). Their indications and uses in the evaluation of nodular goiter are summarized in the following paragraphs (5– 7).
History and physical examination in nodular goiter
A targeted history should be taken to determine whether there are any mechanical symptoms such as exertional dyspnea and/or dysphagia and to identify any features that would arouse a suspicion of cancer. The patient should be asked about
the duration and progression of thyroid enlargement,
any prior irradiation of the head-and-neck region (which would increase the risk of thyroid cancer),
new hoarseness,
any family history of thyroid cancer that would point to a possible hereditary predisposition, e.g., in type 2 multiple endocrine neoplasia.
The absence of suggestive symptoms, however, does not rule out thyroid cancer (6).
By palpating the thyroid gland, the examiner can judge the extent of enlargement and seek physical evidence of malignancy (a hard thyroid mass that does not move on swallowing; enlarged cervical lymph nodes) or, alternatively, of other diseases that might be misdiagnosed as nodular goiter. For example, discomfort on light touch and pain on local pressure are typical of de Quervain’s thyroiditis (6).
Laboratory tests in nodular goiter
Not only the serum TSH concentration, but also the serum calcitonin concentration should be measured in every patient with euthyroid nodular goiter, so that medullary thyroid carcinoma (MTC) will not be overlooked (5, 7– 8). If the basal calcitonin level is reproducibly elevated (baseline values vary depending on the particular test used), then further testing should be done to rule out other causes of hypercalcitoninemia: the Thyroid Section of the German Society of Endocrinology (Deutsche Gesellschaft für Endokrinologie, DGE) recommends a stimulation test with pentagastrin or calcium (8, e3).
Testing for thyroid auto-antibodies (thyroperoxidase [TPO] and TSH-receptor antibodies) is recommended only if thyroid function is abnormal or if the ultrasonographic examination arouses the suspicion of an autoimmune thyropathy (6, 7). Thyroglobulin measurement is not indicated in nodular goiter (6, 7).
The clinical history in nodular goiter.
Progression of thyroid enlargement?
Prior irradiation of the head-and-neck region?
New hoarseness?
Family history of thyroid cancer with a possible hereditary predisposition?
Ultrasonography in the differential diagnosis of nodular goiter
Ultrasonography is used to screen for “suspect,” i.e., potentially cancerous, nodules that require further evaluation by either fine-needle biopsy (which yields a specimen for cytological examination) or open surgical resection (after which the entire nodule can be examined histopathologically) (5, 6, 9). Ultrasonographic findings such as a solid, hypoechoic nodule, microcalcifications, an intranodular vascularization pattern, and poorly demarcated tumor borders are more common in malignant than in benign nodules. Each of these individual findings is of low specificity for malignancy and must not be misinterpreted as hard evidence from which an ultrasound-based, quasi-histological diagnosis can be made (Table 2) (6, 9). According to published data, the presence of multiple suspect ultrasonographic findings in a thyroid nodule is 83% to 99% sensitive and 56% to 85% specific for malignancy (9). In recent years, thyroid ultrasonography and color-coded duplex ultrasonography have been increasingly supplemented by elastography for the differential diagnosis of nodular goiter (10). Thyroid cancer typically has an altered tissue consistency that manifests itself as diminished compressibility in an elastographic study (10, e4).
Table 2. Ultrasonographic criteria for thyroid cancer.
| Criterion | Positive predictive value*1 (%) | Negative predictive value*2 (%) |
| Hypoechoic area | 74–94 | 11–68 |
| Microcalcifications | 42–94 | 24–71 |
| Poorly demarcated border / no halo effect | 39–98 | 9–60 |
| Intranodular vascularization | 86–97 | 24–42 |
| Anteroposterior diameter/ transverse diameter >1 | 75 | 67 |
*1Probability that thyroid cancer will be diagnosed if the criterion is met
*2Probability that thyroid cancer will be ruled out if the criterion is not met (9)
Ultrasonography in the differential diagnosis of nodular goiter.
Ultrasonography is used to screen for “suspect,” i.e., potentially cancerous, nodules that need further evaluation by either fine-needle biopsy (for cytology) or surgical resection (for histology).
The differential diagnosis of multinodular goiter is particularly challenging. Suspect nodules must be identified by a combination of ultrasonography and scintigraphy, with special attention to ultrasonographically abnormal areas that are either non-autononomous or cold (6).
Thyroid cancer.
According to published data, the presence of multiple suspect ultrasonographic findings in a thyroid nodule is 83–99% sensitive and 56–85% specific for malignancy.
Scintigraphy in the differential diagnosis of nodular goiter
Focally autonomous areas can be detected much earlier by scintigraphy than by TSH measurement (11). In Germany—a country with previous iodine deficiency—baseline scintigraphy is performed for all nodules larger than 10 mm in size, regardless of the TSH value, so that autonomous thyroid function (“hot” nodules) will not be missed (6, 11). In the international guidelines, scintigraphy is recommended in all cases of multiple thyroid nodules, regardless of dietary iodine intake, to ensure the detection of all functionally non-autonomous nodules that will need to be examined for potential malignancy (6).
If the patient is in a euthyroid metabolic state and scintigraphy reveals focally increased uptake (a so-called “warm” nodule), a scintigraphic suppression scan is often needed to differentiate true autonomous thyroid function from volume artefacts (size ratio of nodule to thyroid lobe) (5). If autonomous function is demonstrated, then the nodule will not need to be examined for potential malignancy, as the vast majority of autonomous adenomas are benign (5, 6).
The differential diagnosis of multinodular goiter.
The differential diagnosis of multinodular goiter is particularly challenging. Suspect nodules are identified by a combination of ultrasonography and scintigraphy.
Fine-needle biopsy in the differential diagnosis of nodular goiter
The main task of fine-needle biopsy (FNB) is to select thyroid nodules that should be histologically examined for malignancy (5, 6, 12– 14). Its twin goals are the early detection of malignant tumors that would otherwise be missed, so that they can be properly treated, and the prevention of needless operations for suspected cancer (for example, operations based solely on the finding of a cold nodule). An experienced team performing FNB of thyroid nodules can diagnose papillary, medullary, poorly differentiated, and anaplastic thyroid carcinomas with high accuracy (12– 14). FNB also usually enables the cytological diagnosis of simple hyperplastic nodules and the evaluation of cystic lesions. The cytological diagnosis of “follicular neoplasia” in a non-autonomous nodule calls for further histological examination (12– 14). In the past, FNB was held to be indicated in every case of a cold thyroid nodule in a euthyroid patient. The current recommendations are more selective: FNB is indicated when autonomous thyroid function has been ruled out and a thyroid nodule is considered suspect for malignancy by ultrasonographic criteria (e.g., a solid, hypoechoic nodule with a poorly demarcated edge [Tables 2 and 3]), and in patients known to be at increased risk for thyroid cancer (prior irradiation, positive family history) (6). The current German recommendation that FNB should not be performed on nodules measuring less than 1 cm is based on practical considerations, as the accuracy of such biopsies is low, and nodules that are so small are usually clinically insignificant (5). Thyroid societies in other countries, however, recommend FNB even on 5-mm nodules if they are suspect by ultrasonographic criteria (6).
Table 3. Classification of thyroid cytology (12).
| Finding | Criteria |
| Inadequate | Material containing few or no thyrocytes from solid or partly liquid nodules (exception: colloid nodules) |
| Negative | Any or all of the changes found in normal and hyperplastic thyroid glands (follicular adenomas with large- and mid-sized follicles),including regressive changes, cysts, and hemorrhagic (pseudo-)cysts, colloid nodules, and thyroiditis; clinical and/or cytological follow-up are sometimes recommended |
| Uncertain/histological*, in need of further evaluation* | Changes that are seen in highly cellular adenomas and follicular carcinomas, including oncocytic variants; corresponds to follicular/oncocytic proliferation or neoplasia; moreover, some of the criteria of other types of malignant tumor may be ‧present, e.g., papillary carcinoma |
| Suspect for (non-follicular) malignancy | Changes highly indicative of malignancy, although not all of the corresponding criteria are met |
| Positive | Changes meeting the criteria for papillary, medullary, or poorly differentiated thyroid carcinoma, or for other types of cancer |
*two categories in the Bethesda classification (14)
Special considerations in the diagnostic evaluation of retrosternal nodular goiter
An iodine isotope study is preferred for the evaluation of retrosternal goiter because iodine is stored in thyrocytes, rather than just accumulating in them temporarily, as technetium-99m pertechnetate does. This property enables the generation of late images that are largely free of background noise. Iodine-123 is generally used (11). Aside from thyroid scintigraphy, magnetic resonance imaging (MRI) and non-contrast computerized tomography (CT) of the neck and chest are suitable techniques for evaluating the potential retrosternal extension of a large goiter (caution: iodinated contrast medium can induce hyperthyroidism in patients with unrecognized autonomous thyroid function). Airway obstruction by a goiter can be reliably assessed with whole-body plethysmography (4).
The indications for fine-needle biopsy.
Fine-needle biopsy is currently held to be indicated only when autonomous thyroid function has been ruled out and a thyroid nodule is considered suspect for malignancy by ultrasonographic criteria.
The treatment of euthyroid goiter with and without nodules
There are hardly any evidence-based recommendations for the treatment of euthyroid diffuse goiter and nodular goiter. Potential management strategies include watchful waiting for asymptomatic patients, drug treatment, radioactive iodine therapy, and surgery. The choice among them is taken individually for each patient in view of the risks, benefits, and availability of the various techniques, the experience of the treating physicians, and the patient’s wishes. Hardly any comparative studies of the effectiveness and side effects of different treatments have been carried out to date.
Euthyroid diffuse goiter.
Hardly any comparative studies of the effectiveness and side effects of different treatments for euthyroid diffuse goiter have been carried out to date.
Euthyroid diffuse goiter
Iodine deficiency is the main cause of euthyroid diffuse goiter; accordingly, correction of the intrathyroid iodine deficiency is the main objective of treatment. In children and prepubertal adolescents, the use of iodide in a dose of 150–200 µg per day is the treatment of choice (15). Iodide monotherapy may also be an effective treatment for pubertal adolescents and young adults, but little scientific evidence is available on this matter. Instead of monotherapy, adults can be treated with a 2:1 combination of iodide and levothyroxine (e.g., 150 µg of iodide and 75 µg of levothyroxine per day), which generally brings about a reduction of the size of the thyroid gland over a period of 12 to 18 months (4). Once the treatment is over, it is important for the patient to keep on consuming adequate amounts of iodine (Table 4) (15). Outdated treatments include TSH suppression and levothyroxine monotherapy for diffuse goiter. The latter only worsens the intrathyroid iodine deficiency, and the thyroid gland starts to enlarge again as soon as the medication is stopped (4).
Table 4. Daily iodine requirement (WHO recommendation).
| Age | Daily iodine requirement |
| 0 to 5 years | 90 µg |
| 6 to 12 years | 120 µg |
| from age 13 | 150 µg |
| Pregnancy | 250 µg |
| Breastfeeding | 250 µg |
Euthyroid nodular goiter—to treat or not to treat?
Treatment is clearly indicated if there is a suspicion of cancer, or if the patient has mechanical symptoms. Only sparse data are available on the natural course of euthyroid goiter as such (4). According to older studies, euthyroid patients with nodules exhibiting autonomous thyroid function risk developing hyperthyroidism at a rate of roughly 4% per year (e6). A further danger for such patients is decompensation of autonomous function consequent to iodine contamination (radiological contrast media or amiodarone) (16). Sandrock followed a group of euthyroid patients with autonomous function who were exposed to an excessive amount of iodine: 31% developed hyperthyroidism in their further course (e6). Other authors have reported lower rates of hyperthyroidism after cardiac catheterization studies and the like (e7). Autonomous thyroid function is generally an indication for treatment not just in patients with hyperthyroidism (whether subclinical or overt), but also in those who are (still) in a euthyroid metabolic state. The indication also depends on the volume of the autonomously functioning region. Treatment is strongly indicated in multimorbid patients and those who are likely to undergo radiological contrast studies in the future (6, 7). The definitive treatment of autonomously functioning thyroid nodules is with surgery or radioactive iodine therapy (6, 7).
The treatment of euthyroid nodular goiter.
Outdated treatments include TSH suppression and levothyroxine monotherapy for goiter. The latter only worsens the intrathyroid iodine deficiency, and the thyroid gland enlarges again when the medication is stopped.
Drug treatment for nodular goiter
Euthyroid patients with nodular goiter have been treated medically in Germany for many years, yet the state of the evidence from studies performed across the world still leaves room for debate (4, 6, 17, 18, e8). In the prospective German LISA trial, whose results were recently published, 1024 patients with thyroid nodules/goiter were treated for a year with TSH-adapted levothyroxine (LT4), an LT4-iodide combination, iodide alone, or placebo (17). After three months of treatment, the levothyroxine dose was adjusted so that the TSH value came into the target range (0.2–0.8 mU/L). A nodular volume reduction of 21.6% was observed under LT4-iodide combination therapy, compared to a 5.2% reduction with placebo. Combination therapy was superior to both levothyroxine monotherapy (12.6% volume reduction) and iodide monotherapy (9% volume reduction). Over the same period of time, the patients’ thyroid volumes were reduced by 10% under LT4-iodide combination therapy, compared to 1.9% with placebo. This is the first large-scale, placebo-controlled trial to demonstrate volume reduction in nodular goiter with levothyroxine–iodide treatment even without TSH suppression. Some practical questions remain to be addressed, including the long-term course of thyroid nodules after the end of treatment, the potential indication for switching to iodide monotherapy for long-term treatment, and the optimal target range for long-term TSH control to achieve a lasting reduction in nodular volume (17).
The advantages of drug therapy are low cost, non-invasiveness, and no need for hospitalization.
The disadvantages of drug therapy are its unclear mechanism of action, the lack of data on long-term success, iatrogenic hyperthyroidism, diminishing compliance over time, and inadequate effectiveness for large, nodular goiters.
Beware of iodine contamination.
Euthyroid patients with hot nodules are at risk of decompensation of autonomous thyroid function as a consequence of iodine contamination (radiological contrast media or amiodarone).
Radioactive iodine therapy
Radioactive iodine therapy is an effective means of reducing the volume of goiters, even if they are large or very large (100 to 300 mL), by 35% to 40% in one year and by 40% to 60% in two years, with resulting improvement of airway function (18–20). It is thus clearly superior to drug treatment for thyroid volume reduction and is a good alternative to goiter surgery, particularly for patients whose livelihood depends on speech (because there is no risk of recurrent laryngeal nerve palsy) and for multimorbid elderly patients (18– 20). Goiter very rarely recurs after radioactive iodine therapy, and, when it does, after a long delay. If the treatment does not reduce the thyroid volume adequately, it can be repeated without difficulty. One should bear in mind, however, that, while the thyroid volume is usually reduced within three months, the latency to successful treatment can also be longer (18– 20).
The extent of thyroid volume reduction is correlated with
the age of the patient,
the duration of goiter (treatment is more effective in younger patients with fewer regressive changes),
the size of the goiter,
the treatment activity level, and
the homogeneity of iodine storage (20).
The potential long-term complications of radioactive iodine therapy for nodular goiter are hypothyroidism requiring substitution (22% to 58% within 5 to 8 years of treatment) and, in rare cases (<5%), autoimmune thyroid disease. Patients with large nodular goiters should be told before treatment that larger treatment volumes necessitate higher levels of radioactivity and, therefore, a longer hospitalization (20).
The advantages of radioactive iodine therapy are the lack of need for general anesthesia and surgery, the paucity of side effects, the possibility of retreatment if needed, and the even greater efficacy in volume reduction that can be achieved when radioactive iodine therapy is given together with recombinant TSH (19).
Radioactive iodine therapy.
Radioactive iodine therapy is clearly superior to drug treatment for thyroid volume reduction and is a good alternative to goiter surgery, particularly for elderly patients and those whose livelihood depends on speech.
The disadvantages of radioactive iodine therapy are a longer hospitalization than is needed for thyroid surgery, the latency to treatment success, transient swelling, (rarely) painful radiation thyroiditis and hyperthyroidism due to thyrocyte degeneration, the new manifestation of autoimmune hyperthyroidism, the need for regular clinical follow-up for the potential development of hypothyroidism, and the individually variable impairment of quality of life under thyroid hormone replacement therapy.
Patient information for radioactive iodine therapy.
Patients with large nodular goiters should be told before treatment that larger treatment volumes necessitate higher levels of radioactivity and, therefore, a longer hospitalization.
Surgery
Thyroid surgery is obligatory whenever cancer is suspected; it rapidly relieves the mechanical symptoms of benign nodular goiter (6, 21). Other indications include retrosternal or mediastinal extension of a goiter and uni- or multifocal autonomy of thyroid function (in the latter case, surgery is an alternative to radioactive iodine therapy). The serum calcitonin concentration should be measured for the detection of medullary thyroid carcinoma before any operation is performed for nodular goiter (8, 21).
Complications of thyroid surgery.
Permanent postoperative hypoparathyroidism, which leads to considerable mortality
Recurrent laryngeal nerve palsy (transient or permanent)
In 2011, the German Working Group for Endocrine Surgery (Chirurgische Arbeitsgemeinschaft Endokrinologie, CAEK) published its practice recommendations on the indications and techniques of surgery for benign nodular goiter (21). It is recommended that the decisions whether to operate and, if so, how much thyroid tissue to resect (hemi-, subtotal, near-total, or total thyroidectomy) should be allowed to depend on the structural changes that are present in the individual case (ideally, no nodules should be left in place that might lead to recurrent disease) and on the risk of complications (recurrent laryngeal nerve palsy, postoperative hypoparathyroidism) (21). The overall rate of postoperative recurrent laryngeal palsy, according to the current literature, is 2.9% (transient) and 0.7% (permanent); factors affecting the rate of recurrent laryngeal palsy include the surgeon’s skill and experience, the extent of resection, whether the operation is an initial procedure or a reoperation, and the presence or absence of thyroid cancer (21, e9– e14). Permanent postoperative hypoparathyroidism is another complication that leads to considerable morbidity (tetany, hypercalcemic crisis, end-organ damage due to calcium deposition) (21, 22). The reported rates of postoperative hypoparathyroidism are mostly in the range of 0.5% to 6.6%, but much higher in some series; the rates in endocrine surgery centers range from 0.9% to 1.6% (21, 22). This is a further reason why, aside from careful consideration of the possible indication for surgery, the actual performance of the procedure should be in the hands of an experienced surgeon, preferably one specialized in endocrine surgery (21). The CAEK recommends autotransplantation of parathyroid tissue into the cervical musculature if it becomes evident during the procedure that the parathyroid glands have been resected (21).
The advantages of surgery are the rapid elimination of mechanical symptoms and autonomous thyroid function and the provision of tissue for histological examination, so that a diagnosis of cancer can be either confirmed or excluded.
The disadvantages of surgery are the need for hospitalization for an invasive procedure, the risks of recurrent laryngeal nerve palsy and hypoparathyroidism, the variable cosmetic result, and the individually variable impairment of quality of life under thyroid hormone replacement therapy.
Assessment of the available treatment options
Treatment is obligatory only when cancer is suspected, when the patient has symptoms, or when autonomous thyroid function is present. Asymptomatic, benign changes of the thyroid generally require no treatment. Medical therapy with levothyroxine and iodide for one year can reduce the size of (small) nodular goiters, and TSH suppression is not necessary for effective treatment; the long-term results of such treatment, however, are not yet fully clear (in particular, the rate of late progression with mechanical symptoms is unknown, as is the long-term risk of malignancy). Iatrogenic hyperthyroidism, whether subclinical or overt, increases the risk of cardiovascular events and osteoporosis and should be avoided at all costs. This is the main reason why levothyroxine treatment is not recommended for elderly patients (6). If volume reduction is the main goal of treatment for symptomatic, euthyroid nodular goiter, the other treatment options are surgery and radioactive iodine therapy (20). The volume-reducing effect of the latter can be potentiated with the use of recombinant TSH (which has not, however, been approved for this purpose) (19). Surgical resection is mandatory whenever cancer is suspected. Autonomous thyroid function calls for ablative treatment either by radioactive iodine therapy or by surgery, even when the patient is (still) in a euthyroid metabolic state, because of its potential long-term sequelae (overt hyperthyroidism, iodine-induced hyperthyroidism) (6).
Clinical follow-up and further care after treatment
Whenever a diffuse or nodular benign goiter is treated conservatively (i.e., with watchful waiting or with medications), clinical follow-up examinations should ensue every 6 to 18 months, so that the following questions can be answered (5– 7):
Has the volume of the thyroid gland changed since the last follow-up?
Have the previously existing thyroid nodules changed in size or shape, and have any new ones appeared?
Has there been any change in thyroid function, e.g., new hyperthyroidism after a radiological contrast study because of previously unrecognized autonomous thyroid function or new hypothyroidism due to autoimmune thyroiditis in a nodular goiter?
Treatment options.
Iatrogenic hyperthyroidism (subclinical or overt) increases the risk of cardiovascular events and osteoporosis and should be avoided at all costs. This is the main reason why drug treatment is not recommended for elderly patients.
History-taking, physical examination, ultrasonography, and TSH measurement generally constitute an adequate clinical follow-up. Any change in the findings warrants a targeted diagnostic evaluation analogous to the initial evaluation of diffuse or nodular goiter. If drug treatment has been decided upon, the patient’s thyroid function should be rechecked regularly (the first time three months after the start of treatment), as epidemiological studies reveal that many patients taking thyroid medication have TSH values outside the normal range (24, e12).
Further care.
Patients who have undergone radioactive iodine therapy need lifelong clinical follow-up, as they may develop hypothyroidism.
Patients who have undergone radioactive iodine therapy need lifelong clinical follow-up, as they may develop hypothyroidism. The rate of development of hypothyroidism depends on the particular thyroid disease that was treated, the radiation dose that was given, and the functional reserve capacity that the patient’s healthy thyroid tissue had before treatment. Hypothyroidism is rare after the treatment of a single focus of autonomous thyroid function; on the other hand, 30% of patients who undergo radioactive iodine treatment for disseminated functional autonomy will develop hypothyroidism within 10 years (20).
After thyroid surgery, the potential need for thyroid hormone substitution should be assessed. This need is a function of the amount of tissue remaining in place after surgery. Total or near-total thyroidectomy should be immediately followed by the initiation of levothy-roxine substitution in a dose that is proportional to the patient’s body weight (1.6–1.8 µg LT4 / kg BW). As iodine deficiency is the most important avoidable cause of goiter, LT4 substitution can be given in combination with iodide (7). The first TSH check can be performed six weeks after levothyroxine substitution is begun (goal: TSH in euthyroid range). Patients who are under treatment with hormone substitution need lifelong follow-up (annual follow-up if they are on a stable dose). TSH suppression must be strictly avoided, as it elevates the risk of atrial fibrillation and unfavorably affects bone metabolism (24).
If the histological examination has revealed thyroid cancer, then specific further treatment is needed (25). For the timely detection of (usually transient) postoperative hypoparathyroidism, the serum calcium concentration and, if indicated, the parathyroid hormone (PTH) concentration should be measured on the first and second days after surgery, and at any later time should symptoms arise (21, 22). Tetany can be prevented by early substitution with 1,25-dihydroxy-cholecalciferol (active vitamin D3, calcitriol) and calcium. Once the calcium values have stabilized, the treatment can often be tapered off in one to two weeks (22).
Hypoparathyroidism lasting longer than six months meets the definition of permanent hypoparathyroidism (22). It is preferably treated with calcitriol, which has a more rapid onset of action than other vitamin D derivatives, as well as a more rapid offset of action in case of an overdose (ca. 2 to 3 days) (22, 23), and does not need to be activated in the kidneys through the effect of PTH. 0.25 to 1 µg of calcitriol b.i.d. or t.i.d. usually suffices (22, 23). In addition, calcium is given by mouth (500 to 1000 mg t.i.d. of calcium carbonate or calcium citrate), in combination with a proton-pump inhibitor. The dose should not exceed 500 to 1000 mg t.i.d., as higher doses can cause diarrhea. The impaired renal reabsorption of calcium is unaffected by treatment, as it depends on PTH, which is deficient; the resulting hypercalciuria can lead to the formation of kidney stones (22). Thus, substitution with vitamin D preparations or analogues is an unsatisfactory compromise solution in the absence of a physiological replacement for parathyroid hormone. The goals of substitution therapy are the prevention of symptoms of hypocalcemia and the avoidance of clinically relevant hypercalciuria by keeping the serum calcium concentration in or just below the low-normal range (serum albumin–corrected concentration ca. 2.02 to 2.12 mmoL/L). Hypercalciuria can also be lessened by concurrent treatment with hydrochlorothiazide (25 mg q.d.), while hyperphosphatemia can be treated with a modified diet, and the use of phosphate binders can also be considered (22). Patients with postoperative hypoparathyroidism need lifelong follow-up: their calcium and phosphate concentrations should be measured weekly at first, then once every three months, and the serum creatinine concentration and urinary calcium excretion should be checked every six months. Further recommendations for follow-up include renal ultrasonography (to detect kidney stones) and ophthalmological examination for cataracts, both at intervals of one to two years (22).
Follow-up checks for hormone substitution.
Patients who are under treatment with hormone substitution need lifelong follow-up. TSH suppression must be strictly avoided, as it elevates the risk of atrial fibrillation and unfavorably affects bone metabolism.
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article.
See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration.
The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 37/2012.
The CME unit “The Acute Scrotum in Childhood and Adolescence“ (Issue 25/2012) can be accessed until
3 August 2012. For issue 33–34/2012, we plan to offer the topic “Drug interactions: principles, examples and clinical consequences.”
Solutions to the CME questions in Issue 25:
Lorenzl S, et al.: Acute Confusional States in the Elderly—Diagnosis and Treatment.
Solutions: 1d, 2c, 3c, 4b, 5c, 6a, 7d, 8d, 9a, 10e
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What percentage of participants without previously known thyroid disease in the Study of Health in Pomerania were found to have a thyroid nodule?
10.1%
20.2%
30.3%
40.4%
50.5%
Question 2
Which of the following should be measured, along with TSH, as part of the basic diagnostic evaluation of any patient with a euthyroid nodular goiter, so that medullary thyroid cancer will be detected if present?
Calcitonin
Thyroglobulin
Thyroperoxidase
Calcium
Magnesium
Question 3
In Germany, what size of a thyroid nodule is considered the threshold value for the performance of baseline scintigraphy, regardless of the TSH concentration?
a) 2 mm
b) 4 mm
c) 6 mm
d) 8 mm
e) 10 mm
Question 4
According to the current German recommendations, what laboratory tests should be performed in the initial diagnostic evaluation of euthyroid nodular goiter?
Thyroid-stimulating hormone (TSH) and calcitonin
Thyroglobulin and FT4
FT3 and FT4
iodinated FT4 and calcitonin
Thyroperoxidase antibodies and thyroglobulin
Question 5
According to the current German recommendations, when is fine-needle biopsy indicated for the differential-diagnostic evaluation of thyroid nodules?
when a nodule measures less than 8 mm
when a nodule is suspect for cancer by ultrasonographic criteria
for any cold thyroid nodule in a euthyroid patient
for any enlarged nodule
when repeated ultrasonography leads to reclassification of the nodule as no longer suspect
Question 6
A patient with tender enlargement of the thyroid presents for a differential-diagnostic evaluation. Laboratory testing reveals systemic inflammation, the patient is febrile, and his general condition is poor. What is the likely diagnosis?
Diffuse goiter
Nodular goiter
Goiter induced by medication (lithium)
Riedel’s thyroiditis
de Quervain’s thyroiditis
Question 7
Which of the following tests are only recommended if thyroid function is abnormal?
Measurement of creatine kinase
Stimulation with pentagastrin or calcium
Fine-needle biopsy
Measurement of thyroperoxidase antibodies and TSH-receptor antibodies
Measurement of thyroglobulin
Question 8
How sensitive is the presence of multiple suspect ultrasonographic findings for the detection of thyroid cancer?
83–99%
72–88%
61–77%
50–66%
30–50%
Question 9
What test enables the earliest possible detection of focal autonomy in a nodular goiter?
TSH measurement
Calcitonin measurement
Scintigraphy
Doppler ultraonography
Computerized tomography
Question 10
What should be the interval between follow-up visits of a patient treated conservatively for benign diffuse or nodular goiter?
4 weeks
2–4 months
4–5 months
6–18 months
24–36 months
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Führer serves as a paid consultant for AstraZeneca and Pfizer. She has received honoraria and reimbursement of travel expenses from Merck, Sanofi-Aventis, Ipsen, Pfizer, Novartis, Amgen, and AstraZeneca. She has also received payment for carrying out clinical trials on behalf of AstraZeneca, Pfizer, Ipsen, Novartis, Bayer, Lilly, Novo Nordisk, Merck, and Sanofi-Aventis.
Prof. Bockisch has received funding from Bayer, Exelixis, and Eisai for clinical trials on the use of tyrosine kinase inhibitors in thyroid carcinoma. Prof. Schmid states that no conflict of interest exists.
References
- 1.Völzke H, Lüdemann J, Robinson DM, et al. The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid. 2003;13:803–810. doi: 10.1089/105072503768499680. [DOI] [PubMed] [Google Scholar]
- 2.Reiners C, Wegscheider K, Schicha H, et al. Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96 278 unselected employees. Thyroid. 2004;14:926–932. doi: 10.1089/thy.2004.14.926. [DOI] [PubMed] [Google Scholar]
- 3.Krohn K, Fuhrer D, Bayer Y, et al. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev. 2005;26:504–524. doi: 10.1210/er.2004-0005. [DOI] [PubMed] [Google Scholar]
- 4.Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- 5.Führer D, Schmid KW. Benign thyroid nodule or thyroid cancer? Internist. 2010;51:611–619. doi: 10.1007/s00108-009-2500-1. [DOI] [PubMed] [Google Scholar]
- 6.Gharib H, Papini E, Paschke R, et al. Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive Summary of recommendations. AACE/AME/ETA Task Force on Thyroid Nodules. J Endocrinol Invest. 2010;33:287–291. doi: 10.1007/BF03346587. [DOI] [PubMed] [Google Scholar]
- 7.Paschke R, Reiners C, Führer D, Schmid KW, Dralle H, Brabant G. Recommendations and unanswered questions in the diagnosis and treatment of thyroid nodules. Opinion of the Thyroid Section of the German Society for Endocrinology. Dtsch Med Wochenschr. 2005;130:1831–1836. doi: 10.1055/s-2005-871906. [DOI] [PubMed] [Google Scholar]
- 8.Karges W, Dralle H, Raue F, et al. Calcitonin measurement to detect medullary thyroid carcinoma in nodular goiter: German evidence-based consensus recommendation. German Society for Endocrinology (DGE) - Thyroid Section. Exp Clin Endocrinol Diabetes. 2004;112:52–58. doi: 10.1055/s-2004-815727. [DOI] [PubMed] [Google Scholar]
- 9.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 10.Bojunga J, Herrmann E, Meyer G, Weber S, Zeuzem S, Friedrich-Rust M. Real-time elastography for the differentiation of benign and malignant thyroid nodules: a meta-analysis. Thyroid. 2010;20:1145–1150. doi: 10.1089/thy.2010.0079. [DOI] [PubMed] [Google Scholar]
- 11.Dietlein M, Dressler J, Eschner W, Leisner B, Reiners C, Schicha H. Procedure guideline for thyroid scintigraphy (3rd version) Nuklearmedizin. 2007;46:203–205. [PubMed] [Google Scholar]
- 12.Schmid KW. Pathogenese, Klassifikation und Histologie von Schilddrüsenkarzinomen. Onkologe. 2010;16:644–656. [Google Scholar]
- 13.Schmid KW, Reiners C. Wann ist die Feinnadelbiopsie der Schilddrüse am effektivsten? Der Pathologe. 2011;32:169–172. doi: 10.1007/s00292-010-1413-z. [DOI] [PubMed] [Google Scholar]
- 14.Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference: The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 16.Rendl J, Saller B. Schilddrüse und Röntgenkontrastmittel: Pathophysiologie, Häufigkeit und Prophylaxe der jodinduzierten Hyperthyreose. Dtsch Arztebl. 2001;98(7) [Google Scholar]
- 17.Grussendorf M, Reiners C, Paschke R, Wegscheider K. Reduction of thyroid nodule volume by levothyroxine and jodine alone and in combination: A randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2011;96:2786–2795. doi: 10.1210/jc.2011-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesche MF, Tiel-V Buul MM, Lips P, Smits NJ, Wiersinga WM. A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab. 2001;86:998–1005. doi: 10.1210/jcem.86.3.7244. [DOI] [PubMed] [Google Scholar]
- 19.Fast S, Hegedüs L, Grupe P, et al. Recombinant human thyrotropin-stimulated radioiodine therapy of nodular goiter allows major reduction of the radiation burden with retained efficacy. J Clin Endocrinol Metab. 2010;95:3719–3725. doi: 10.1210/jc.2010-0634. [DOI] [PubMed] [Google Scholar]
- 20.Dietlein M, Dressler J, Grünwald F, et al. Guideline for radioiodine therapy for benign thyroid diseases (4thversion) Nuklearmedizin. 2007;46:220–223. [PubMed] [Google Scholar]
- 21.Musholt TJ, Clerici T, Dralle H, et al. German Association of Endocrine Surgeons practice guidelines for the surgical treatment of benign thyroid disease. Interdisciplinary task force guidelines of the German Association of Endocrine Surgeons. Langenbecks Arch Surg. 2011;396:639–649. doi: 10.1007/s00423-011-0774-y. [DOI] [PubMed] [Google Scholar]
- 22.Shoback D. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 23.Schäffler A. Hormone replacement after thyroid and parathyroid surgery. Dtsch Arztebl Int. 2010;107:827–834. doi: 10.3238/arztebl.2010.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 25.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- e1.Völzke H, Ittermann T, Albers M, Friedrich N, Nauck M, Below H. Five-year change in morphological and functional alterations of the thyroid gland—the study of health in Pomerania. Thyroid. 2012 doi: 10.1089/thy.2011.0525. Apr 18 (epub) [DOI] [PubMed] [Google Scholar]
- e2.Krohn K, Maier J, Paschke R. Mechanisms of disease: Hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 2007;3:713–720. doi: 10.1038/ncpendmet0621. [DOI] [PubMed] [Google Scholar]
- e3.Kratzsch J, Petzold A, Raue F, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin Chem. 2011;57:467–474. doi: 10.1373/clinchem.2010.151688. Epub 2010 Dec 15. [DOI] [PubMed] [Google Scholar]
- e4.Hegedüs L. Can elastography stretch our understanding of thyroid histomorphology? J Clin Endocrinol Metab. 2010;95:5213–5215. doi: 10.1210/jc.2010-2411. [DOI] [PubMed] [Google Scholar]
- e5.Hegedüs L, Bonnema SJ. Approach to management of the patient with primary or secondary intrathoracic goiter. J Clin Endocrinol Metab. 2010;95:5155–5162. doi: 10.1210/jc.2010-1638. [DOI] [PubMed] [Google Scholar]
- e6.Sandrock D, Olbricht T, Emrich D, Benker G, Reinwein D. Long-term follow-up in patients with autonomous thyroid adenoma. Acta Endocrinol. 1993;128:51–55. doi: 10.1530/acta.0.1280051. [DOI] [PubMed] [Google Scholar]
- e7.Nolte W, Muller R, Siggelkow H, Emrich D, Hufner M. Prophylactic application of thyrostatic drugs during excessive iodine exposure in euthyroid patients with thyroid autonomy: a randomized study. Eur J Endocrinol. 1996;134:337–341. doi: 10.1530/eje.0.1340337. [DOI] [PubMed] [Google Scholar]
- e8.Papini E, Petrucci L, Guglielmi R, et al. Long-term changes in nodular goiter: a 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab. 1998;83:780–783. doi: 10.1210/jcem.83.3.4615. [DOI] [PubMed] [Google Scholar]
- e9.Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery. 2003;133:180–185. doi: 10.1067/msy.2003.61. [DOI] [PubMed] [Google Scholar]
- e10.Randolph GW, Dralle H, et al. International Intraoperative Monitoring Study Group. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121(Suppl 1):1–16. doi: 10.1002/lary.21119. [DOI] [PubMed] [Google Scholar]
- e11.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]