Abstract
Background: Some intervention studies have suggested that dairy products may influence body weight, but the results remain controversial.
Objective: We identified and quantified the effects of dairy consumption on body weight and fat mass from randomized controlled trials (RCTs).
Design: We conducted a comprehensive search of PubMed and EMBASE databases (to April 2012) of English reports of RCTs regarding dairy consumption on body weight, body fat, or body weight and body fat in adults. The results across studies were pooled by using a random-effects meta-analysis.
Results: Twenty-nine RCTs were included with a total of 2101 participants. Overall, consumption of dairy products did not result in a significant reduction in weight (−0.14 kg; 95% CI: −0.66, 0.38 kg; I2 = 86.3%). In subgroup analysis, consumption of dairy products reduced body weight in the context of energy restriction or short-term intervention (<1 y) trials but had the opposite effect in ad libitum dietary interventions or long-term trials (≥1 y). Twenty-two RCTs that reported results on body fat showed a modest reduction in the dairy group (−0.45 kg; 95% CI: −0.79, −0.11 kg; I2 = 70.9%), and further stratified analysis indicated significant beneficial effects of dairy intervention on body fat in energy-restricted or short-term trials but not in long-term or ad libitum studies.
Conclusions: This meta-analysis does not support the beneficial effect of increasing dairy consumption on body weight and fat loss in long-term studies or studies without energy restriction. However, dairy products may have modest benefits in facilitating weight loss in short-term or energy-restricted RCTs.
See corresponding editorial on page 687.
INTRODUCTION
The prevalence of overweight and obesity has increased dramatically in the United States and around the world (1, 2). Thus, weight control is a national and global priority. It has been postulated that the consumption of dairy products may facilitate body weight and fat loss (3) because dairy products contain calcium, protein (casein and whey), and other bioactive compounds that may favorably affect energy balance.
Many randomized controlled trials (RCTs) have been conducted to determine the effect of dairy foods on weight loss and body composition. However, results have been inconsistent (4–9). Discrepancies in results might be due to small sample sizes, insufficient study durations, differences in study design, and the diversity of study populations. Thus, a meta-analysis is needed to increase the statistical power and enhance the precision of estimates across multiple modest-sized trials. Recently, a meta-analysis (10) on this topic was published, but a number of eligible studies were not included, and the result from one trial was repeatedly used. These methodologic issues may have led to biased results, and incomplete study selection may have impaired the statistical power to detect influential factors on the pooled estimates, such as study duration. Therefore, to achieve a more precise estimation of effects across trials, we performed a systematic review and meta-analysis on RCTs to evaluate whether increasing the consumption of dairy products could promote weight loss.
METHODS
Data sources and searches
This meta-analysis was conducted after a review protocol (11). We searched PubMed (http://ww.nlm.nih.gov/pubs/factsheets/pubmed.html) and EMBASE (http://www.embase.com) databases for clinical trials published from January 1966 to April 2012 that described the effects of dairy products on body weight and composition in adults. We specified 2 comprehensive search themes. The first theme identified relevant terms for dairy by combining exploded versions of the Medical Subject Headings terms dairy products, dairy, milk, calcium, cheese, and yogurt and corresponding key words in titles and abstracts. The second theme identified terms related to body weight by combining weight loss, weight reduction, weight change, body fat, or adiposity and corresponding key words in titles and abstracts. The third theme identified terms related to randomized controlled trials by combining the Medical Subject Headings term intervention studies and corresponding key words in titles and abstracts. Additional articles were identified from the reference lists of included studies and relevant reviews. See “Supplemental data” in the online issue for a detailed search strategy.
Study selection
We included RCTs with either a parallel or a crossover design that were conducted in adults aged ≥18 y. Dairy products should have been used as the main intervention but not as part of a multicomponent dietary supplement in either experimental or control groups, the intervention in control groups was not soy milk, and participants had to consume dairy products for ≥4 wk. Furthermore, data on changes of body weight or fat mass in both experimental and control groups were extracted from the report or obtained from the authors. We included English-language articles only.
Data extraction and quality assessment
We extracted the following information from each study: authors, publication year, geographic location, funding sources, sample size and attrition, intervention and control regimens, study duration, study design (crossover or parallel), analysis strategy (intent-to-treat or per-protocol analysis), and participant information (age, sex, and baseline intake). We used the Jadad score to assess study quality (12). Trials scored one point for each item addressed in the study design, including random assignment, blinding, description of withdrawals and dropouts, methods of random assignment, and double-blinding status, which generated a scale from 0 to 5. Higher numbers represented the better quality of a given study. Because double blinding was almost not possible in this type of trial, studies with a Jadad score ≥3 were defined as high quality, and the rest of the studies were classified as low quality.
Most of the RCTs showed the intervention dose in servings per day. In 6 studies (13–18) in which dairy interventions were not shown in servings per day, we standardized units to servings per day, which was equivalent to 240 mL or 8 oz in volume or 452 mg Ca or 8.4 g protein in nutrient content per day according to the USDA national nutrient database for standard reference (19). In 2 studies (17, 20), participants were randomly assigned to a control group or medium- or high-dairy groups; thus, we combined medium- and high-dairy groups as the intervention arm with relevant data provided by the authors.
We extracted the means and SDs of changes from baseline to endpoint (both intervention and control arms) from all studies. SDs were calculated from SEs or CIs when necessary; for articles with missing SDs for measurements of change (4, 13, 16), change-from-baseline SDs were imputed by using the correlation coefficient method referenced in the Cochrane Handbook for Systematic Reviews of Interventions (21). We used the average correlation coefficient of 0.96 between baseline and endpoint measurements, which was estimated from all other included studies with available data. A sensitivity analysis indicated that results were not substantially altered by removing the 3 studies with imputed values.
Meta-analysis and statistical analysis
The estimate of the principal effect was defined as the mean difference (net change in kilograms) in body weight and body fat mass between participants assigned to dairy products and participants assigned to control regimens. Cochran's Q test was conducted to test the statistical heterogeneity of treatment effects between studies (P < 0.1). We also examined the I2 statistic, and I2 > 50% was considered to indicate a significant heterogeneity across trials (22). Results were presented by using the random-effects model because high heterogeneities were shown in most cases, and we repeated the analysis by fixed-effect models if there was a low heterogeneity (I2 < 50%) (21). Potential publication bias was examined by using funnel plots in which SEMs of the studies were plotted against their corresponding effect sizes (21). Meta-regression was implemented to examine characteristics of studies that were hypothesized to influence observed treatment effects (23), including energy restriction (yes or no), intervention dose (<3 or ≥3 servings/d), study duration (<1 or ≥1 y), baseline BMI (in kg/m2; <25 or ≥25), funding sources, study quality (high or low), and sex (men, women, or both). Sensitivity analyses were also conducted to evaluate the impact of individual studies on overall pooled estimates and heterogeneity. The meta-analysis was performed with STATA software (version 11.0; StataCorp).
RESULTS
Results of literature search
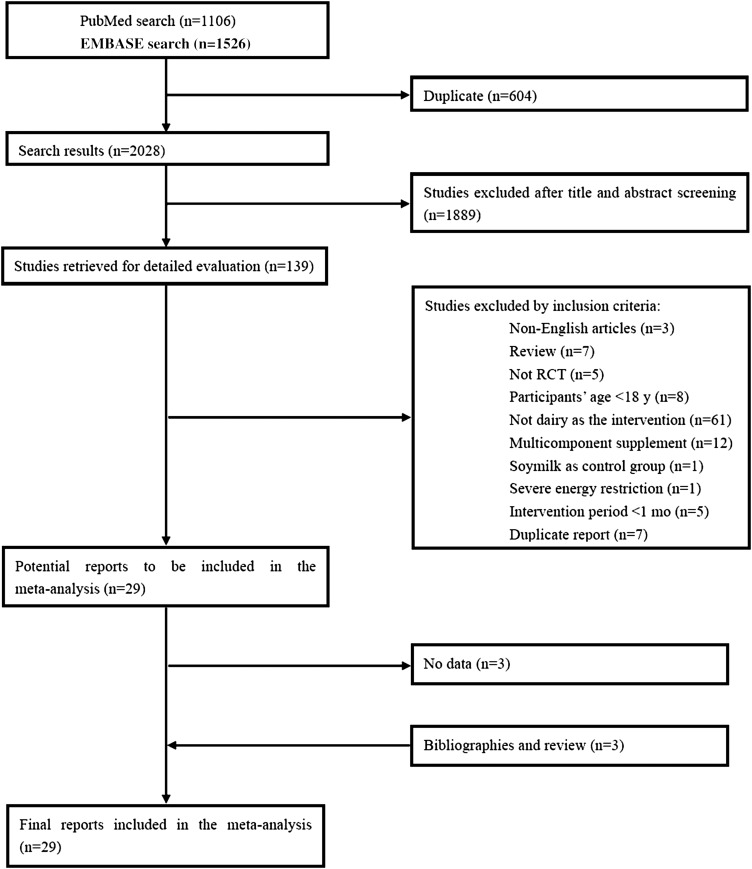
The literature search yielded 2028 citations (1106 from PubMed and 1526 from EMBASE with 604 duplicate records); among the citations, 139 articles remained for detailed full-text evaluation after title and abstract screening. We excluded 110 studies on the basis of our study selection criteria, and details of the study flow are depicted in Figure 1. One study (24) was excluded because participants were on a severe energy-deficit diet by reducing 7 MJ/d (1673 kcal/d) from their preintervention energy amounts. Finally, 29 citations met the inclusion criteria and were included in the meta-analysis.
FIGURE 1.
Summary of evidence search and selection. Searched databases included PubMed (http://ww.nlm.nih.gov/pubs/factsheets/pubmed.html) and EMBASE (http://www.embase.com). RCT, randomized controlled trial.
Characteristics of studies
Primary characteristics of the 29 included trials are shown in Table 1. A total of 2441 participants were randomly assigned to intervention or control regimens in these trials with a completion rate of 84.4% (n = 2060). The sample size varied from 20 to 265 participants with a mean age range from 20.0 to 62.0 y (median: 41.4 y). The mean baseline BMI ranged from 20.2 to 43.0, and 24 trials enrolled overweight or obese participants. Of the 29 trials, 14 trials were conducted exclusively in women (5 trials conducted in postmenopausal women, 8 trials conducted in premenopausal women, and 1 trial that did not mention menopausal status), 1 trial was conducted in men, and the other 14 trials were conducted in both sexes, with 2 trials that reported results separately by sex (4, 14).
TABLE 1.
Characteristics of included studies1
| First author, year of publication (reference) | Enrollment (completers) | Intervention |
Baseline intake | Intervention dose4 | Jadad score | |||||||
| Age2 | Men | BMI3 | Control group | Dairy group | Duration | Design | Energy control | |||||
| n | y | % | kg.m2 | Servings/d | Servings/d | mo | kcal/d | |||||
| Baran, 1990 (13) | 59 (37) | 30–42 | 0 | NA | Usual diet | Dairy supplement of 500–600 mg Ca/d | 1.22 | 1.22 | 36 | 3 | RP | No |
| Barr, 2000 (3) | 205 (200) | 55–85 | 35.5 | 25.9 | Usual diet | Three 8-oz servings of skim or 1% fluid milk to the usual diet5 | <1.5 | 2.25 | 3 | 3 | RP | No |
| Bowen, 2005 (14) | 69 (50) | 20–65 | 40 | 35.0 | Mixed diet containing 500 mg Ca/d | High-dairy diet containing 2400 mg Ca/d | NA | 4.2 | 4 | 3 | RP | First 12 wk ER + 4 wk EB |
| Buchowski, 2010 (15) | 40 (34) | 21–50 | 29.4 | 32.0 | Nondairy Ca: 500 mg/1500mg | Dairy Ca: 500 mg/1500 mg | NA | 2.21 | 3 | 4 | RC | 100–200 ER |
| Chee, 2003 (30) | 200 (173) | 50–65 | 0 | 24.0 | Habitual diet | Extra 50 g high-calcium skimmed-milk powder with 400 mL H2O | <2 | 1.67 | 24 | 3 | RP | No |
| Faghih, 2011 (25) | 50 (42) | 20–50 | 0 | 30.4 | Restricted diet | 3 servings (220 mL) low-fat milk (1.5%) | NA | 3 | 2 | 2 | RP | 500 ER |
| Ghadirian, 1995 (16) | 265 (158) | 50–90 | 0 | NA | Dairy-free diet | Dairy food diet providing 30 g protein | ≤1.72 | 3.57 | 1 | 2 | RP | No |
| Gilbert, 2011 (26) | 41 (25) | 25–50 | 0 | 33.0 | 1000 mg/d Ca and energy-equivalent placebo | Milk supplement (1% fat) | ≤1.77 | 2.37 | 6 | 4 | RP | 600 ER |
| Gunther, 2005a (17) | 155 (135) | 18–30 | 0 | 22.5 | Habitual diet | Medium dairy containing 1050 mg Ca/d or high dairy containing 1350 mg Ca/d | ≤1.77 | 2.65 | 12 | 3 | RP | No |
| Gunther, 2005b (18) | 26 (19) | 18–26 | 0 | 22.0 | Habitual diet | Nonfat or low-fat milk supplement containing 1000–1400 mg/d | ≤1.77 | 2.65 | 12 | 2 | RP | No |
| Harvey-Berino, 2005 (33) | 53 (44) | 18–60 | 7.5 | 30.0 | <2 dairy servings/d | 3–4 dairy servings/d | <1 | 2.5 | 12 | 4 | RP | 500 ER |
| Josse, 2011 (20) | 55 (46) | 19–45 | 0 | 32.0 | 0–1 serving/d | 3–4 and 6–7 servings dairy products/d | low | 5.24 | 4 | 3 | RP | 500 ER |
| Kukuljan, 2009 (27) | 180 (175) | 50–79 | 100 | 27.5 | Habitual diet | 400 mL reduced-fat (∼1%) milk | NA | 1.67 | 12 | 3 | RP | No |
| Lau, 2001 (31) | 200 (185) | 55–59 | 0 | 24.0 | Habitual diet | Extra 50 g high-calcium, low-fat, low-lactose milk powder with 400 mL H2O (1200 mg Ca) | NA | 1.67 | 24 | 2 | RP | No |
| Manios, 2009 (5) | 82 (75) | 55–65 | 0 | 30.5 | Habitual diet | 3 portions of low-fat dairy products, milk, and yogurt | NA | 3 | 12 | 2 | RP | No |
| Palacios, 2011 (6) | 20 (16) | 22–50 | 18.8 | 38.3 | Habitual diet | 4 daily servings of dairy products | 1.55 | 4 | 5.25 | 4 | RP | No |
| Rosado, 2011 (29) | 93 (69) | 25–45 | 0 | 34.8 | An energy-restricted diet with no intake of milk | Additional 3 servings of low-fat milk | <3 | 3 | 4 | 3 | RP | 500 ER |
| Stancliffe, 2011 (36) | 40 (40) | 22–52 | 47.5 | 36.7 | <0.5 dairy serving providing 600 mg Ca/d | >3.5 dairy servings containing 1200 mg Ca/d | NA | 3 | 3 | 3 | RP | 0 |
| Thomas, 2010 (40) | 35 (29) | 29–45 | 0 | 29.1 | Low-calcium diet 3 d/wk | High-dairy-based calcium diet (≥1200 mg/d), 3 d/wk | ≤1 | 3 | 4 | 3 | RP | 250 ER |
| Thomas, 2011 (32) | 35 (29) | 29–45 | 0 | 29.1 | 6-oz isoenergetic sucrose beverage 3 d/wk5 | 6-oz serving of fat-free yogurt supplement 3 d/wk5 | NA | 0.64 | 4 | 2 | RP | 249 ER |
| Thompson, 2005 (34) | 59 (48) | 25–70 | 13.3 | 35.0 | <2 dairy servings/d | 4 dairy servings (≥2 fluid milk)/d | NA | 3 | 12 | 2 | RP | 500 ER |
| Van Loan, 2011 (37) | 78 (71) | 20–50 | NR | 32.5 | <1 dairy serving providing 460 mg Ca/d | >4 dairy servings containing 1339 mg Ca/d | ≤1 | 3 | 3 | 3 | RP | 500 ER |
| van Meijl, 2010 (39) | 40 (35) | 18–70 | 28.6 | 32.0 | 600 mL fruit juice and 43 g fruit biscuits/d | 500 mL low-fat (1.5%, wt/wt) milk and 150 g low-fat (1.5%, wt/wt) yogurt | <2 | 2.67 | 1 | 1 | RC | No |
| Wagner, 2007 (28) | NA (30) | 19–53 | 0 | 33.0 | Placebo capsules | Extra dairy servings of 1% milk containing 800 mg Ca/d | NA | 2.76 | 3 | 3 | RP | 500 ER exercise 3 times/wk |
| Wennersberg, 2009 (38) | 121 (113) | 30–65 | 32.7 | 30.0 | Habitual diet | 3–5 dairy servings | NA | 4 | 6 | 2 | RP | No |
| Zemel, 2004 (9) | 28 (21) | 18–60 | 15.6 | 34.9 | <1 dairy serving containing 500 mg Ca/d and a daily placebo supplement | 3 dairy servings containing 1200 mg Ca/d, with placebo | 1.2 | 2.5 | 6 | 3 | RP | 500 ER |
| Zemel, 2005a (35)6 | 39 (34) | 26–55 | 32.4 | 34.5 | <1 dairy serving containing 500 mg Ca/d | 3 dairy servings containing 1200 mg Ca/d, at least 1 fluid milk | NA | 2.5 | 6 | 3 | RP | No |
| Zemel, 2005a (35)6 | 36 (29) | 26–55 | 13.8 | 35.5 | <1 low-fat dairy serving containing 500 mg Ca/d | 3 dairy servings containing 1200 mg Ca/d, at least 1 fluid milk | NA | 2.5 | 6 | 3 | RP | 500 ER |
| Zemel, 2005b (7) | 38 (34) | 18–50 | 20.6 | 33.5 | <1 dairy serving providing 500 mg Ca/d | Three 6 oz fat-free yogurt, 1100 mg Ca/d5 | 1.2 | 2.5 | 3 | 3 | RP | 500 ER |
| Zemel, 2009 (8) | 70 (64) | 18–35 | 22.8 | 29.1 | <1 dairy serving providing 500 mg Ca/d | 3 dairy servings containing 1200 mg Ca/d | 1.33 | 2.5 | 3 | 1 | RP | 500 ER |
EB, energy balance; ER, energy restriction; NA, not applicable; RC, randomized crossover design; RP, randomized parallel design.
All values are ranges.
All values are means of baseline BMI.
Difference of total dairy intake between dairy intervention and control groups.
Eight ounces = 236.6 mL.
Zemel, 2005a (35) is listed twice because one subtrial was energy restricted and the other subtrial was for weight maintenance.
Intervention regimens were diverse. Low-fat fluid milk was evaluated in 7 trials (4, 17, 25–29); skimmed-milk powder was used in 2 trials (30, 31); yogurt was used in 2 trials (7, 32); yogurt, cheese, and milk were freely chosen as the intervention in 11 trials (6, 8, 9, 14, 17, 33–38); a yogurt and milk combination was used in 2 trials (5, 24, 39); and the remaining 5 studies (13, 15, 16, 20, 40) did not specify the intervention regimen. The intervention dose (total dairy intake) varied from 1 to 6.5 servings/d. A habitual diet or a calorie-restricted diet with lower dairy consumption was chosen as the control regimen in most studies. An isocaloric sucrose beverage or fruit juice was provided to participants in control groups in 2 studies (32, 39).
The trials lasted from 1 to 24 mo, with a median duration of 4 mo. Most of the trials used parallel study designs, and 2 trials (15, 39) used crossover designs. The quality of selected trials was diverse, with 19 studies classified as high quality (Jadad score ≥3) and 10 studies classified as low quality.
In 17 weight-loss trials, participants were instructed to reduce their daily energy intakes to a certain amount below their energy requirements. Caloric requirements were estimated individually on the basis of resting metabolic rates and physical activity levels, and energy deficits from 100 to 600 kcal/d were subtracted from estimated caloric requirements in 16 of 17 studies. The remaining one study (14) administrated isocaloric low-energy diet plans for all participants regardless of their individual energy requirements. Participants were instructed to keep physical activity levels constant in the majority of trials except in 2 studies, one of which (27) had a 2-by-2 factorial design that included an exercise component, and in the other study (28), milk was given in addition to exercise, whereas participants in the control arm received only an exercise intervention. Most studies were conducted in the United States (n = 17), and other studies were conducted in Europe (n = 2), Canada (n = 3), Australia (n = 2), Mexico (n = 1), Asia (n = 3), and Puerto Rico (n = 1).
Changes in weight and body fat
In 5 studies (26, 28, 29, 33, 34), data were analyzed by using the intention-to-treat principle, whereas the other studies analyzed data in completers only. For this reason, a total of 2101 participants in 29 studies were included in the analysis of weight change, and 1536 participants in 22 studies were included in the analysis of changes in body fat.
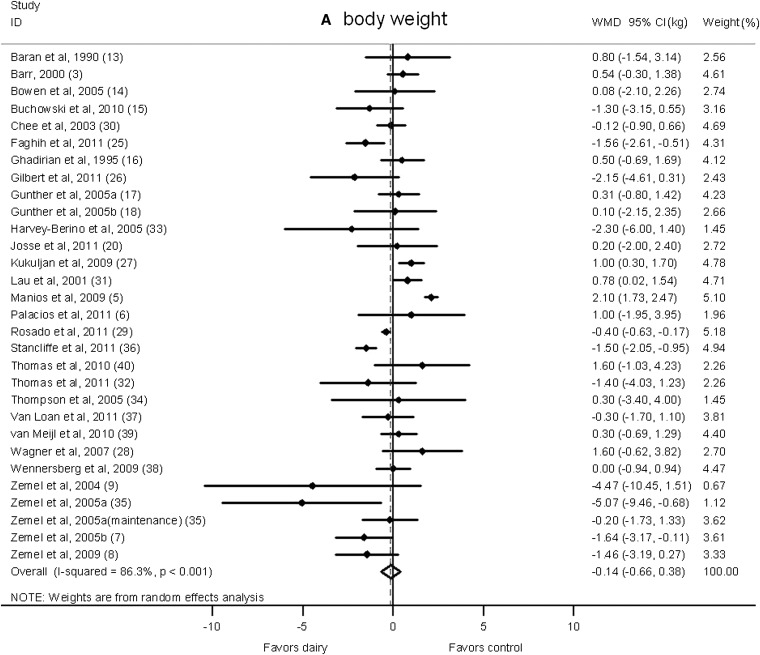
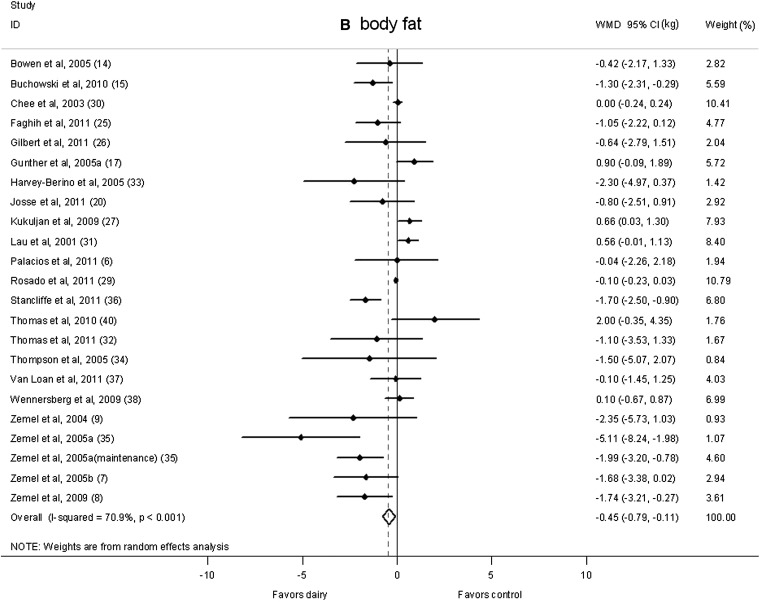
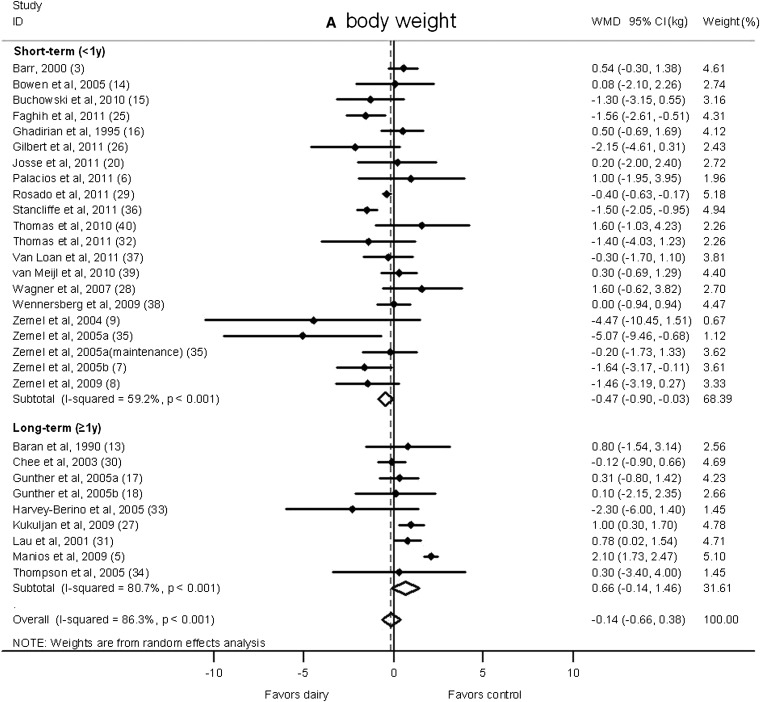
There was no significant difference in body weight changes between the dairy intervention and control groups (−0.14 kg; 95% CI: −0.66, 0.38 kg; Figure 2A); however, a significant reduction that favored dairy products in body fat was shown (−0.45 kg; 95% CI: −0.79, −0.11 kg; Figure 2B). A significant heterogeneity was observed for both body weight (I2 = 86.3%) and body fat (I2 = 70.9%).
FIGURE 2.
Net change (95% CI) in body weight (A) and fat (B) associated with dairy interventions expressed as the change (kg) during the intervention with dairy products minus the change during control regimen. Horizontal lines denote the 95% CIs; solid diamonds represent the point estimate of each study. The open diamond represents the pooled estimate of the intervention effect. The dashed line denotes the point estimate of the pooled result. ID, identification; WMD, weighted mean difference.
Subgroup analysis
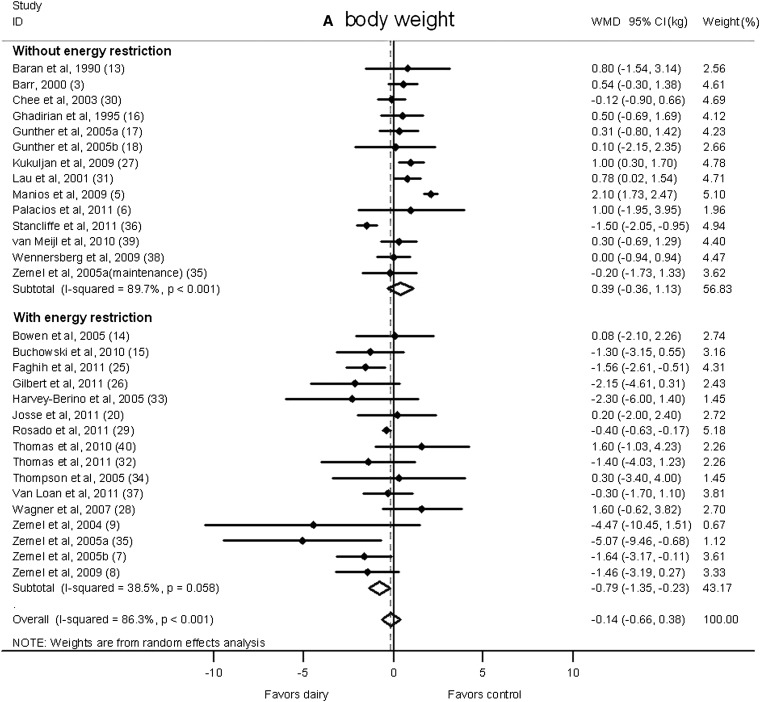
Meta-regression analysis showed that energy restriction (P = 0.008) and study duration (P = 0.008) significantly influenced pooled estimates. Therefore, we conducted subgroup analyses according to these variables.
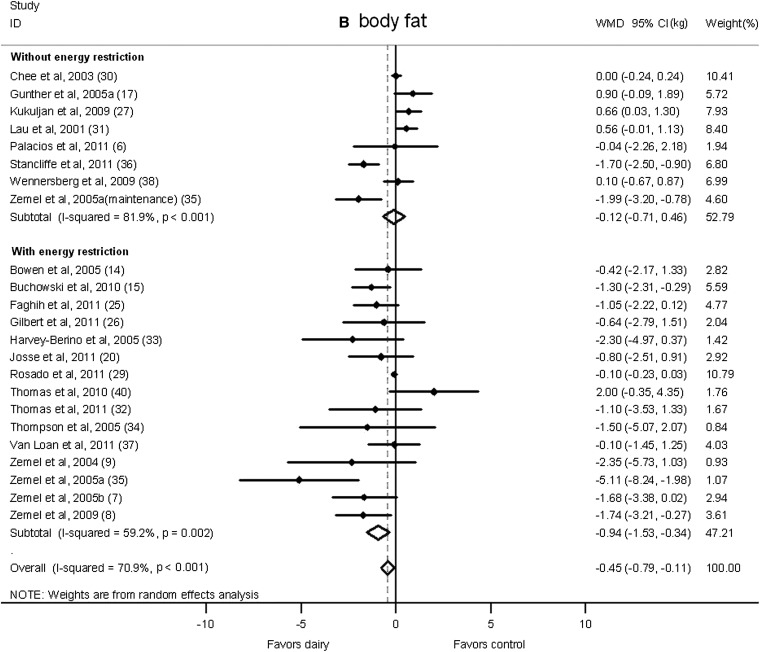
A significant reduction in body weight in the dairy group was shown in studies that imposed energy restriction (−0.79 kg; 95% CI: −1.35, −0.23 kg; I2 = 38.5%; Figure 3A). On the contrary, in studies without energy restriction, no significant effect of dairy intervention was observed (0.39 kg; 95% CI: −0.36, 1.13 kg; I2 = 89.7%). Similarly, body fat declined significantly in energy-restricted trials (−0.94 kg; 95% CI: −1.53, −0.34 kg; I2 = 59.2%; Figure 3B) but not in studies without energy restriction (−0.12 kg; 95% CI: −0.71, 0.46 kg; I2 = 81.9%).
FIGURE 3.
Net change (95% CI) by energy restriction in body weight (A) and fat (B) associated with dairy interventions expressed as the change (kg) during the intervention with dairy products minus the change during the control regimen. Horizontal lines denote the 95% CIs; solid diamonds represent the point estimate of each study. The open diamond represents the pooled estimate of the intervention effect. The dashed line denotes the point estimate of the pooled result. ID, identification; WMD, weighted mean difference.
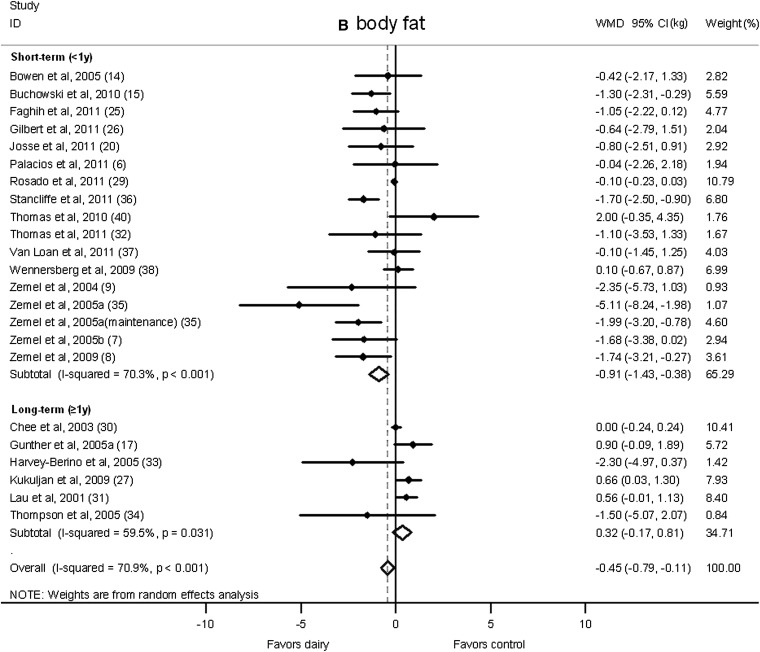
We also classified studies according to duration as short term (<1 y duration) or long term (≥1 y duration; Figure 4). Dairy products significantly reduced body weight in the short-term interventions (−0.47 kg; 95% CI: −0.90, −0.03 kg; I2 = 59.2%) but moderately increased weight gain in long-term interventions (0.66 kg; 95% CI: −0.14, 1.46 kg; I2 = 80.7%). Furthermore, a significant reduction in body fat in the dairy group was shown in short-term studies (−0.91 kg; 95% CI: −1.43, −0.38 kg; I2 = 70.3%), whereas no significant change was observed in long-term trials (0.32 kg; 95% CI: −0.17, 0.81 kg; I2 = 59.5%).
FIGURE 4.
Net change (95% CI) by study duration (short-term: <1 y; long-term: ≥1 y) in body weight (A) and fat (B) associated with dairy interventions expressed as the change (kg) during the intervention with dairy products minus the change during control regimens. Horizontal lines denote the 95% CIs; solid diamonds represent the point estimate of each study. The open diamond represents the pooled estimate of the intervention effect. The dashed line denotes the point estimate of the pooled result. ID, identification; WMD, weighted mean difference.
Stratified analysis by both energy restriction and duration indicated that dairy products significantly facilitated weight loss in short-term trials with energy restriction, whereas a marginally significant weight gain was observed in long-term trials without energy restriction. For body fat, a significant reduction was shown in short-term studies with energy restriction, whereas a marginally significant weight gain was observed in long-term trials without energy restriction. No significant difference was observed in weight or body fat change between dairy and control groups in short-term RCTs without energy restriction or long-term energy-restricted RCTs. See Figure 1 under “Supplemental data” in the online issue for forest plots.
Sensitivity analysis
We conducted several sensitivity analyses by excluding 1 RCT that provided isoenergetic low-energy diet plans (14), 2 RCTs (27, 28) that included a physical activity–intervention component, 3 RCTs (3, 13, 16) for which change-from-baseline SDs were imputed, 3 RCTs of <3-mo duration (16, 25, 39), and 10 RCTs for which the Jadad score was <3, respectively. For all analyses, results were not substantially changed (data not shown).
We also carried out sensitivity analyses to explore heterogeneity. After excluding the study of Stancliffe et al (36) from short-term trials without energy restriction, the heterogeneity in that subgroup decreased dramatically (I2 = 78.4–0.0%), and a similar impact of the study of Manios et al (5) was observed on the heterogeneity of long-term studies without energy restriction (I2 = 63.4–3.0%).
Publication bias
On the basis of a funnel plot (see Figure 2 under “Supplemental data” in the online issue) and Begg's test, no significant publication bias was shown in the meta-analysis of body weight (P = 0.44) or body fat (P = 0.19) changes.
DISCUSSION
This meta-analysis does not support the beneficial effect of increasing dairy consumption on body weight and fat loss in long-term studies (≥1 y) or studies without energy restriction. However, dairy products may have modest benefits in facilitating weight loss in short-term or energy-restricted RCTs.
The dairy–weight loss hypothesis has also been investigated in a number of prospective epidemiologic studies. In a review (41) of 9 prospective cohort studies on dairy consumption and overweight and obesity in adults, the authors concluded that these studies provide evidence of a suggestive but not consistent protective effect of dairy consumption on risk of overweight and obesity. It should be noted that prospective cohort studies can be susceptible to residual or unmeasured confounding by individual dietary components. Specifically, milk consumption has often been associated with a better overall dietary profile (42) and inversely associated with the consumption of sugar-sweetened beverages, especially soda and fruit juice. A recent study (43) in 120,877 US adults from 3 large cohorts showed a null association between changes in consumption of most dairy foods and long-term weight gains, whereas the increase of yogurt intakes was inversely associated with weight gains. Among studies included in our meta-analysis, only 2 trials used yogurt as the intervention; one 3-mo RCT in 34 participants indicated a significant benefit of 3 servings fat-free yogurt/d on weight loss (body weight: −0.71 kg; 95% CI: −1.41, −0.01 kg; body fat: −0.68 kg; 95% CI: −1.37, 0.01 kg) (7), and the other 2-mo RCT in 29 individuals reported a nonsignificant weight reduction of 3 servings fat-free yogurt/wk (body weight: −0.38 kg; 95% CI: −1.12, 0.35 kg; body fat: −0.32 kg; 95% CI: −1.06, 0.41 kg) (32). Long-term, high-quality RCTs are warranted to further assess the effect of yogurt consumption on weight loss.
A meta-analysis (10) of RCTs on dairy intervention and weight loss was recently published, but it included only 14 RCTs with 883 participants compared with 29 RCTs with 2101 participants in our analysis. We have enrolled all RCTs included in the previous meta-analysis except one study (44), which was a substudy of another RCT (8), and the 2 publications were repeatedly used in the previous meta-analysis; in addition, we were able to obtain additional data from investigators for 2 studies (6, 20) in which imputed data had been used in the previous meta-analysis. Our literature search yielded 16 more RCTs that were not included in the previous meta-analysis, and these RCTs were deemed eligible for the meta-analysis on the basis of inclusion criteria. Therefore, our meta-analysis provides a more comprehensive view of the current literature. Because of the difference in the total number of included studies, the study weight from each study was significantly changed. In particular, study weights of several RCTs (7–9, 34–36) from Zemel's group were much lower in our meta-analysis than in the previous meta-analysis. Therefore, our results were stable and less influenced by individual studies. Overall, our analysis showed somewhat different results for weight change (−0.14 kg; 95% CI: −0.66, 0.38 kg) than the previous meta-analysis showed (−0.61 kg; 95% CI: −1.29, 0.07 kg). In addition, the larger number of studies included in our meta-analysis enabled us to identify a significant heterogeneity according to trial duration in addition to energy restriction that was reported in the previous meta-analysis. Although hypocaloric interventions and short-term trials tended to induce favorable effects of dairy on weight loss, ad libitum interventions and long-term trials showed less weight loss in dairy-intervention than in control groups. A long-term and sustained weight loss is of greater public health and clinical significance than a short-term weight loss is, and the results of this meta-analysis do not support increasing dairy consumption as an effective way for long-term weight control.
The reasons for the heterogeneity of effects in different subgroups are not entirely clear. One possible reason might have been either differential compliance with the intervention protocol between short and long trials or its relation with energy restriction. Unfortunately, most studies did not provide adequate data on compliance. In addition, an extra dairy intake in ad libitum dietary intervention might have led to an increased energy intake, which would have resulted in weight gains or have offset the potential protective effect of the dairy intervention. For example, in the study of Barr (3), the total energy intake from two 3-d dietary records showed that participants assigned to the dairy intervention group had ∼100-kcal/d higher calorie intakes during the study period than at baseline, whereas no substantial change in the mean energy intake was observed in individuals in the control arm. In contrast, caloric requirements were estimated individually in most energy-restricted trials on the basis of resting metabolic rates and physical activity levels, and thus, energy intakes were better controlled. Therefore, the potential benefits of dairy on body weight and body fat in energy-restriction interventions could be interpreted as the effects of the substitution of dairy products for certain other foods.
There are several postulated mechanisms for the effect of dairy products on body weight and fat. An increased calcium intake may be beneficial for weight loss because a high calcium intake can reduce lipogenesis and stimulate lipolysis, probably via the suppressing formation of 1,25-dihydroxyvitamin D and secretion of parathyroid hormone or calciotropic hormones (45). Also, calcium has been shown to combine with fatty acids in the intestine to form insoluble soaps, which leads to reduced absorption of fat (46). Several RCTs have been conducted to investigate the effects of calcium supplementation on body weight, and a recent meta-analysis (47) of 7 calcium supplementation trials showed a small but significant reduction in body weight that favored calcium over a placebo (mean difference: −0.74 kg; 95% CI: −1.00, −0.48 kg). However, the largest study (48) included in the meta-analysis did not find a significant effect of 2-y intervention using 1500-mg Ca supplements/d on body weight or fat mass in overweight or obese adults. Therefore, whether calcium supplementation has a long-term effect on body weight remains unclear. Except for calcium, other dairy constituents have also been proposed to facilitate weight and body fat loss. For example, whey protein may have some beneficial effects on muscle sparing and lipid metabolism (49, 50), and conjugated linolenic acid may regulate adipogenesis, inflammation, and lipid metabolism (51). Taken together, future research is needed to further illustrate potential mechanisms of dairy products on body weight relevant to energy restriction and intervention duration.
Several issues warrant additional discussion. First, the effects of dairy on body weight and fat were not uniform because of the substantial heterogeneity in individual studies that were due to differences in study populations, study designs, and intervention methods and durations. Second, most of the studies did not use a double-blinded design because of practical reasons. Indeed, we showed that the weight- and fat-reducing effects of dairy were related to energy restriction and study duration. However, trials that imposed energy restrictions were small in size and relatively short in duration. In addition, influences of other factors such as the quality of products and amounts of specific bioactive components and their bioavailability could not be fully determined because of a lack of information in most existing studies. Efforts should be made to include a detailed nutrient content and compliance rate in future studies.
In conclusion, our meta-analysis does not support the beneficial effect of increasing dairy consumption on body weight and fat management in long-term studies or studies without energy restriction. However, dairy products may have modest benefits in facilitating weight loss when energy is restricted, but this effect seems to be short and not sustainable.
Acknowledgments
The authors thank Jo-Anne Gilbert, Leonie van Meijl, Cristina Palacios, and Andrea Josse for providing their unpublished data. We appreciate Eric Ding for his valuable comments.
The authors’ responsibilities were as follows—MC, AP, VSM, and FBH: designed the research; MC and AP: performed the systematic review and analyzed data; AP: reviewed data; FH: had primary responsibility for the final content of the manuscript; and all authors: wrote, reviewed, and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7 [DOI] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med 2007;356:213–5 [DOI] [PubMed] [Google Scholar]
- 3.Barr SI. Increased dairy product or calcium intake: is body weight or composition affected in humans? J Nutr 2003;133:245S–8S [DOI] [PubMed] [Google Scholar]
- 4.Barr SI, McCarron DA, Heaney RP, Dawson-Hughes B, Berga SL, Stern JS, Oparil S. Effects of increased consumption of fluid milk on energy and nutrient intake, body weight, and cardiovascular risk factors in healthy older adults. J Am Diet Assoc 2000;100:810–7 [DOI] [PubMed] [Google Scholar]
- 5.Manios Y, Moschonis G, Koutsikas K, Papoutsou S, Petraki I, Bellou E, Naoumi A, Kostea S, Tanagra S. Changes in body composition following a dietary and lifestyle intervention trial: the postmenopausal health study. Maturitas 2009;62:58–65 [DOI] [PubMed] [Google Scholar]
- 6.Palacios C, Bertran JJ, Rios RE, Soltero S. No effects of low and high consumption of dairy products and calcium supplements on body composition and serum lipids in Puerto Rican obese adults. Nutrition 2011;27:520–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes (Lond) 2005;29:391–7 [DOI] [PubMed] [Google Scholar]
- 8.Zemel MB, Teegarden D, Van Loan M, Schoeller DA, Matkovic V, Lyle RM, Craig BA. Dairy-rich diets augment fat loss on an energy-restricted diet: a multicenter trial. Nutrients 2009;1:83–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res 2004;12:582–90 [DOI] [PubMed] [Google Scholar]
- 10.Abargouei AS, Janghorbani M, Salehi-Marzijarani M, Esmaillzadeh A. Effect of dairy consumption on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Obes (Lond) 2012; Jan 17 (Epub ahead of print; DOI:10.1038/ijo.2011.269) [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 13.Baran D, Sorensen A, Grimes J, Lew R, Karellas A, Johnson B, Roche J. Dietary modification with dairy products for preventing vertebral bone loss in premenopausal women: a three-year prospective study. J Clin Endocrinol Metab 1990;70:264–70 [DOI] [PubMed] [Google Scholar]
- 14.Bowen J, Noakes M, Clifton PM. Effect of calcium and dairy foods in high protein, energy-restricted diets on weight loss and metabolic parameters in overweight adults. Int J Obes (Lond) 2005;29:957–65 [DOI] [PubMed] [Google Scholar]
- 15.Buchowski MS, Aslam M, Dossett C, Dorminy C, Choi L, Acra S. Effect of dairy and non-dairy calcium on fecal fat excretion in lactose digester and maldigester obese adults. Int J Obes (Lond) 2010;34:127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghadirian P, Shatenstein B, Verdy M, Hamet P. The influence of dairy products on plasma uric acid in women. Eur J Epidemiol 1995;11:275–81 [DOI] [PubMed] [Google Scholar]
- 17.Gunther CW, Legowski PA, Lyle RM, McCabe GP, Eagan MS, Peacock M, Teegarden D. Dairy products do not lead to alterations in body weight or fat mass in young women in a 1-y intervention. Am J Clin Nutr 2005;81:751–6 [DOI] [PubMed] [Google Scholar]
- 18.Gunther CW, Lyle RM, Legowski PA, James JM, McCabe LD, McCabe GP, Peacock M, Teegarden D. Fat oxidation and its relation to serum parathyroid hormone in young women enrolled in a 1-y dairy calcium intervention. Am J Clin Nutr 2005;82:1228–34 [DOI] [PubMed] [Google Scholar]
- 19.Nutrient Data Laboratory (US), Consumer and Food Economics Institute (US). USDA nutrient database for standard reference. Riverdale, MD.: USDA, Nutrient Data Laboratory, Agricultural Research Service, 1999 [Google Scholar]
- 20.Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr 2011;141:1626–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Chichester, United Kingdom; Hoboken, NJ: Wiley-Blackwell, 2008 [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624 [DOI] [PubMed] [Google Scholar]
- 24.Summerbell CD, Watts C, Higgins JP, Garrow JS. Randomised controlled trial of novel, simple, and well supervised weight reducing diets in outpatients. BMJ 1998;317:1487–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faghih S, Abadi AR, Hedayati M, Kimiagar SM. Comparison of the effects of cows’ milk, fortified soy milk, and calcium supplement on weight and fat loss in premenopausal overweight and obese women. Nutr Metab Cardiovasc Dis 2011;21:499–503 [DOI] [PubMed] [Google Scholar]
- 26.Gilbert JA, Joanisse DR, Chaput JP, Miegueu P, Cianflone K, Almeras N, Tremblay A. Milk supplementation facilitates appetite control in obese women during weight loss: a randomised, single-blind, placebo-controlled trial. Br J Nutr 2011;105:133–43 [DOI] [PubMed] [Google Scholar]
- 27.Kukuljan S, Nowson CA, Bass SL, Sanders K, Nicholson GC, Seibel MJ, Salmon J, Daly RM. Effects of a multi-component exercise program and calcium-vitamin-D3-fortified milk on bone mineral density in older men: a randomised controlled trial. Osteoporos Int 2009;20:1241–51 [DOI] [PubMed] [Google Scholar]
- 28.Wagner G, Kindrick S, Hertzler S, DiSilvestro RA. Effects of various forms of calcium on body weight and bone turnover markers in women participating in a weight loss program. J Am Coll Nutr 2007;26:456–61 [DOI] [PubMed] [Google Scholar]
- 29.Rosado JL, Garcia OP, Ronquillo D, Hervert-Hernandez D, Caamano Mdel C, Martinez G, Gutierrez J, Garcia S. Intake of milk with added micronutrients increases the effectiveness of an energy-restricted diet to reduce body weight: a randomized controlled clinical trial in Mexican women. J Am Diet Assoc 2011;111:1507–16 [DOI] [PubMed] [Google Scholar]
- 30.Chee WS, Suriah AR, Chan SP, Zaitun Y, Chan YM. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int 2003;14:828–34 [DOI] [PubMed] [Google Scholar]
- 31.Lau EM, Woo J, Lam V, Hong A. Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. J Bone Miner Res 2001;16:1704–9 [DOI] [PubMed] [Google Scholar]
- 32.Thomas DT, Wideman L, Lovelady CA. Effects of a dairy supplement and resistance training on lean mass and insulin-like growth factor in women. Int J Sport Nutr Exerc Metab 2011;21:181–8 [DOI] [PubMed] [Google Scholar]
- 33.Harvey-Berino J, Gold BC, Lauber R, Starinski A. The impact of calcium and dairy product consumption on weight loss. Obes Res 2005;13:1720–6 [DOI] [PubMed] [Google Scholar]
- 34.Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res 2005;13:1344–53 [DOI] [PubMed] [Google Scholar]
- 35.Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res 2005;13:1218–25 [DOI] [PubMed] [Google Scholar]
- 36.Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J Clin Nutr 2011;94:422–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Loan MD, Keim NL, Adams SH, Souza E, Woodhouse LR, Thomas A, Witbracht M, Gertz ER, Piccolo B, Bremer AA, et al. Dairy foods in a moderate energy restricted diet do not enhance central fat, weight, and intra-abdominal adipose tissue losses nor reduce adipocyte size or inflammatory markers in overweight and obese adults: a controlled feeding study. J Obes 2011;2011:989657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wennersberg MH, Smedman A, Turpeinen AM, Retterstol K, Tengblad S, Lipre E, Aro A, Mutanen P, Seljeflot I, Basu S, et al. Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study. Am J Clin Nutr 2009;90:960–8 [DOI] [PubMed] [Google Scholar]
- 39.van Meijl LE, Mensink RP. Effects of low-fat dairy consumption on markers of low-grade systemic inflammation and endothelial function in overweight and obese subjects: an intervention study. Br J Nutr 2010;104:1523–7 [DOI] [PubMed] [Google Scholar]
- 40.Thomas DT, Wideman L, Lovelady CA. Effects of calcium and resistance exercise on body composition in overweight premenopausal women. J Am Coll Nutr 2010;29:604–11 [DOI] [PubMed] [Google Scholar]
- 41.Louie JC, Flood VM, Hector DJ, Rangan AM, Gill TP. Dairy consumption and overweight and obesity: a systematic review of prospective cohort studies. Obes Rev 2011;12:e582–92 [DOI] [PubMed] [Google Scholar]
- 42.Holmes BA, Kaffa N, Campbell K, Sanders TA. The contribution of breakfast cereals to the nutritional intake of the materially deprived UK population. Eur J Clin Nutr 2012;66:10–7 [DOI] [PubMed] [Google Scholar]
- 43.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smilowitz JT, Wiest MM, Teegarden D, Zemel MB, German JB, Van Loan MD. Dietary fat and not calcium supplementation or dairy product consumption is associated with changes in anthropometrics during a randomized, placebo-controlled energy-restriction trial. Nutr Metab (Lond) 2011;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr 2005;24(Suppl):537S–46S [DOI] [PubMed] [Google Scholar]
- 46.Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, Tremblay A, Astrup A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 2009;10:475–86 [DOI] [PubMed] [Google Scholar]
- 47.Onakpoya IJ, Perry R, Zhang J, Ernst E. Efficacy of calcium supplementation for management of overweight and obesity: systematic review of randomized clinical trials. Nutr Rev 2011;69:335–43 [DOI] [PubMed] [Google Scholar]
- 48.Yanovski JA, Parikh SJ, Yanoff LB, Denkinger BI, Calis KA, Reynolds JC, Sebring NG, McHugh T. Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Ann Intern Med. 2009;150(12):821–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr 2010;104:716–23 [DOI] [PubMed] [Google Scholar]
- 50.Pihlanto-Leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: concentration and characterization of active peptides. J Dairy Res 2000;67:53–64 [DOI] [PubMed] [Google Scholar]
- 51.Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M. Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem 2010;21:171–9 [DOI] [PMC free article] [PubMed] [Google Scholar]